Introduction
Adolescent depression has been linked to a wide range of negative outcomes including suicide (Hawton et al., Reference Hawton, Saunders and O'Connor2012), substance use (Henry et al., Reference Henry, Feehan, McGee, Stanton, Moffitt and Silva1993) and various social impairments (Rudolph, Reference Rudolph, Nolen-Hoeksema and Hilt2009). Increasing evidence suggests that depression is also associated with an elevated risk of violent outcomes. The increased risk of violence has been reported in several longitudinal studies (Capaldi and Stoolmiller, Reference Capaldi and Stoolmiller1999; Kofler et al., Reference Kofler, McCart, Zajac, Ruggiero, Saunders and Kilpatrick2011; Fazel et al., Reference Fazel, Wolf, Chang, Larsson, Goodwin and Lichtenstein2015; Yu et al., Reference Yu, Aaltonen, Branje, Ristikari, Meeus, Salmela-Aro, Goodwin and Fazel2017) including using sibling and twin designs (Fazel et al., Reference Fazel, Wolf, Chang, Larsson, Goodwin and Lichtenstein2015). In addition, other designs, including cross-sectional and prevalence studies in selected samples, are consistent with this link (Arseneault et al., Reference Arseneault, Moffitt, Caspi, Taylor and Silva2000; Coid et al., Reference Coid, Yang, Roberts, Ullrich, Moran, Bebbington, Brugha, Jenkins, Farrell, Lewis and Singleton2006; Fairchild et al., Reference Fairchild, van Goozen, Stollery, Brown, Gardiner, Herbert and Goodyer2008; Ferguson et al., Reference Ferguson, San Miguel and Hartley2009; Piko and Pinczés, Reference Piko and Pinczés2014). However, inconsistent findings have been reported, as several studies found no clear associations between depression and violent outcomes (Chen et al., Reference Chen, Huang, Wang and Chang2012; van Dorn et al., Reference van Dorn, Volavka and Johnson2012; Marsh et al., Reference Marsh, Craven, Parker, Parada, Guo, Dicke and Abduljabbar2016). Clarifying moderators of the links between depression and violent outcomes is needed and may assist in the design of effective prevention and intervention programmes.
One potentially important moderator of the link between depression and violent outcomes could be differences in stress sensitivity (Raine, Reference Raine2002; Hellhammer et al., Reference Hellhammer, Wüst and Kudielka2009). Blunted hypothalamic–pituitary–adrenal (HPA) activity is a component of stress hypoarousal that may reduce the ability to share the distress of others (von Polier et al., Reference von Polier, Herpertz-Dahlmann, Konrad, Wiesler, Rieke, Heinzel-Gutenbrunner, Bachmann and Vloet2013; Johnson et al., Reference Johnson, Caron, Mikolajewski, Shirtcliff, Eckel and Taylor2014). Cortisol is the primary hormonal end product of the HPA axis and its response or activity is an important biological indicator of self-regulation, playing a central role in the regulation of emotional and behavioural responses to environmental stressors. Low cortisol activity is an indication of blunted HPA activity and is hypothesized to be linked to antisocial behaviours (Raine, Reference Raine2002). The cortisol awakening response (CAR) refers to the marked morning rhythm normally exhibited by cortisol within the first hour after awakening, and is characterized by a rapid increase in levels upon awakening, peaking at around 30 min post-awakening, and declining thereafter (Wüst et al., Reference Wüst, Federenko, Hellhammer and Kirschbaum2000). The overall volume of cortisol released over the waking period [area under the curve ground (AUCg)] and the absolute changes in cortisol levels post-awakening [area under the curve increase (AUCi)] provide useful and reliable markers of HPA activity (Pruessner et al., Reference Pruessner, Wolf, Hellhammer, Buske-Kirschbaum, von Auer, Jobst, Kaspers and Kirschbaum1997).
Support for the association between CAR and antisocial behaviours comes from studies that report lower morning cortisol levels and increased risk of violent outcomes in clinical samples with attention-deficit hyperactivity and conduct disorders (Pajer et al., Reference Pajer, Gardner, Rubin, Perel and Neal2001; Freitag et al., Reference Freitag, Hänig, Palmason, Meyer, Wüst and Seitz2009) and general community samples (Shirtcliff et al., Reference Shirtcliff, Granger, Booth and Johnson2005; Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013). In particular, several longitudinal studies have reported that low morning cortisol levels predicted adolescent aggressive behaviours 5 years later (Shoal et al., Reference Shoal, Giancola and Kirillova2003) and persistent aggressive behaviours over 3 years (Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013). However, this is not a consistent finding. For instance, studies in predominantly male samples of adolescent offenders and disruptive youth have shown non-significant links (Dabbs et al., Reference Dabbs, Jurkovic and Frady1991; Scerbo and Kolko, Reference Scerbo and Kolko1994).
Previous studies have shown that psychological factors such as depression and biological features such as CAR can independently lead to aggressive and violent behaviours. From a psychobiological point of view, aggressive and violent behaviours can also be regarded as the outcome of the interaction between psychological and biological factors (Quay, Reference Quay1993). Thus, depression is likely to interact with CAR in predicting adolescent violent outcomes. Based on prior research (Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013; Fazel et al., Reference Fazel, Wolf, Chang, Larsson, Goodwin and Lichtenstein2015), we propose the following hypotheses on the patterns of interaction. Specifically, when adolescents have higher levels of depressive symptoms, they have an increased risk of violent outcomes, in particular when their CAR decreases. The theoretical basis for this is that as depressive symptoms include pervasive low mood, negativity, irritability, agitation and pessimism, when these symptoms are experienced in combination with low CAR, adolescents will have more difficulty dealing with or regulating these symptoms and be more likely to act out their distress with violence and aggression towards others. In contrast, high CAR may act as a protective factor in the link between depressive symptoms and violence. In particular, it might serve as a biological buffer to prevent individuals from acting out depressive symptoms with violent behaviours. Thus, we hypothesize that depression is not associated with violent outcomes when CAR is higher. We tested these hypotheses with the data from a longitudinal study of adolescents. Understanding the interplay between cortisol response and depressive symptoms is important as it could contribute to the possibility of using biomarkers as part of prognostic assessments in mental health (Vitacco et al., Reference Vitacco, Shirtcliff, Dismukes and Johnson2015).
Methods
Sample
Participants were 358 adolescents (205 boys) who took part in cortisol awaking measurements at wave 3 of the ongoing longitudinal RADAR Young study (N = 497, Research on Adolescent Development And Relationships). RADAR Young is a cohort study focusing on adolescent developmental outcomes including internalizing and externalizing problem behaviours and physiological developments. The current study was based on the data from the third to fifth annual waves. The mean age of the participants in wave 3 was 15.0 years (ranging from 14.0 to 17.6; s.d. = 0.5). All participants identified themselves as Dutch. In this sample, 10.5% were from low socioeconomic status family, which was defined as having a father and mother who were unemployed or held a manual job (Statistics Netherlands, 1993). In addition, analyses of all variables used in this study revealed a normed χ2 (χ2/df) of 1.04, which indicates that the pattern of the missing data was not materially different from a missing completely at random pattern (Bollen, Reference Bollen1989).
Procedure
Participants were recruited from various Dutch elementary schools. Across 3 years at a similar time of each year, adolescents filled out questionnaires on socio-demographic, depression and violence measures during annual home visits, supervised by trained research assistants. In addition to the administration of the behavioural measurements, research assistants gave detailed verbal and written instructions for cortisol measurements. The RADAR study has been approved by the medical ethics committee of the University Medical Center in Utrecht, The Netherlands.
Measurements
Depressive symptoms
The Reynolds Adolescent Depression Scale, second edition (RADS-2) (Reynolds, Reference Reynolds2002) was used to measure depression symptoms. This self-report questionnaire includes 23 items (e.g. ‘I feel nobody cares about me.’) Adolescents responded to the questionnaire on a four-point Likert scale, ranging from 1 (almost never) to 4 (usually). Scores can range from 23 to 92. Previous research has shown good psychometric properties of RADS-2 (e.g. test–retest reliabilities >0.7 in diverse samples) (Reynolds, Reference Reynolds, Hersen, Segal and Hilsenroth2004). In the current sample, the Cronbach's α of this scale was 0.9 in all three annual waves and the average test–retest reliability with a 1-year interval was 0.7 across waves.
Violent behaviours
Adolescents’ violent behaviours in the last 12 months were measured on a self-reported scale based on a large international comparative study on delinquency (Enzmann et al., Reference Enzmann, Marshall, Killias, Junger-Tas, Steketee and Gruszczynska2010). Violent behaviours were measured with five items including: stealing from person with threat/force, assaulting, injuring someone with a weapon, and beating and/or kicking (with/without) causing injury. Adolescents responded on a five-point scale, ranging from 0 (never) to 4 (more than ten times). Scores can range from 0 to 20. The Cronbach's αs of this scale were 0.5 at wave 3, 0.7 at wave 4 and 0.6 at wave 5 and the average test–retest reliability with a 1-year interval was 0.5 across 3 years.
Physical aggression
Physical aggression was measured with a self-reported questionnaire (Linder et al., Reference Linder, Crick and Collins2002) via six items (e.g. ‘I push or punch others to get what I want.’) Adolescents responded to these items on a seven-point Likert scale, ranging from 1 (not at all true) to 7 (very true). Scores can range from 6 to 42. Prior research has indicated good reliability and validity (Linder et al., Reference Linder, Crick and Collins2002). The Cronbach's α for the scale was 0.9 in all three waves and the average test–retest reliability with a 1-year interval was 0.7 across the three waves.
Cortisol awakening responses
CARAUCg and CARAUCi were measured in the saliva that was collected by passive drooling, immediately after awakening (Cort0), 30 min (Cort30) and 60 min (Cort60) later. Cortisol sampling took place in February and March of each consecutive year, as soon as possible after assessing depression and violent outcomes from wave 3 to 5. The saliva sampling was scheduled on a typical weekday during the school year. Participants were instructed to rinse their mouths with water before sampling, and not to eat, drink, smoke or brush their teeth before completing Cort60. They were requested to collect their saliva through a small straw into a polypropylene tube, and label these tubes with the time and date of sampling. After collection, participants were asked to store the samples in the refrigerator and send them by mail to the research centre the same day. At the research centre, the cortisol collections were stored uncentrifuged at −20 °C until analysis. Salivary cortisol levels were analysed using electrochemiluminescence immunoassay (E170 Roche, Switzerland). The lower detection limit was 0.5 nmol/l, and the mean intra-assay and inter-assay coefficients of variation were 3% and 12%, respectively. Cases were excluded from analyses if the cortisol data used incorrect sampling time, or if it was unclear how it was sampled (i.e. not registered) or contaminated (e.g. by smoking or brushing teeth). In the current study, 358 participants provided qualified data. CARAUCg is a summary parameter of the repeated measurements of CAR. Thus, it is an estimation of total adrenal cortisol secretion during the first hour after awakening. CARAUCi is the absolute change in cortisol levels during the first hour post-awakening. We calculated the CARAUCg and CARAUCi with the formula provided by Pruessner et al. (Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). Specifically, CARAUCg = (Cort30 + Cort0)/2 + (Cort60 + Cort30)/2 and CARAUCi = (Cort30 + Cort0)/2 + (Cort60 + Cort30)/2 − (3–1) × Cort0.
Time dynamic covariates
As alcohol drinking, substance use and stressful life experiences might affect both CAR (Clow et al., Reference Clow, Thorn, Evans and Hucklebridge2004; Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013) and violent outcomes (Hoffmann and Cerbone, Reference Hoffmann and Cerbone1999), the effects of these factors were adjusted. Alcohol use over the last 4 weeks was assessed with a question with six response options, ranging from ‘none’ to ‘daily’. Substance use was defined as illicit drug use that was assessed with six questions (e.g. How many times have you used XTC/marijuana/cocaine/mushrooms/amphetamine/heroin in the last 12 months?). Responses range from ‘0 time’ to ‘40 times or more’. Stressful experiences included sexual assault, physical assault and being threatened with violence, and were measured with the International Crime Victims Survey (Nieuwbeerta, Reference Nieuwbeerta2002). Participants were asked to indicate their stressful life experiences with five items (e.g. Has anyone ever touched you against your will in any sexual way in the past year?). Responses include ‘yes’ and ‘no’. These time-varying factors were measured at the same three annual waves as the other predictors.
Statistical analyses
We adopted a within-individual design applying fixed-effects methods to examine the interaction effects between depressive symptoms and CAR on adolescent violent outcomes. Unlike between-individual approaches, estimators in the within-individual model rely only on within-individual changes over time. Adolescence offers a promising period for using this design as individuals pass through significant changes in all the key studied variables (Moffitt, Reference Moffitt1993; Shirtcliff et al., Reference Shirtcliff, Allison, Armstrong, Slattery, Kalin and Essex2012; Thapar et al., Reference Thapar, Collishaw, Pine and Thapar2012; Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013).
In addition, in the within-individual model, each individual acts as their own control. As no changes over time occur in time-invariant variables, the effects of them are automatically controlled for in the within-individual design (Gunasekara et al., Reference Gunasekara, Richardson, Carter and Blakely2013). Further details of the rationale and regression equations of this method are reported elsewhere (Allison, Reference Allison2009). As many time-invariant confounding factors, such as genetic and early environmental factors (Risch et al., Reference Risch, Herrell, Lehner, Liang, Eaves, Hoh, Griem, Kovacs, Ott and Merikangas2009; Roisman et al., Reference Roisman, Barnett-Walker, Owen, Bradley, Steinberg, Susman, Booth-LaForce, Belsky and Houts2009; Byrd and Manuck, Reference Byrd and Manuck2014; St Clair et al., Reference St Clair, Croudace, Dunn, Jones, Herbert and Goodyer2015), might be linked to the studied variables, this method provides parameter estimates that are less subject to bias (Allison, Reference Allison2009; Gunasekara et al., Reference Gunasekara, Richardson, Carter and Blakely2013).
We further extended our model to adjust for time-varying factors including alcohol drinking, hard drug use and stressful life events at each of the three measurement points. In addition, as gender differences have been suggested in the developmental changes of and interactions among studied variables (Dodge et al., Reference Dodge, Coie, Lynam, Damon and Lerner2006; Card et al., Reference Card, Stucky, Sawalani and Little2008; Avenevoli et al., Reference Avenevoli, Swendsen, He, Burstein and Merikangas2015), as well as in other unmeasured time-varying variables such as sex hormones including testosterone and oestradiol (Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol, Jansen, van Engeland and Doreleijers2007; Mehta et al., Reference Mehta, Welker, Zilioli and Carré2015; Tackett et al., Reference Tackett, Reardon, Herzhoff, Page-Gould, Harden and Josephs2015), we conducted within-individual analyses for boys and girls separately. We also tested three-way interactions between depression, CAR and gender to assess whether the gender differences were statistically significant. Furthermore, we examined whether the interaction effects between depression and CAR differed by age. In addition, as within-individual correlations between CARAUCi and CARAUCg were small to moderate (r was 0.42 in girls and 0.16 in boys; Table 1), we included CARAUCg and CARAUCi in the same model. Data were analysed using STATA SE version 14 (StataCorp, 2015).
Table 1. Descriptives of and correlations between depressive symptoms, cortisol awakening responses (CAR), violence behaviours and physical aggression

s.d., standard deviation; CARAUCg, cortisol awakening response area under the curve ground; CARAUCi, cortisol awakening response area under the curve increase. The descriptives and correlations were based on 445 observations in 153 girls and 595 in 205 boys. Data for stressful life events were not included in the correlational matrix due to their binary feature. Coefficients in bold are significant (p < 0.05). Correlations regarding girls’ data are presented below the diagonal, regarding boys’ data above the diagonal.
When the interaction effects between depression and CAR were significant, we examined the shape of the interaction by probing the interaction effects. To examine this, simple slopes for each interaction were presented [at 1 standard deviation (s.d.) above and below mean of the moderators (CARAUCg and CARAUCi)]. Moreover, we applied the Johnson–Neyman technique, using the computational tool of Preacher et al. (Preacher et al., Reference Preacher, Curran and Bauer2006) to calculate the values of moderators (CARAUCg and CARAUCi) at which the regression of outcomes (violence and aggression) on predictors (depressive symptoms) moves from non-significance to significance (http://www.quantpsy.org/interact/mlr2.htm) (Hayes and Matthes, Reference Hayes and Matthes2009).
Results
Descriptive statistics
Table 1 shows an overview of means of and bivariate intercorrelations among depressive symptoms, CAR and violent outcomes for adolescents across three waves. This descriptive information and correlations were based on observations (including repeated measurements of individuals) across three time points. In this community sample, 24% of adolescents perpetrated violent behaviours over 3 years. There were positive correlations between depression and violent outcomes. In general, there was no association between CAR and depression, expect for a small correlation between depression and CARAUCg in boys (r = 0.10) and no association between CAR and violent outcomes, expect for small correlations between CARAUCg and aggression in girls (r = 0.11) and CARAUCi and violence in boys (r = 0.09). Table 2 and Table 3 present the results of our final models examining the interaction effects between CAR and depressive symptoms on adolescent violent outcomes.
Table 2. Interaction effects between depressive symptoms and cortisol awakening response (CAR) on adolescent violence

CARAUCg, cortisol awakening response area under the curve ground; CARAUCi, cortisol awakening response area under the curve increase.
***p < 0.001; **p < 0.01; *p < 0.05.
Table 3. Interaction effects between depressive symptoms and cortisol awakening response (CAR) on adolescent physical aggression

CARAUCg, cortisol awakening response area under the curve ground; CARAUCi, cortisol awakening response area under the curve increase.
**p < 0.01; *p < 0.05.
Interaction effects between CAR and depressive symptoms on adolescent violent outcomes
Three of the four models showed significant interaction effects between CAR and depressive symptoms in predicting adolescent violent outcomes at the within-individual level over 3 years, showing that increases in depressive symptoms were associated with elevated risk of violent outcomes when CAR was low. The interaction effects between CARAUCi and depressive symptoms on violent outcomes including both violence and aggression were significant in girls but not in boys (Table 2). The interaction effect in predicting violence was marginally stronger in girls than boys [B (s.e.) = −0.20 (0.11), β = −0.13, p = 0.07]. In addition, there was a significant interaction effect between CARAUCg and depressive symptoms in predicting aggression in boys, whereas no such interaction was present in girls (Table 3). Three-way interaction analyses indicated that this effect was significantly stronger in boys than girls [B (s.e.) = 1.02 (0.36), β = 0.20, p < 0.01].
These interaction effects indicated that the associations between depressive symptoms and adolescent violent outcomes differed for varying levels of CARAUCi in girls and CARAUCg in boys. Region of significance tests revealed that when CARAUCi levels were ⩽−1.09 s.d. (standard deviation) and ⩽−1.71 s.d. below the mean, higher depressive symptoms significantly predicted higher levels of violence and aggression in girls, respectively. The reverse was true for the associations between depression and the two violent outcomes when CARAUCi was higher than certain levels. Specifically, when CARAUCi levels were ⩾0.65 s.d. and ⩾0.85 s.d. above the mean, higher depressive symptoms significantly predicted lower levels of girls’ violence and aggression, respectively. In addition, CARAUCg moderated the association between depressive symptoms and aggressive behaviours in boys. When the CARAUCg level was ⩽−0.24 s.d. below the mean, depressive symptoms were positively related to aggressive behaviours in boys, whereas when the CARAUCg level was ⩾3.04 s.d. above the mean, depressive symptoms were negatively associated with aggressive behaviours. The interactive effects were visualized by showing simple slopes for high in CAR (at 1 s.d. above the mean) and low in CAR (at 1 s.d. below the mean) (Fig. 1a–c). The simple slopes for girls with high and low CARAUCi are depicted in Fig. 1a and b. The simple slopes for boys with high and low CARAUCg are depicted in Fig. 1c.
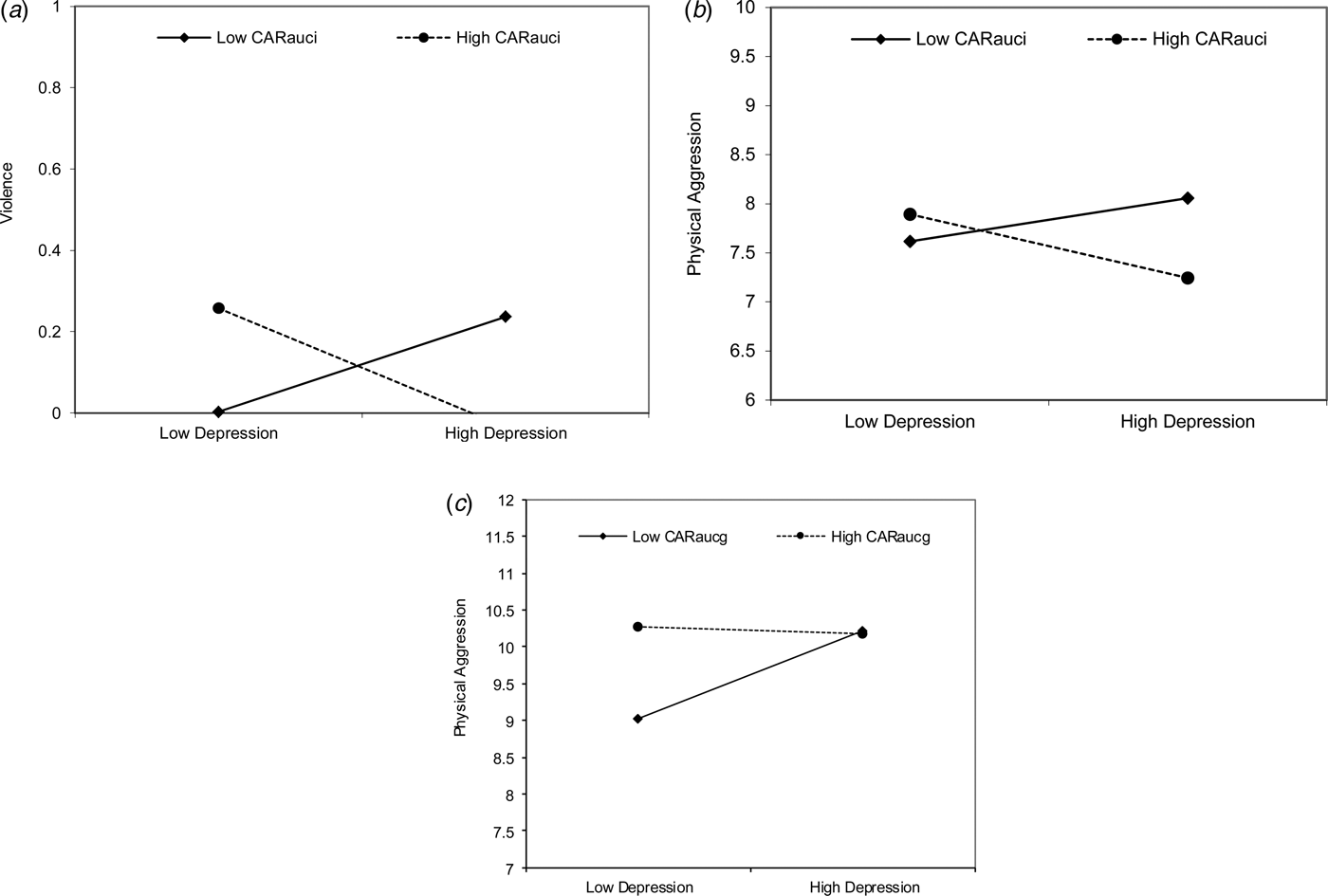
Fig. 1. (a) Interaction effects between depression and CARAUCi in predicting violence in girls. (b) Interaction effects between depression and CARAUCi in predicting aggression in girls. (c) Interaction effects between depression and CARAUCg in predicting aggression in boys. (a–c) CARAUCi, cortisol awakening response area under the curve increase; CARAUCg, cortisol awakening response area under the curve ground. Low, one s.d. below mean; High, one s.d. above mean.
We found that the interaction effect between depression and CARAUCg in predicting aggression in boys varied by age. The three-way interaction effect between depression, CARAUCg and age was: B (s.e.) = −0.59 (0.26), β = −0.10, p = 0.03, which suggested a stronger interaction effect when boys were older. No other age effects were found in the interaction effects.
In addition, we did sensitivity analyses to test whether the interaction effects between depression and CAR existed for subtypes of aggressive behaviours. We found that the depression × CARAUCg interaction effects occurred in predicting both proactive [B (s.e.) = −0.38 (0.12), β = −0.15, p < 0.01] and reactive physical aggression [B (s.e.) = −0.26 (0.14), β = −0.07, p = 0.06] in boys. Further, there were depression × CARAUCi interaction effects on both proactive [B (s.e.) = −0.33 (0.10), β = −0.20, p < 0.01] and reactive physical aggression [B (s.e.) = −0.23 (0.12), β = −0.10, p = 0.06] in girls.
Discussion
In this study of 358 adolescents who were assessed annually for 3 years, we examined interactions between depressive symptoms and CAR in predicting adolescent violent outcomes using within-individual models. These models, where the effects are estimated based on variations within the same person, allowed for time-invariant factors to be accounted for, such as genetic background and early environmental experiences. We found that CAR moderated the links between depressive symptoms and adolescent violent outcomes. When CARAUCi were low, increases in depressive symptoms were positively associated with increases in violent and aggressive behaviours at the within-individual level in girls. A similar interaction pattern appeared between depressive symptoms and CARAUCg in predicting aggressive behaviours at the within-individual level in boys.
One explanation for the moderating effect of CAR is that cortisol could be associated with psychological variables, such as callous and unemotional traits, which are on the pathway between depression and violent outcomes. Low cortisol has been reported in individuals with psychopathic and callous-unemotional traits (von Polier et al., Reference von Polier, Herpertz-Dahlmann, Konrad, Wiesler, Rieke, Heinzel-Gutenbrunner, Bachmann and Vloet2013; Johnson et al., Reference Johnson, Caron, Mikolajewski, Shirtcliff, Eckel and Taylor2014). Hence, it is possible that low cortisol may lead to disinhibition (Freitag et al., Reference Freitag, Hänig, Palmason, Meyer, Wüst and Seitz2009) and possibly more expression of callous-unemotional traits (von Polier et al., Reference von Polier, Herpertz-Dahlmann, Konrad, Wiesler, Rieke, Heinzel-Gutenbrunner, Bachmann and Vloet2013; Johnson et al., Reference Johnson, Caron, Mikolajewski, Shirtcliff, Eckel and Taylor2014) which in turn may be linked to higher risk of violent outcomes in depressed individuals. However, this potential pathway will need to be validated. Furthermore, low CAR has been linked to conduct disorders (Pajer et al., Reference Pajer, Gardner, Rubin, Perel and Neal2001), which are associated with various violent behaviours (Arseneault et al., Reference Arseneault, Moffitt, Caspi, Taylor and Silva2000). Thus, conduct disorders could potentially function as a mediator through which the risk of violent outcomes increases when an individual's depressive symptoms increased and CAR decreased. Finally, blunted CAR has been associated with poor behavioural regulation when confronting environmental stressors (Raine, Reference Raine2002). Dysfunctional regulation might also lead individuals to act out violently when depressive symptoms are higher and CAR is lower. More research is required to unpack different potential mechanisms behind the interaction effects.
Our study showed differential patterns of interaction effects between CAR and depression among boys and girls. The moderating role of CARAUCi in the effects of depressive symptoms on violence and aggression was significant only in girls, whereas the moderating effects of CARAUCg appeared in predicting aggression in boys. One possibility could be that other sex hormones, such as testosterone, play a role in the interaction between depression and CAR. The participants in this study were followed from ages 15 to 17, which is an important period of sex hormonal development in adolescence with oestrogen and progesterone increasing in girls and testosterone rising in boys (Rowe et al., Reference Rowe, Maughan, Worthman, Costello and Angold2004). Prior studies have showed that low cortisol predicts high levels of physical aggression particularly when the levels of testosterone are high (Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol, Jansen, van Engeland and Doreleijers2007). It is possible that sex hormones interact with CAR and lead to differential interaction patterns between depression and CAR. For instance, it could be that depressive symptoms increase the risk of violent outcomes, but only when cortisol is low and testosterone is high. Future studies in this area should consider including sex hormones.
The negative link between depression and aggression in boys only appeared when CARAUCg was ⩾3.04 s.d. above the mean. This suggests that the protective or buffering effects of high CARAUCg on the effect of depression on aggression in boys was not as strong as that of CARAUCi on the effect in girls. That is, boys would only score low in aggression when CAR and depressive symptoms are low, whereas girls could also score low in violent outcomes when CAR and depressive symptoms are high. Further work to understand the mechanisms underlying these gender differences is required.
We found that the interaction effects between depression and CARAUCg on aggression in boys differed by age. That is, when CARAUCg was low, higher depression would lead to more aggression when the boys were older, compared with when they were younger. No age effects were found in the interaction between depression and CARAUCi in predicting violent outcomes in girls. This might be related to gender differences in development during adolescence. On average, the onset of puberty is earlier and maturation is achieved sooner in girls than boys (Colom and Lynn, Reference Colom and Lynn2004). It is possible that the age effect already occurred for girls, and the age range in the current study did not capture this.
Overall, the findings suggest that prevention and intervention efforts could consider the interplay between biomarkers (such as CAR) and mental health (including depressive symptoms) in predicting the risk of violence. It suggests that biological markers can provide additional information that may assist in the assessment of risk of violence and aggression, beyond current approaches that rely on historical and clinical factors.
Our study has several strengths including the longitudinal design. Repeated measures of both predictors and outcomes allowed for a within-individual design. In this study, the interaction effects referred to whether variations in CARs moderated the link between changes in depression and violent outcomes at the within-individual level. This approach enabled us to take into account time-invariant confounders such as genetic and early environmental factors (e.g. childhood adversity), which have shown consistent associations with the studied variables (Chida and Steptoe, Reference Chida and Steptoe2009; Lewis and Plomin, Reference Lewis and Plomin2015; Sitnick et al., Reference Sitnick, Shaw, Weaver, Shelleby, Choe, Reuben, Gilliam, Winslow and Taraban2017). In addition, compared with a single measurement of CAR, our repeated measures across three annual times increased the reliability of CAR (Hellhammer et al., Reference Hellhammer, Fries, Schweisthal, Schlotz, Stone and Hagemann2007), especially given low stability of morning cortisol levels (Shirtcliff et al., Reference Shirtcliff, Granger, Booth and Johnson2005).
However, several limitations should be noted. First, the measurement of depressive symptoms and violent outcomes was based on self-report data, which might be subject to socially desirable response bias. Presence of such bias could lead to under-reporting of violence and aggression reflected by the reliability of the violent behaviours measure (which was below 0.7 in two out of three waves). However, adolescent self-report, particularly of internalizing problems such as depression, remains an important source of information (Sourander et al., Reference Sourander, Helstelä and Helenius1999), and the measures we used demonstrate good external validity. Second, although we have tried to account for residual confounds using within-individual analyses and additionally controlled for alcohol drinking, substance use and stressful life events over time, other relevant time-varying confounding factors such as sex hormones might have been missed (Popma et al., Reference Popma, Vermeiren, Geluk, Rinne, van den Brink, Knol, Jansen, van Engeland and Doreleijers2007; Mehta et al., Reference Mehta, Welker, Zilioli and Carré2015; Tackett et al., Reference Tackett, Reardon, Herzhoff, Page-Gould, Harden and Josephs2015). More studies are needed to test for bias from unmeasured time-varying confounding. Third, we noted an average decrease in CARAUCi for boys. Although this may be an artefact of a delay in sampling after awakening, negative CARAUCi could occur in accurate sampling, which has been reported (Bäumler et al., Reference Bäumler, Kirschbaum, Kliegel, Alexander and Stalder2013; Miller et al., Reference Miller, Plessow, Kirschbaum and Stalder2013; Smyth et al., Reference Smyth, Clow, Thorn, Hucklebridge and Evans2013). Furthermore, it has been shown that in general CARAUCi becomes less negative with age (Platje et al., Reference Platje, Jansen, Raine, Branje, Doreleijers, de Vries-Bouw, Popma, van Lier, Koot and Meeus2013). Finally, this is the first investigation, to our knowledge, of the depression–cortisol interaction effects on adolescent violent outcomes. Future research is needed to replicate these findings in different samples (e.g. clinical populations) and with different measurements of cortisol activity (e.g. hair cortisol) and reactivity (e.g. responses to social challenges) to triangulate the results.
In conclusion, this study demonstrated that CAR, including both CARAUCg and CARAUCi, moderated the association between depressive symptoms and adolescent violent outcomes. The findings suggest that the CAR should be investigated further as a potential biological marker for violence in adolescents with high levels of depressive symptoms.
Financial support
Data from RADAR Young (Research on Adolescent Development And Relationships) were used. RADAR has been financially supported by main grants from the Netherlands Organisation for Scientific Research (NWO; GB-MAGW 480-03-005, GB-MAGW 480-08-006), and Stichting Achmea Slachtoffer en Samenleving (SASS), a grant from the NWO to the Consortium Individual Development (CID; 024.001.003), and various other grants from the NWO, the VU University Amsterdam, and Utrecht University. The first author of this study (R. Yu) is funded by a Rubicon Research Fellowship (446-15-002) from the NWO. The last author of this study (S. Fazel) is funded by the Wellcome Trust Senior Research Fellowship (202836/Z/16/Z). The funders of this research had no role in the study design, analysis and interpretation of data, writing the report, or in the decision to submit the paper for publication.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.






