The Question: What is the therapeutic dose and duration of treatment for esketamine (Spravato) nasal spray in depression?
The Psychopharmacological Dilemma: Finding an effective treatment regimen utilizing esketamine (Spravato) spray for treatment-resistant depression (TRD)
 Pretest self-assessment question
Pretest self-assessment question
How are the antidepressant effects of esketamine (Spravato) pharmacodynamically mediated?
N-methyl-D-aspartate receptor (NMDAR) antagonism
Increases brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin (mTOR) activity
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR) antagonism
Dopamine-2 receptor (D2) agonism
A and B
All of the above
 Patient evaluation on intake
Patient evaluation on intake
A 42-year-old woman with a chief complaint of being “depressed, hanging on barely”
Chronically depressed with comorbid panic disorder (PD), uses polypharmacy and vagal nerve stimulation (VNS), all of which seem to be failing over last several weeks
Presents for first esketamine (Spravato) spray treatment today
Recurrent MDD now in recurrence with extensive treatment failures
Panic disorder is remitted though
Currently exhibits anhedonia, dysphoric mood and constricted congruent affect which are affecting her performance at work
MDD symptoms escalating with occasional passive suicidal ideation which is worrisome
Compliant with previous medication management
Reports no side effects on current regimen
No contraindications to esketamine (Spravato) nasal spray treatment, has prior authorization, and has read the patient guide regarding treatment
 Psychiatric history
Psychiatric history
Long history of MDD with some resultant anxiety, with fear of others’ judgments, and also suffers from PD with mild agoraphobic symptoms
Previously endorsed symptoms of fatigue, hypersomnia, social isolation, low motivation, appetite disturbance, feelings of guilt and low self-worth, and negative self-thinking
VNS implanted in 2007 with adequate response for several years, but response now fading
Prior to today, failed 30+ medications (including electroconvulsive therapy [ECT], VNS, IV ketamine [weekly dosing], and psychotherapy)
 Social and personal history
Social and personal history
Former smoker, quit several years ago
° Recently restarted smoking with onset of recurrent MDD symptoms
° Prior history of light daily cigarette-smoking
Rare alcohol use
No recreational drug use
Single parent with grown son
Works as a nurse per diem
 Medical history
Medical history
Osteoarthritis
Gastroesophageal reflux disease (GERD)
Kidney stones
Raynaud phenomenon
 Family history
Family history
History of depression and anxiety, but extent is unknown
Patient’s family places emphasis on appearances and impressions, which causes her to fear making mistakes and looking foolish
Anxiety disorder in her paternal aunt and grandmother
 Medication history
Medication history
Previous therapeutic failures:
SSRIs: selective serotonin reuptake inhibitors
° Fluoxetine (Prozac)
° Sertraline (Zoloft)
SNRIs: serotonin–norepinephrine reuptake inhibitors
° Venlafaxine (Effexor XR)
° Duloxetine (Cymbalta)
SARIs: serotonin antagonist / reuptake inhibitors
° Trazodone (Desyrel)
° Nefazodone (Serzone)
NDRI: norepinephrine–dopamine reuptake inhibitor
° Bupropion (Wellbutrin XL)
NaSSA: norepinephrine antagonist / selective serotonin antagonist
° Mirtazapine (Remeron)
TCAs: tricyclic antidepressants
° Doxepin (Adapin)
° Desipramine (Norpramin)
° Amitriptyline (Elavil)
° Clomipramine (Anafranil)
MAOIs: monoamine oxidase inhibitors
° Selegiline (Emsam)
° Tranylcypromine (Parnate)
SPARIs: serotonin partial agonist / reuptake inhibitors
° Vilazodone (Viibryd)
Augmentations
° Benzodiazepines (BZs)
▪ Lorazepam (Ativan)
▪ Diazepam (Valium)
▪ Clonazepam (Klonopin)
▪ Estazolam (Prosom)
▪ Zolpidem (Ambien)
Atypical antipsychotics
° Quetiapine (Seroquel)
° Aripiprazole (Abilify)
° Olanzapine (Zyprexa)
Anticonvulsant
° Divalproex (Depakote)
° Lamotrigine (Lamictal)
Stimulant
° Dextroamphetamine (Dexadrine)
° d/l-amphetamine (Adderall)
° Methylphenidate (Ritalin)
° Dexmethylphenidate (Focalin)
° Modafinil (Provigil)
° Armodafinil (Nuvigil)
Nutraceutical
° N-acetylcysteine (NAC)
° L-methylfolate (Deplin)
D2/D3 partial agonist
° Pramipexole (Mirapex)
Others
° Buspirone (Buspar), a serotonin 1A (5-HT1A) partial receptor agonist
° Lithium (Eskalith), a calcium modulating mood stabilizer
° Liothyronine (Cytomel), a thyroid augmentation
° Atomoxetine (Strattera), a norepinephrine reuptake inhibitor (NRI)
Interventional
° VNS
° ECT
° Ketamine (Ketalar) IV failed (6 sessions, 0.5 mg/kg once weekly)
 Psychotherapy history
Psychotherapy history
Limited success in weekly psychotherapy
Admits that she has given up quickly with psychotherapy when she feels uncomfortable
Not currently in psychotherapy
 Current medications
Current medications
Vortioxetine (Trintellix) 20 mg/d (SPARI)
Alprazolam (Xanax) 0.75 mg/d, a BZ
Cariprazine (Vraylar) 0.75 mg/d, an atypical antipsychotic
Lisdexamfetamine (Vyvanse) 70 mg/d, a stimulant
L-methylfolate (Deplin) 15 mg/d, a nutraceutical one carbon cycle enhancer
Vagus nerve stimulator
 Question
Question
Considering the patient’s multiple failed treatments, including previous failure of ketamine IV, do you think it is likely that the esketamine (Spravato) intranasal spray treatment will be effective?
Yes: as the ketamine (Ketalar) IV was given weekly, and esketamine (Spravato) will use a loading dose with multiple sessions within the first few weeks
No: as ketamine (Ketalar) IV is absorbed more efficiently than intranasal esketamine (Spravato), it is unlikely that intranasal esketamine (Spravato) will be effective at this time
 Attending physician’s mental notes: initial psychiatric evaluation
Attending physician’s mental notes: initial psychiatric evaluation
Although intranasal absorption of a drug is much less compared to IV administration, esketamine has four-fold more potency for NMDAR antagonism compared to racemic ketamine
Given the ease of intranasal administration compared to IV treatments, it is worth evaluating intranasal esketamine spray as a treatment option for TRD even if ketamine (Ketalar) IV failed
Additionally, her ketamine (Ketalar) IV treatments did not use a loading dose. Therefore, it was possibly subtherapeutic. A loading dose will be used for the esketamine (Spravato) treatments
Patient with chronic MDD and anxiety is on polypharmacy and VNS, but now has recurrence of a new MDD episode without many new options or new treatments available in the current pipeline
Esketamine (Spravato) is newly available and likely worth a trial, even if palliative
 Case outcome: first esketamine (Spravato) treatment
Case outcome: first esketamine (Spravato) treatment
Patient was prepped and dosed with the usual starting 56 mg esketamine (Spravato) spray over several minutes and tolerated administration well with initial blood pressure of 126/78
40 minutes after administration, patient reported mild sedation and some “loopy” feelings for several minutes but was not sedated nor dissociative, with a blood pressure of 110/70
° Interestingly, blood pressure lowered here when there are regulatory warnings for likely increases during treatment sessions
2 hours after administration, patient reported no side effects and was ready to go home, with a final blood pressure of 112/68
° Patients are obligated to have someone else drive them home after sessions
 Question
Question
 Attending physician’s mental notes: first psychiatric follow-up visit
Attending physician’s mental notes: first psychiatric follow-up visit
Effects of esketamine (Spravato) and ketamine (Ketalar) are primarily mediated by its antagonistic NMDAR activity, causing its anesthetic and analgesic effects
Esketamine/ketamine also can have antagonistic activity at monoaminergic, muscarinic, and nicotinic receptors, causing a range of adverse effects that should be monitored for after administration
Patient tolerated first esketamine (Spravato) spray well and had minimal issues with the usual side effects of sedation, nausea, hypertension, and dissociation
Due to recurrent MDD, we agreed to continue current medications and increase dosing of esketamine (Spravato) sprays to the higher available 84 mg/d dose and continue them twice weekly
Reported feelings of anxiety are stable and only situational; panic has not worsened due to nasal sprays
 Case outcome: weeks 2 through 8
Case outcome: weeks 2 through 8
Over the next 4 weeks, patient received seven more esketamine (Spravato) 84 mg spray treatments, where the usual is to receive twice a week for the first month and then once weekly for the second month
For the following 4 weeks, patient received four esketamine (Spravato) spray treatments of 84 mg each, completing the US Food and Drug Administration (FDA) approved protocol
During this course, patient continued her previous oral medication regimen as well:
▪ Vortioxetine (Trintellix) 20 mg/d
▪ Cariprazine (Vraylar) 0.75 mg/d
▪ Lisdexamfetamine (Vyvanse) 70 mg/d
▪ L-methylfolate (Deplin) 15 mg/d
Patient MDD symptoms went into full remission
However, once esketamine (Spravato) spray was discontinued at the end of 8 weeks, patient had return of MDD symptoms at 12 days without ongoing nasal sprays
 Question
Question
What options exist for patients who relapse when esketamine (Spravato) sessions end?
Start new 12-dose course of esketamine (Spravato)
Start maintenance treatments with esketamine (Spravato) every 7–10 days
Increase dose of esketamine (Spravato) above regulatory limits
 Attending physician’s mental notes (weeks 2 through 8)
Attending physician’s mental notes (weeks 2 through 8)
The approved dosing guidelines for esketamine (Spravato) spray are an induction spray twice weekly for 4 weeks
Based on patient’s response and tolerability, this dose can be increased from 56 mg to 84 mg
After evaluating its therapeutic benefit for 4 weeks, the established dose (56 mg or 84 mg) can be continued weekly as maintenance therapy up to 8 weeks
Maintenance therapy for MDD with IV ketamine (Ketalar) is well established, providing support to the use of esketamine (Spravato) spray off label for maintenance therapy, which has not been well delineated or FDA approved
Since our patient had remission of MDD symptoms with esketamine (Spravato) spray, likely is best to continue ongoing maintenance treatments every 7–10 days
 Case outcome: follow-up visit at 2 months
Case outcome: follow-up visit at 2 months
Patient re-started weekly 84 mg dosing of esketamine (Spravato) spray and MDD symptoms remitted again
Patient reported that esketamine (Spravato) spray would alleviate depressive symptoms for 7–10 days, but then would lose effectiveness and depressive symptoms would return before next scheduled dose
° Reports sadness, amotivation, fatigue, and inability to get out of bed when the esketamine (Spravato) spray effect diminishes
Otherwise tolerating esketamine (Spravato) spray treatment well with no side effects
 Attending physician’s mental notes: second interim follow-up visit (month 2)
Attending physician’s mental notes: second interim follow-up visit (month 2)
Because of her intermittent return of symptoms, her current oral medication regimen seems to be inadequate despite ongoing esketamine (Spravato) use
Need to consider novel treatments of off-label therapies with a similar mechanism of action to esketamine (Spravato) spray, which may help ultimately to taper off esketamine (Spravato) spray
 Question
Question
How would you alter her medication regimen to alleviate her return of MDD symptoms between esketamine (Spravato) doses?
Decrease length of time between esketamine (Spravato) spray treatments and continue its use indefinitely
Add dextromethorphan / quinidine sulfate (Nuedexta) as it manipulates glutamate activity as well
Recommend new trial of psychotherapy
Recommend new trial of ketamine IV (Ketalar)
Recommend new series of ECT
 Attending physician’s mental notes: second interim follow-up visit (month 2) (continued)
Attending physician’s mental notes: second interim follow-up visit (month 2) (continued)
Patient likely cannot afford indefinite weekly treatments of esketamine (Spravato) spray and declines psychotherapy. She also declines ECT as it would interfere with her work
As esketamine (Spravato) spray is helping to decrease her MDD symptoms, another glutamate medication may mimic the effects of esketamine (Spravato) spray and allow increased time between nasal spray sessions
Decided to add dextromethorphan / quinidine sulfate (Nuedexta) as it will weakly antagonize NMDA receptors (NMDARs), somewhat similar to the effect of esketamine (Spravato) spray
° This agent is approved for use in treating pseudobulbar affect
Will keep esketamine (Spravato) spray weekly, but patient will take dextromethorphan / quinidine sulfate (Nuedexta) only on days 5/6 when depressive symptoms start appearing again, hopefully avoiding MDD relapse
 Case outcome: follow-up visit at 3 months
Case outcome: follow-up visit at 3 months
Reports depressive symptoms are recurrent between sessions, but mild and possibly improving with esketamine (Spravato) spray plus dextromethorphan / quinidine sulfate (Nuedexta) treatment augmentation
Patient expressed concern about affording esketamine (Spravato) spray treatment long term and is worried about having to stop treatment abruptly
Chooses to increase length between sessions to 2 weeks as she feels she can only afford 20 more sessions
 Attending physician’s mental notes: follow-up visit (month 3)
Attending physician’s mental notes: follow-up visit (month 3)
Esketamine (Spravato) spray every 2 weeks still allows MDD relapses to occur
Dextromethorphan / quinidine sulfate (Nuedexta) now increased to daily use without side effects
Still difficult to wean off esketamine (Spravato) spray without MDD relapse
 Question
Question
What would you do next?
Stop dextromethorphan / quinidine sulfate (Nuedexta) and try to find another novel glutamate-based treatment
Review chart and remove current non-effective medications and restart medications that seemed to help in the past
 Case outcome: follow-up at 3 months
Case outcome: follow-up at 3 months
Dextromethorphan / quinidine sulfate (Nuedexta) has not been able to replace esketamine (Spravato) and was discontinued as it was ineffective
Confirmed alprazolam (Xanax) was not being taken, as some accounts suggest benzodiazepine use may lower ketamine/esketamine effectiveness
Main residual MDD symptoms are increased fatigue and amotivation and she has failed multiple stimulant treatments; will try medications that will increase norepinephrine activity perhaps instead of dopamine
She has never taken the more highly noradrenergic SNRI levomilnacipran (Fetzima), so will titrate that next
 Case outcome: follow-up at 6 months
Case outcome: follow-up at 6 months
With levomilnacipran (Fetzima) 120 mg/d treatment, she only requires esketamine (Spravato) spray now every 30–45 days
Maintenance began with nasal sprays every 7 days, then 10 days, then 15 days, and so on until 45+ days was achieved
Nasal sprays continue to be spaced farther apart in this manner
Lisdexamfetamine (Vyvanse) effectively lowered from 70 mg/d down to 20 mg/d
Vortioxetine (Trintellix) lowered to 10 mg/d to avoid serotonin toxicity when combining with newer levomilnacipran (Fetzima)
Current medications now include:
° Vortioxetine (Trintellix) 10 mg/d
° Lisdexamfetamine (Vyvanse) 20 mg/d
° Levomilnacipran (Fetzima) 120 mg/d
° Esketamine (Spravato) 84 mg every 2 months
Vagal nerve stimulator battery depleted and not replaced due to its ineffectiveness
 Case debrief
Case debrief
This patient has clear TRD and had failed practically every treatment available, including psychotherapy, polypharmacy, and intervention with ECT, VNS, and ketamine (Ketalar) IV
Interestingly, nasal esketamine (Spravato) helped to gain temporary remission from MDD when the IV racemic ketamine treatment failed previously
° Assume this is due to the fact that the nasal spray used an induction or loading strategy with multiple treatments per week, whereas the IV used an older once-weekly protocol
Patients often do not remit in a sustained or durable fashion from their depression symptoms after acute IV treatment, nor do they from the nasal sprays
In this case, ineffective medications were streamlined and taken away (l-methylfolate [Deplin], cariprazine [Vraylar]) or lowered (vortioxetine [Trintellix], lisdexamfetamine [Vyvanse]), and new medications (dextromethorphan/quinidine [Nuedexta] and levomilnacipran [Fetzima]) were tried to gain a more durable response to allow a tapering off of esketamine (Spravato) nasal sprays, seemingly with good effectiveness
This approach allowed for minimizing esketamine (Spravato) nasal spray treatments, to occur every 3 months instead of every week
 Take-home points
Take-home points
Incidence of TRD is common, 30% or greater prevalence
Some definitions suggest this is a failure to respond to two different antidepressants from different classes
Many patients are actually more treatment resistant than this, as this case illustrates
It is unclear when patients become truly refractory, but the goal of treatment regardless should be to keep trying for symptom remission prior to assuming a more palliative psychiatric approach
Interestingly, nasal esketamine (Spravato) can work even if IV ketamine has previously failed
Finally, many patients who use esketamine (Spravato) will likely need maintenance treatment, whereas the most recent 4-year data suggests that about 70% of patients who respond will durably maintain their responses
 Performance in practice: confessions of a psychopharmacologist
Performance in practice: confessions of a psychopharmacologist
What could have been done better here?
Perhaps continuing esketamine (Spravato) in a weekly maintenance fashion for those patients with clearly recurrent TRD is warranted instead of stopping at 8 weeks per indication, as TRD patients are known to relapse and have recurrences at a greater rate than others with alternative antidepressant treatments
What are possible action items for improvement in practice?
Review the literature on maintenance IV ketamine (Ketalar) use for TRD
Review similar for esketamine (Spravato)
Review literature and texts to determine whether there are other oral medications with similar mechanism of action to ketamine and esketamine for possible cross-titration
° Dextromethorphan/quinidine (Nuplazid) for pseudobulbar affect
° Dextromethorphan/bupropion (Auvelity) for MDD
 Mechanism of action moment
Mechanism of action moment
° Acts to blockade excitatory synaptic glutamate activity, due to its antagonistic effects on NMDA glutamate receptors (NMDARs) (Figure 1.1)
▪ At higher doses is responsible for loss of alertness/responsiveness when used for anesthesia
▪ At moderate to higher doses is responsible for feelings of dissociation and delirium
° Lower doses may cause an increase in glutamate cycling and extracellular glutamate availability as NMDARs are blocked, allowing more activity downstream, with now greater binding at glutamate AMPAR sites.
▪ As stated above, NMDAR antagonism increases secondary activation of AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid) glutamate receptors
▪ This increased AMPA binding activity may lead to activation of improved neuroplasticity-related signaling pathways that utilize mechanistic target of rapamycin (mTOR) and BDNF, causing increased synaptogenesis (Figure 1.3)
Increased synaptogenesis likely combats the prolonged stress model of depression, which describes MDD as being associated with neuronal atrophy and synaptic depression in the prefrontal cortex and hippocampus
In this manner, use of ketamine may allow improvement in neurophysiologic communication and adaptive changes within the depressed brain to improve CNS functioning and hinder phenotypic depressive symptoms from manifesting
▪ Ketamine additionally acts as:
▪ A norepinephrine transporter (NET), serotonin transporter (SERT)
Ketamine inhibits NET and SERT function, increasing the amount of norepinephrine and serotonin at the synapse, contributing to antidepressant effects, and can lead to µ-opioid receptor activation (Figure 1.4)
likely also contributes to antidepressant effects
Esketamine: S-enantiomer of ketamine
° It is four times more potent for antagonizing the NMDA receptor compared to ketamine
▪ Provides option of administering lower dose of esketamine, which would reduce the dose-dependent dissociative side effects associated with ketamine use
° A systematic review of 24 different trials concluded that IV ketamine demonstrated a more significant overall response and remission rate compared to placebo
Dextromethorphan/bupropion (Auvelity):
° Dextromethorphan has activity at the same NMDARs as ketamine, supporting that it may have a similar effect
▪ It is a weaker NMDA receptor antagonist, however
▪ Inhibits SERT/NET:
Increasing amounts of serotonin and norepinephrine at the synapse, providing antidepressant effects via reuptake inhibition
▪ σ1 agonist:
Neuroprotective actions and antidepressant effects
▪ μ-opioid receptors:
Although structurally similar to other opioid agonists, dextromethorphan does not have relevant activity at opioid receptors
° Dextromethorphan (Figure 1.5) is combined with bupropion for MDD so it cannot be easily metabolized into its cough suppressing metabolite, thus increasing its plasma concentrations and bioavailability
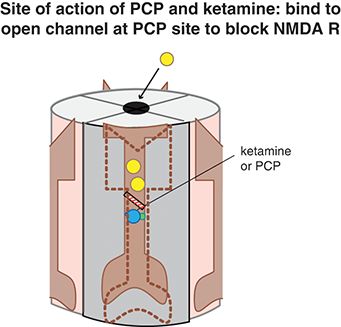
Figure 1.1 Site of action of ketamine. Ketamine binds to the open channel conformation of the NMDAR. Specifically, it binds to a site within the calcium channel of this receptor, which is often termed the PCP site because it is also where phencyclidine (PCP) binds. Blockade of NMDARs may prevent the excitatory actions of glutamate, which is postulated to be a therapeutic mechanism for treating depression.
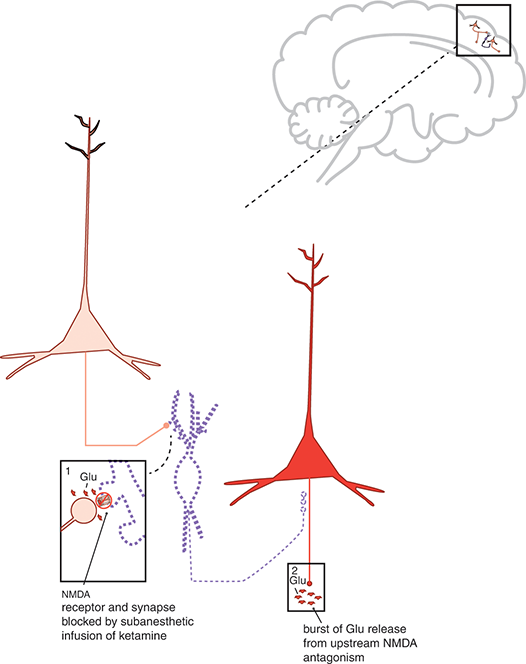
Figure 1.2 Mechanism of action of ketamine. Shown here are two cortical glutamatergic pyramidal neurons and a GABAergic interneuron. (1) If an NMDAR on a GABAergic interneuron is blocked by ketamine, this prevents the excitatory actions of glutamate (Glu) there. Thus, the GABA neuron is inactivated and does not release GABA (indicated by the dotted outline of the neuron). (2) GABA binding at the second cortical glutamatergic pyramidal neuron normally inhibits glutamate release: thus, the absence of GABA there means that the neuron is disinhibited, and glutamate release is increased.
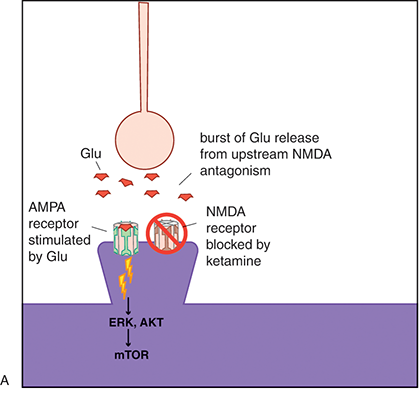
(A) It may be that blockade of the NMDA receptor leads to rapid activation of AMPA and mTOR signaling pathways.
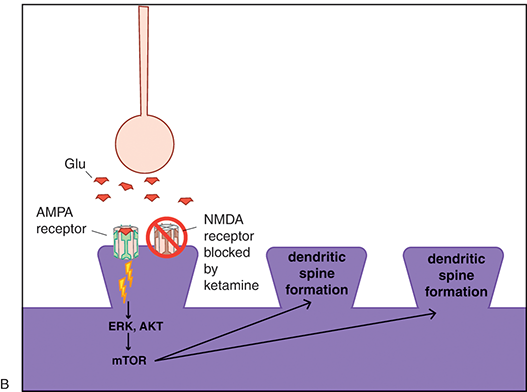
(B) This in turn would lead to rapid AMPA-mediated synaptic potentiation. Traditional antidepressants also cause synaptic potentiation; however, they do so via downstream changes in intracellular signaling. This may therefore explain the difference in onset of antidepressant action between ketamine and traditional antidepressants.
Figure 1.3 Ketamine, AMPA receptors, and mTOR. Glutamate activity heavily modulates synaptic potentiation; this is specifically modulated through NMDA and AMPARs. Ketamine is an NMDAR antagonist; however, its rapid antidepressant effects may also be related to indirect excitatory effects on AMPA receptor signaling and the mTOR pathway.
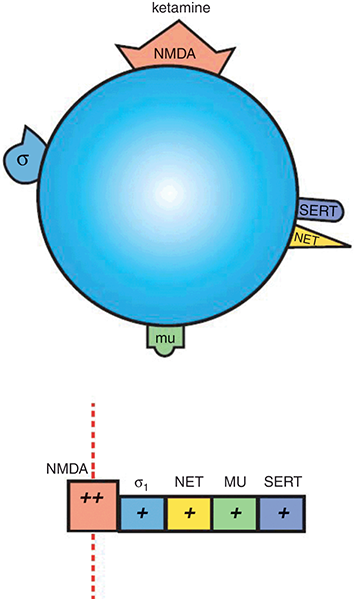
Figure 1.4 Ketamine. Ketamine is an NMDAR antagonist, with additional weak actions at σ1 receptors, NET, µ-opioid receptors, and SERT.
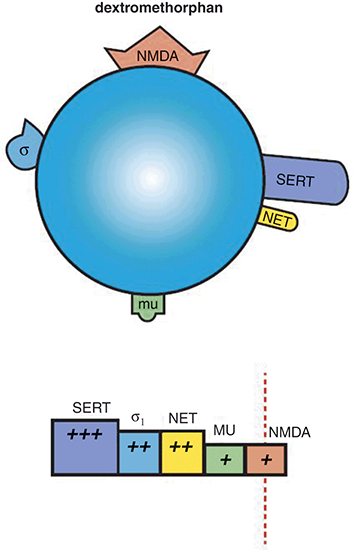
Figure 1.5 Dextromethorphan. Dextromethorphan is a weak NMDAR antagonist, with stronger binding affinity for SERT, σ1 receptors, and NET. It also has some affinity for µ-opioid receptors. Dextromethorphan (in combination with quinidine, which increases its bioavailability) may have therapeutic utility in depression as well, especially in patients that respond well to ketamine, as dextromethorphan and ketamine have similar mechanisms of action.
 Tips and pearls
Tips and pearls
What does each esketamine (Spravato) spray 2-hour session involve?
To avoid nausea, patient should not eat at least 2 hours prior to esketamine (Spravato) spray administration and avoid liquids 30 minutes prior
If patient requires a nasal corticosteroid or decongestant, allow this at least 1 hour prior to esketamine administration
Assess blood pressure prior to administration
° If elevated (> 140/90) consider risks/benefits of short-term elevation of blood pressure (10–40 mm) with an esketamine (Spravato) spray
° Sometimes patients are asked to avoid use of stimulants the day of treatment
° Sometimes patients are asked to taper off or lower dosing of opioids and benzodiazepine sedatives as they may lower effectiveness of the nasal sprays and cause respiratory suppression
Patient should gently blow nose prior to administration of first device
Patient should recline at 45-degree angle and close opposite nostril when lightly breathing in dose
Each nasal spray device has two sprays (one for each nostril)
° Both sprays are metered and deliver total of 28 mg
° Use two devices for 56 mg total dose, or three devices for 84 mg total dose
° A 5-minute rest between using each device to allow adequate absorption is suggested
40 minutes after dosing, reassess blood pressure (timing corresponds to peak plasma concentration) and respiration
Monitor patient for 2 hours after administration for signs of sedation, dissociation, or other significant adverse effects
If blood pressure is decreasing and patient is stable 2 hours after administration, may discharge patient with someone else driving
There is a Risk Evaluation and Mitigation Strategy (REMS) monitoring program that requires the treating clinician to register the patient in the REMS system prior to the start of treatment and then during each session to capture the information above on an individual worksheet that must be submitted to the coordination center
 Two-minute tutorial
Two-minute tutorial
What is palliative psychiatry?
Palliative psychiatry: an approach that improves the quality of life of patients and their families in facing the problems associated with severe persistent mental illness when a remission of symptoms in the future is unlikely
° Emphasizes prevention and relief of suffering and treatment of associated physical, mental, social, and spiritual needs
Focuses on harm reduction and on avoidance of burdensome psychiatric interventions with questionable impact especially where remarkable side effect burdens may result
Difficult situations that might benefit from palliative psychiatry:
° Treatment-refractory depression, schizophrenia, post-traumatic stress disorder (PTSD)
° Severe anorexia in which patients have repeat hospitalizations with involuntary feeding
Patients who cycle through different treatment options without any improvement of symptoms can feel demoralized, as they feel they are caught in a cycle of false hope
Palliative psychiatry suggests that in patients with treatment-resistant chronic mental illness, shifting the focus from finding a “cure” to acceptance of chronicity and refocusing on improving quality of life may be more beneficial
° Focusing on improving patient’s quality of life improves therapeutic alliance between patient and practitioner
° Palliative psychiatry is not withholding treatment – instead redefining goals, such as maintaining meaningful relationships with family and others in the patient’s interpersonal environment
° In this case, there were very few if any treatments that had not been tried in the seeking of remission. Esketamine (Spravato) was released and deemed to be novel so it was utilized with good effectiveness so far
° In the absence of a novel treatment, the clinician in this case likely may have switched to a more palliative, coping strategy approach
 Post-test question
Post-test question
The most theorized mechanism of action of esketamine suggests that initial antagonism of NMDA leads to agonism of AMPA receptors. There seems to be downstream increased activity of mTOR and BDNF, suggesting this agent is improving synaptic plasticity and neuroanatomic circuitry communication across a variety of brain areas.


 Patient evaluation on intake
Patient evaluation on intake  Medical history
Medical history  Current medications
Current medications  Attending physician’s mental notes: initial psychiatric evaluation
Attending physician’s mental notes: initial psychiatric evaluation  Take-home points
Take-home points  Performance in practice: confessions of a psychopharmacologist
Performance in practice: confessions of a psychopharmacologist  Mechanism of action moment
Mechanism of action moment  Tips and pearls
Tips and pearls 





