TTTS is a severe complication of MC twin pregnancies and one of the most lethal conditions in perinatal medicine. The optimal management is still a major challenge for both obstetricians and neonatologists. TTTS is the result of unbalanced inter-twin blood transfusion through placental vascular anastomoses and is characterized by the presence of oligohydramnion in the donor twin and polyhydramnion in the recipient twin.
Treatment options in TTTS include serial amnioreduction to drain the excess amniotic fluid in the amniotic sac of the recipient, and fetoscopic laser coagulation of placental vascular anastomoses. Twins treated with serial amnioreduction are less likely to survive compared to twins treated with laser surgery (Rossi & D'Addario, Reference Rossi and D'Addario2008) and are born on average at 29 weeks’ gestation, compared to 32–33 weeks’ gestation after laser surgery. In addition, treatment with amnioreduction is associated with a significant higher risk of severe cerebral injury compared to laser surgery (van Klink et al., Reference van Klink, Koopman, van Zwet, Oepkes, Walther and Lopriore2013). The preferred treatment for TTTS is therefore fetoscopic laser coagulation of the anastomoses, with an overall survival rate increasing in the past decade from 55% to 74% (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004; Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014).
With this improving survival rate, attention is shifting towards the long-term outcome in surviving children. An increasing number of studies are gradually shedding more light on the wide range of long-term morbidity associated with TTTS. This review will focus on the long-term neurodevelopmental outcome in TTTS survivors treated with either serial amnioreduction or fetoscopic laser surgery.
Cerebral Injury on Neuroimaging in the Neonatal Period
The reported incidence of cerebral injury after amnioreduction ranges from 6% to 38% compared to 8% to 18% following laser surgery (Cincotta et al., Reference Cincotta, Gray, Gardener, Soong and Chan2009; Lenclen et al., Reference Lenclen, Paupe, Ciarlo, Couderc, Castela, Ortqvist and Ville2007; Lopriore et al., Reference Lopriore, Wezel-Meijler, Middeldorp, Sueters, Vandenbussche and Walther2006; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Several types of cerebral injury have been described, including cystic periventricular leukomalacia (PVL), cerebral white-matter cysts, severe intraventricular hemorrhage (IVH), ventricular dilatation, cerebral atrophy, and arterial ischemic stroke (Lopriore et al., Reference Lopriore, Wezel-Meijler, Middeldorp, Sueters, Vandenbussche and Walther2006). Donors and recipients appear to be equally at risk (Lopriore et al., Reference Lopriore, Wezel-Meijler, Middeldorp, Sueters, Vandenbussche and Walther2006). Cerebral injury may result from antenatal injury and/or postnatal injury, partly due to extreme prematurity, an important risk factor for cystic PVL and IVH. Antenatal injury may result from impaired cerebral perfusion due to hemodynamic imbalance and inter-twin shifts of blood through the vascular anastomoses, leading to hypoxic-ischemic insults. Hypoxic-ischemic damage caused by cerebral hypoperfusion is probably the main cause for cerebral injury in donor twins. Hyperviscosity and polycythemia causing vascular sludging may be an important cause for cerebral injury in recipients.
In a meta-analysis performed by our group, we found a 7-fold higher risk of severe cerebral injury in TTTS twins treated with amnioreduction compared to twins treated with laser surgery (van Klink et al., Reference van Klink, Koopman, van Zwet, Oepkes, Walther and Lopriore2013). Given the high incidence and range of cerebral injuries in TTTS, routine standardized ante- and postnatal cerebral imaging protocols are strongly recommended to accurately evaluate origin, timing, and type of neurological damage. In case of cerebral injury, MRI may play an important role in investigating the correlation between antenatal and postnatal imaging findings. Increased awareness may improve neonatal and pediatric care for these children. The clinical relevance of neuroimaging findings should then be determined using long-term neurodevelopmental outcome data of all TTTS survivors until at least childhood.
Long-Term Neurodevelopmental Outcome in TTTS Survivors
Advancing techniques, increasing survival rates and improving short-term outcome necessitate a greater knowledge on the impact of TTTS and its management on long-term neurodevelopment. A better understanding of the impact on child development over time will allow more accurate counseling of parents and targeted interventions to optimize child development when needed. This requires international collaboration, to obtain large enough sample sizes and statistical power, using a standardized follow-up regimen including uniform and clearly defined criteria for long-term NDI. NDI is a standard composite outcome defined as at least one of the following: CP, severe motor and/or cognitive developmental delay, bilateral blindness, or deafness requiring amplification with hearing aids. Determining NDI involves a follow-up regimen that includes a physical and neurologic examination and an assessment of cognitive and motor development using developmental tests such as the Bayley Scales of Infant and Toddler Development by certified examiners.
The incidence and type of NDI in TTTS survivors treated with amnioreduction or laser surgery reported in the literature is described in the two following sections and summarized in Tables 1 and 2.
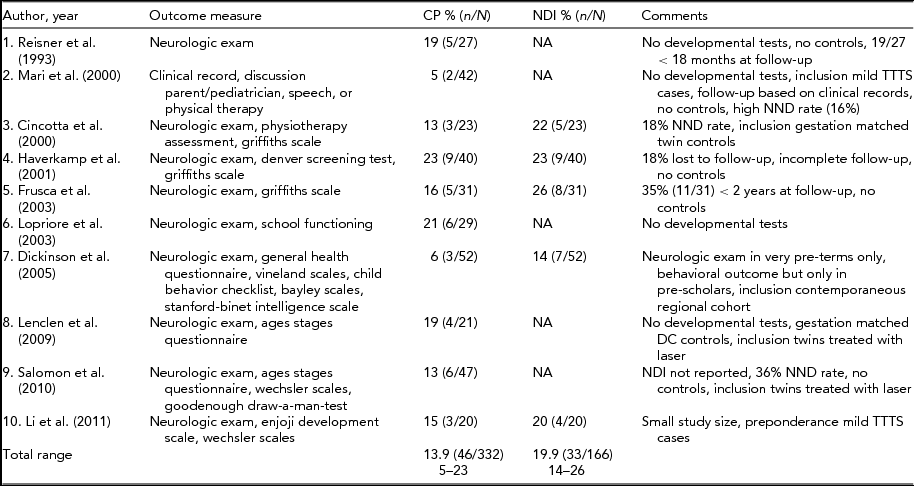
TABLE 1 Long-Term Neurodevelopmental Outcome in TTTS Treated with Amnioreduction
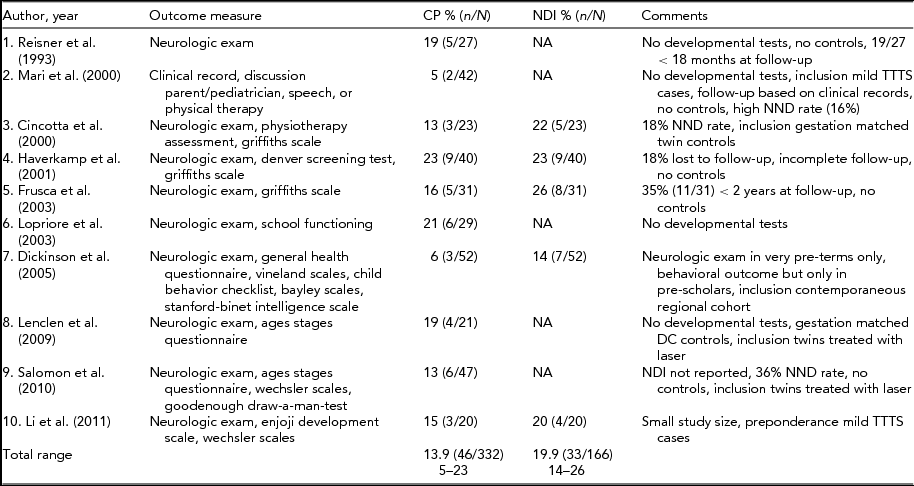
The first part of the Eurofetus trial is not included in this table because the children are more fully described in the follow-up of this trial. CP = cerebral palsy; NDI = neuro developmental impairment defined as CP, severe cognitive and/or motor delay (<2 SD), blindness and/ or deafness; NND = neonatal death; DC = dichorionic.
TABLE 2 Long-Term Neurodevelopmental Outcome in TTTS Treated With Laser Surgery
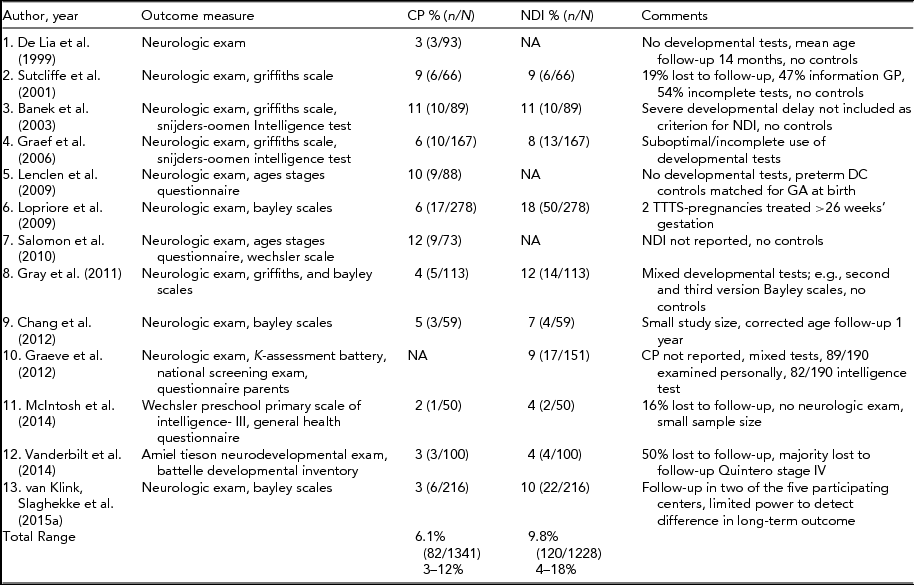
Two follow-up studies (Lopriore et al., Reference Lopriore, Middeldorp, Sueters, Oepkes, Vandenbussche and Walther2007; van Klink et al., Reference van Klink, Koopman, van Zwet, Middeldorp, Walther, Oepkes and Lopriore2014) in TTTS after laser are not included in this table because the included children are more fully described in the multi-center follow-up of this studies (Lopriore et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009; van Klink et al., Reference van Klink, Slaghekke, Balestriero, Scelsa, Introvini, Rustico and Lopriore2015b). CP = cerebral palsy; NDI = neuro developmental impairment defined as CP, severe cognitive and/or motor delay (<2 SD), blindness and/ or deafness; NND = neonatal death; GP = general practitioner; GA = gestational age.
Long-Term Neurodevelopmental Outcome in TTTS Treated With Amnioreduction
The reported incidence of CP and NDI after amnioreduction ranges from 5% to 23% and from 6% to 26%, respectively (Cincotta et al., Reference Cincotta, Gray, Phythian, Rogers and Chan2000; Dickinson et al., Reference Dickinson, Duncombe, Evans, French and Hagan2005; Frusca et al., Reference Frusca, Soregaroli, Fichera, Taddei, Villani, Accorsi and Martelli2003; Haverkamp et al., Reference Haverkamp, Lex, Hanisch, Fahnenstich and Zerres2001; Lenclen et al., Reference Lenclen, Ciarlo, Paupe, Bussieres and Ville2009; Lopriore et al., Reference Lopriore, Nagel, Vandenbussche and Walther2003; Mari et al., Reference Mari, Detti, Oz and Abuhamad2000; Reisner et al., Reference Reisner, Mahony, Petty, Nyberg, Porter, Zingheim and Luthy1993). This large discrepancy is due to considerable differences in methodology between the studies and heterogeneity within the case series. In the majority of studies, cohorts included only a small number of children (range: 20–52 children). As a consequence, the investigators were unable to assess whether NDI was due to confounders such as prematurity or growth restriction (Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). A clear definition of impairment, in terms of NDI, was often not reported, and detailed information on individual observations of impairment was often lacking. Finally, not all studies included standardized developmental tests (Lenclen et al., Reference Lenclen, Ciarlo, Paupe, Bussieres and Ville2009; Lopriore et al., Reference Lopriore, Nagel, Vandenbussche and Walther2003; Mari et al., Reference Mari, Detti, Oz and Abuhamad2000; Reisner et al., Reference Reisner, Mahony, Petty, Nyberg, Porter, Zingheim and Luthy1993). Table 1 summarizes the follow-up studies after amnioreduction.
In the Eurofetus trial, the short-term neurologic outcome of TTTS survivors treated with amnioreduction was less favorable compared to laser surgery. However, the long-term outcome was similar between the two treatment groups (Salomon et al., Reference Salomon, Ortqvist, Aegerter, Bussieres, Staracci, Stirnemann and Ville2010; Senat et al., Reference Senat, Deprest, Boulvain, Paupe, Winer and Ville2004). Unfortunately, the long-term evaluation did not take into account that in a relatively large percentage (22%; 20/93) of live-born neonates in the amnioreduction group, intensive care treatment was withdrawn due to severe cerebral injury. Had these children survived, the differences in long-term neurodevelopmental outcome between both groups could have been much more evident (Lopriore et al., Reference Lopriore, Walther and Oepkes2011).
Long-Term Neurodevelopmental Outcome in TTTS Treated With Laser Surgery
The reported incidence of CP and NDI after laser surgery ranges from 3% to 12% and from 4% to 18%, respectively (Banek et al., Reference Banek, Hecher, Hackeloer and Bartmann2003; Chang et al., Reference Chang, Chao, Chang, Lien, Hsieh and Wang2012; De Lia et al., Reference De Lia, Kuhlmann and Lopez1999; Graef et al., Reference Graef, Ellenrieder, Hecher, Hackeloer, Huber and Bartmann2006; Graeve et al., Reference Graeve, Banek, Stegman-Woessner, Maschke, Hecher and Bartmann2012; Gray et al., Reference Gray, Poulsen, Gilshenan, Soong, Cincotta and Gardener2011; Lenclen et al., Reference Lenclen, Ciarlo, Paupe, Bussieres and Ville2009; Lopriore et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009; McIntosh et al., Reference McIntosh, Meriki, Joshi, Biggs, Welsh, Challis and Lui2014; Salomon et al., Reference Salomon, Ortqvist, Aegerter, Bussieres, Staracci, Stirnemann and Ville2010; Sutcliffe et al., Reference Sutcliffe, Sebire, Pigott, Taylor, Edwards and Nicolaides2001; Vanderbilt et al., Reference Vanderbilt, Schrager, Llanes, Hamilton, Seri and Chmait2014). Table 2 summarizes the follow-up studies after laser surgery. Again, care must be taken when comparing the results of these studies, as this large discrepancy is due to different methodology, differences in (neonatal) death rates, considerable heterogeneity within the small case series and lack of uniform outcome measures and criteria.
In 1999, De Lia et al. reported major impairment in 5% (5/93) of TTTS survivors after laser surgery at a mean age of 14 months (De Lia et al., Reference De Lia, Kuhlmann and Lopez1999). No developmental tests were employed. Sutcliffe et al. (Reference Sutcliffe, Sebire, Pigott, Taylor, Edwards and Nicolaides2001) found CP in 9% (6/66) of TTTS survivors treated with laser. Details on the number of children with severe cognitive delay were not reported. Two follow-up studies from Germany reported NDI in 11% (10/89) and 8% (13/167) of survivors at 2 years of age (Banek et al., Reference Banek, Hecher, Hackeloer and Bartmann2003; Graef et al., Reference Graef, Ellenrieder, Hecher, Hackeloer, Huber and Bartmann2006). NDI was more likely in children born <32 weeks’ gestation. In both studies, the definition of impairment did not include severe cognitive delay. Hence, children with severe cognitive delay but without CP were not included in the group with NDI. Neurodevelopmental outcome of 190 of these 256 children was re-evaluated at a median age of 6 years and 5 months (Graeve et al., Reference Graeve, Banek, Stegman-Woessner, Maschke, Hecher and Bartmann2012). NDI, now defined as major neurologic deficiencies (CP) and severe cognitive delay as measured with a standardized intelligence test, was detected in 9% (17/190). The authors concluded that neurodevelopmental outcome at 6 years was not different from outcome at 2 years. Of note, a significantly higher number of children with NDI were born very or extremely preterm, when compared to the children with normal development.
In a long-term follow-up study in France, Lenclen et al. (Reference Lenclen, Ciarlo, Paupe, Bussieres and Ville2009) reported NDI in 11% (10/88; CP: n = 9; blindness: n = 1). Developmental test scores were similar in TTTS survivors compared to preterm dichorionic twins. Low gestational age at birth was significantly associated with NDI (relative risk [RR]: 1.20 for each week, 95% CI: 1.1–1.4; p = .02). In 2009, three European fetal therapy centers (Barcelona, Leuven, and Leiden) performed a multicenter follow-up study to investigate risk factors for NDI in TTTS treated with laser (Lopriore et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009). Overall, the incidence of NDI was 18% (50/278). Four risk factors were found to be significantly associated with increased risk for NDI: gestational age at laser surgery (odds ratio [OR] 1.30, 95% CI: 1.0–1.7; p = .05), Quintero stage (OR 3.55 for each increment in stage, 95% CI: 1.1–11.8; p = .04), lower gestational age at birth (OR 1.39 for each week, 95% CI 1.1–1.8; p = .01), and lower birth weight (OR 1.18 for each 100-g decrease, 95% CI 1.1–1.3; p = < .01; Lopriore et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009). Gray et al. (Reference Gray, Poulsen, Gilshenan, Soong, Cincotta and Gardener2011) found a 4% (5/113) incidence of CP and NDI was diagnosed in 12% (14/113) of TTTS survivors. Four children with CP also had severe cognitive delay, whereas nine children without CP were diagnosed with severe cognitive delay, using standardized developmental tests. Advanced Quintero stage was a risk factor for NDI (OR: 13.02; 95% CI: 1.92–88.33; p = < .001). At a corrected age of 1 year at follow-up, Chang et al. (Reference Chang, Chao, Chang, Lien, Hsieh and Wang2012) reported CP in 5% (3/59) and NDI in 7% (4/59) of TTTS survivors treated with laser. Univariate analyses revealed that low gestational age at birth was a significant predictor of impairment (OR: 0.63; 95% CI not reported; p = .018). Although standardized neurological assessment and developmental tests were employed, the timing of follow-up was too early for a reliable diagnosis of CP or developmental delay.
McIntosh et al. (Reference McIntosh, Meriki, Joshi, Biggs, Welsh, Challis and Lui2014) investigated the long-term neurodevelopmental outcome of a cohort of 3- to 5-year-old survivors of TTTS treated with laser. NDI was reported in 4% (2/50) children tested with the Wechsler Preschool Primary Scale of Intelligence and a general health questionnaire. Most recently, Vanderbilt et al. (Reference Vanderbilt, Schrager, Llanes, Hamilton, Seri and Chmait2014) reported NDI in 4% (4/100 TTTS survivors), as one child had a Battelle Developmental Inventory score < 70 and three children were diagnosed with CP. Lower maternal education (β = 0.60; p = < .01), higher Quintero stage (β = -0.36; p = < .01) and lower gestational age at birth (β = 0.30; p = < .01) were associated with worse cognitive scores. The results could, however, be severely hampered due to a 50% lost-to-follow-up rate. High lost-to-follow-up rates can lead to biased reports of impairment, likely resulting in underestimation, since parents with low educational levels and those with children with severe developmental delay are most likely to drop out of follow-up programs (Aylward et al., Reference Aylward, Hatcher, Stripp, Gustafson and Leavitt1985; Wolke et al., Reference Wolke, Sohne, Ohrt and Riegel1995). In addition, in countries where families have to travel long distances to the follow-up clinic, parents are more likely to refrain from participating. Assessments using (web-based) questionnaires such as the Ages and Stages Questionnaire could be more feasible to avoid large lost to follow-up rates.
Randomized Controlled Trials in TTTS
Despite the fact that the preferred treatment for TTTS is fetoscopic laser coagulation, severe postoperative complications can occur when inter-twin vascular anastomoses remain patent, including twin-anemia polycythemia sequence (TAPS) or recurrent TTTS. To minimize the occurrence of residual anastomoses, a modified laser surgery technique, the Solomon technique, was developed, in which the entire vascular equator is coagulated (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014). In the Solomon randomized controlled trial, this technique was associated with a significant reduction in TAPS and recurrence of TTTS when compared to the standard laser surgery technique (Slaghekke et al., Reference Slaghekke, Lopriore, Lewi, Middeldorp, van Zwet, Weingertner and Oepkes2014).
In a follow-up study, we evaluated the long-term neurodevelopmental outcome in surviving children with TTTS included in the trial (van Klink, Slaghekke et al., Reference van Klink, Slaghekke, Balestriero, Scelsa, Introvini, Rustico and Lopriore2015b). Routine, standardized follow-up in survivors, at least 2 years after the estimated date of delivery, was performed at two of the five centers participating in the Solomon trial: Buzzi Hospital Milan (Italy) and Leiden University Medical Center (the Netherlands). The primary outcome, survival without NDI, was detected in 95/141 (67%) in the Solomon group and in 99/146 (68%) in the standard group (p = .92). NDI in long-term survivors included for follow-up was detected in 12/107 (11%) in the Solomon and in 10/109 (9%) in the standard group (p = .61; see Table 2).
Several explanations can be considered to explain the lack of difference between the two treatment groups in this follow-up study. First, the Solomon trial was primarily designed and powered to detect a difference in short-term outcome (van Klink, Slaghekke et al., Reference van Klink, Slaghekke, Balestriero, Scelsa, Introvini, Rustico and Lopriore2015b). Follow-up was only available from two of the five centers participating in the Solomon trial and results cannot be generalized to the total trial population. A second explanation could be that timely detection and adequate management and treatment (intrauterine transfusion, laser surgery re-intervention) in patients with short-term complications (TAPS or recurrent TTTS) in the standard group reduced the risk for long-term impairment. The lack of difference in Bayley scores could also be related to early interventions for children with developmental impairment. However, no difference in the rate of early interventions including physical therapy (39% vs. 41%, p = .89), speech-language therapy (9% vs. 12%, p = .65) and psychological interventions (4% vs. 7%, p = .54), was found between the Solomon and standard group, respectively. In view of the reduction of short-term complications and absence of increased adverse long-term effects, our data support the use of the Solomon technique in the treatment of TTTS.
Other Interventions and Complications in Monochorionic Pregnancies
When fetoscopic laser surgery is not feasible or in cases with severe complications leading to the impending death of one twin, selective reduction can be offered. Up to now, no long-term outcome data of TTTS survivors following selective feticide of the co-twin are available. In a recent follow-up study, long-term NDI was detected in 7% (5/74) of co-twin survivors treated with selective reduction for severe complications, including TTTS, TAPS, twin-reversed arterial perfusion (TRAP), and selective fetal growth restriction (sFGR; van Klink, Koopman et al., Reference van Klink, Koopman, Middeldorp, Klumper, Rijken, Oepkes and Lopriore2015a). Of the 25 cases treated with selective reduction for TTTS, 2 (8%) were diagnosed with NDI. Large, multicenter follow-up studies are required to reliably assess long-term outcome in TTTS pregnancies treated with selective reduction.
The optimal management of other severe complications in MC pregnancies, including TAPS, TRAP, and sFGR, is not clear and international consensus on the best treatment strategy is lacking. Multicenter efforts are of utmost importance to study the natural history in complicated pregnancies with fetal disorders and the effect of interventions to determine optimal management (timing and type of intervention). The ideal study design to evaluate new interventions in fetal therapy is an adequately powered randomized controlled trial with ‘survival without NDI’ as a primary outcome. Long-term follow-up, at a minimum of 2 years of age, should be an integrated component of fetal therapy in all fetal medicine centers around the world. In addition, worldwide registries to record and evaluate the outcome in large groups of children after fetal therapy are of paramount importance to increase current knowledge in specific subgroups. It is crucial to continuously assess child development, including formal psychological testing and standardized measures of well documented psychometric quality, with increasing reliability of results with increasing age of surviving children following fetal therapy.
Conclusions
TTTS is associated with an increased risk of neonatal mortality and morbidity, including severe cerebral injury. Twins treated with amnioreduction are less likely to survive compared to twins treated with laser surgery. The incidence of long-term NDI in TTTS treated with amnioreduction is high, on average 20%. The long-term outcome in TTTS treated with laser is more favorable; NDI is reported in 10%. This significant difference in outcome is partly due to the higher rate of severe prematurity in TTTS treated with amnioreduction. The association between low gestational age at birth and NDI is not surprising, as prematurity is a well-recognized risk factor for adverse neurodevelopmental outcome. Other important risk factors are advanced gestational age at intervention, higher Quintero stage, severe cerebral injury, low birth weight, and low parental educational level (Lopriore et al., Reference Lopriore, Ortibus, Acosta-Rojas, Le Cessie, Middeldorp, Oepkes and Lewi2009; van Klink et al., Reference van Klink, Koopman, van Zwet, Middeldorp, Walther, Oepkes and Lopriore2014). However, special care must be taken when comparing the results of long-term, follow-up studies, as discrepancies exist at least partly due to different methodology, differences in neonatal death rates, the considerable heterogeneity within small case series, and lack of uniform outcome criteria.
In conclusion, regardless of antenatal treatment, all TTTS survivors are at increased risk for NDI and require long-term follow-up. The age of follow-up assessment in most studies is 2 years, which is still of limited value since developmental problems often become more apparent at a later age, especially at school age. Therefore, follow-up until at least school age is recommended. Continuing close collaboration between obstetricians, neonatologists and child developmental specialists is crucial in order to improve care of children with TTTS (Lopriore et al., Reference Lopriore, Middeldorp, Sueters, Vandenbussche and Walther2005).