INTRODUCTION
Prevalence of head louse infestation has increased in many countries, exacerbated by the spread of insecticide resistance. At the same time, public concern about the safety of insecticides has led to greater use of alternative therapies, such as combing or using herbal extracts. Regardless of the therapy used, it is essential to ensure that a patient has an active infestation before initiating any treatment in order to avoid unnecessary exposure to potentially harmful chemicals. Too often, in schools and the community diagnoses are made based on finding only louse eggs, which may or may not be viable, or the empty eggshells (nits) that remain after louse nymphs have emerged [1, Reference Williams2].
Making a diagnosis of head louse infestation by finding live lice is often not easy until a high level of infestation is reached. Even experienced public health nurses often have never seen live lice in situ on the scalp [1]. Diagnosis of head louse infestation by visual inspection of the hair and scalp, sometimes aided by use of applicator sticks to part hair or magnifiers to facilitate viewing, mainly relies upon finding eggs or nits, rather than the trophic stages of adult or nymphal lice. Identification of viable eggs is difficult even for experienced investigators and it is often assumed that eggs close to the scalp have been laid recently. This assumption of viability is uncertain so false-positive diagnoses may be common [Reference Williams2, Reference Pollack, Kiszewski and Spielman3].
Several investigators have used combing as a diagnostic tool to find live lice and some techniques appear more effective than others [Reference Mumçuoglu4, Reference De Maeseneer5]. For mass screening in schools the diagnostic intervention needs to be rapid and not require more than basic equipment or be overly disruptive of school routine. We evaluated a new plastic head louse detection comb against visual inspection for diagnosing head louse infestation.
MATERIALS AND METHODS
Setting and participants
This study was conducted in two rural communities in Manisa province, western Turkey. These were selected because the schools were of a manageable size to screen all children during one day and, being schools for small communities, had a high attendance record, ensuring all students were likely to be in school on study days and available for follow-up. Each community was served by one elementary school for children aged 7–14 years. The smaller school in the village of Yagcilar (School Y) had 129 pupils and took children from a single village whereas the other school in the larger village of Osmancali (School O) had 332 pupils from more than 20 villages and communities in the locality; children were bussed in to attend each day.
Approval for the study was granted by the ethical committee of the Medical Faculty of Celal Bayar University, and for screening for lice by the school authorities. Verbal assent was obtained from each student prior to examination for head lice. Written informed consent was obtained from parents/guardians for each case requiring treatment. On receipt of the consent form the treatment was given to the family for application by them at home.
This study was conducted between 29 November and 17 December 2004. The sequence of procedures and the number of children participating at each stage are shown in Figure 1.
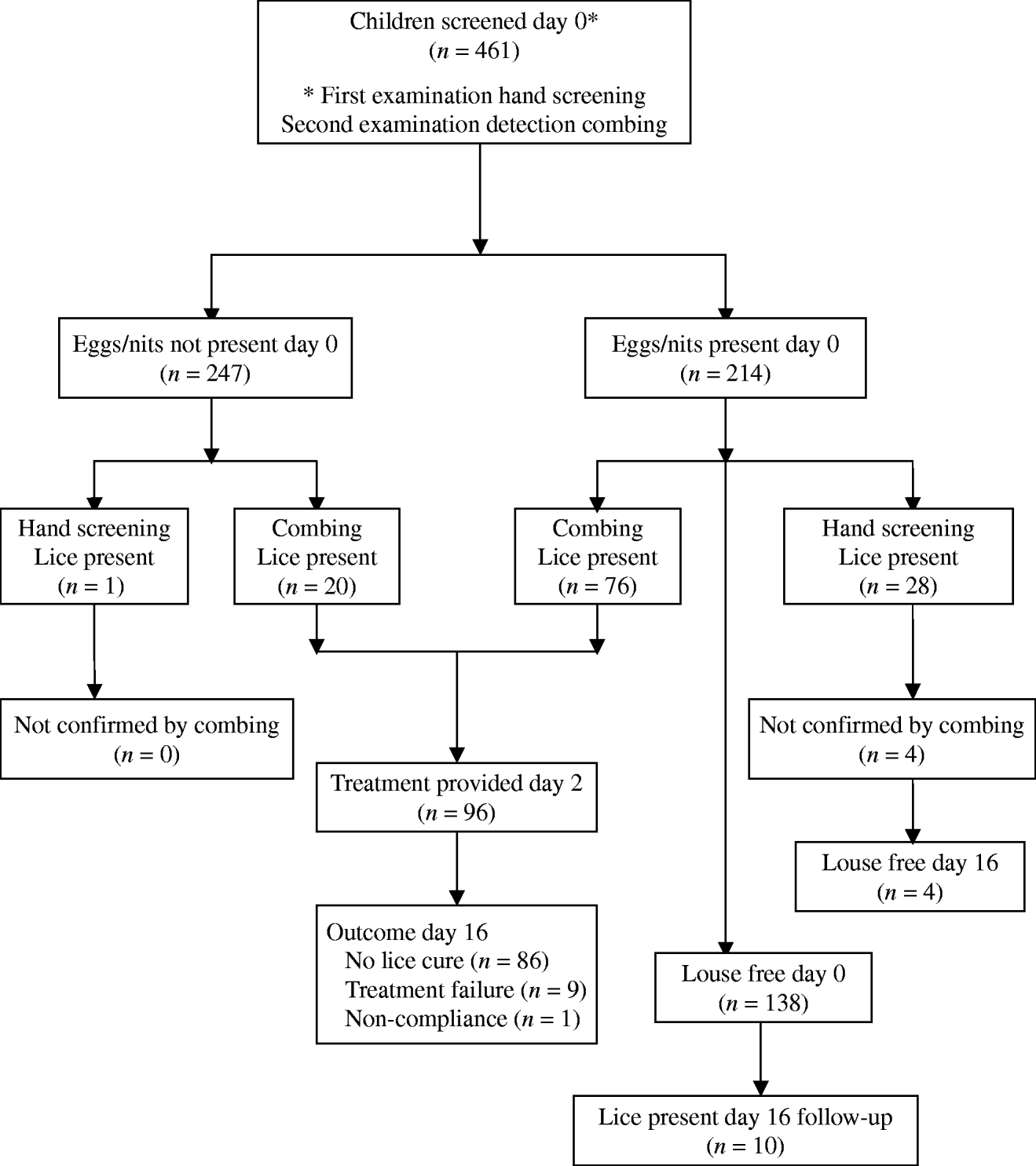
Fig. 1. Flowchart to show diagnostic and treatment outcomes.
Screening for head lice
All seven investigators who screened students for infestation were experienced dermatologists and/or ectoparasitologists familiar with screening for head lice and various life stages of the parasite.
For this study we used a head louse detection comb (‘PDC’, KSL Consulting, Helsinge, Denmark) made from acrylonitrile butadiene styrene (ABS). The face of the comb is flat giving the leading face of the parallel sided teeth square-cut edges, 0·2 mm apart (Fig. 2). The tips of the teeth are rounded and bevelled on the back, which previous experience in a clinical study showed was less likely to scratch the scalp compared with a similar comb used previously. The ‘PDC’ is a registered Class I medical device in the European Union.

Fig. 2. ‘PDC’ plastic head louse detection comb.
Every child in attendance on the day of the study was examined by two diagnostic methods. First they underwent visual screening, in which the investigator systematically parted the hair using fingers and thumbs over the whole of the head, and the hair and scalp were examined by eye for signs of lice or louse eggs for a maximum of 3 min. If evidence of active infestation was found before that time the examination was discontinued and the diagnosis recorded. The presence of louse eggs and lice was recorded separately. No distinction was made during screening between nits and eggs. A subjective evaluation of the density of infestation with louse eggs and nits was also made based on a 3-point scale: light ⩽20 eggs/nits, medium >20–50 eggs/nits, heavy >50 eggs/nits. If an investigator suspected that he had found a louse or louse-like object amongst the hair, but was unable to see it clearly either because it had moved away or was entangled in a hair tress, it was recorded but extensive efforts were not made to extract it or remove it from the head.
After visual screening, a different team of investigators, unaware of the results, examined each child using a head louse detection comb (‘PDC’). Combing employed a systematic approach starting on one side of the head and working around to the other side. Combing was continued until the whole of the scalp had been combed, also for a maximum of 3 min, or until one louse was found, whichever occurred sooner. The combing technique involved inserting the comb into dry hair until the tips of the teeth were in contact with the skin then drawing the comb smoothly through the hair to the end of the tress. Each section of hair was combed 3–4 times before moving to the adjacent section. If a suspected louse was observed on the teeth of the comb while being drawn through the hair, the investigator trapped it against the face of the comb using a thumb. This prevented lice being repelled by static electricity as the comb was withdrawn from the hair. After removal, suspected insects were examined on the surface of the comb to confirm the diagnosis.
Treatment
Children found to have live head lice by means of detection combing were given a letter informing their parents that they had an active infestation. Included with the letter was a consent form. On receipt of a signed consent form, permethrin 1% creme rinse (Zalvor, GlaxoSmithKline, Istanbul, Turkey) was provided, and parents were instructed to use a 10-min application before rinsing from the hair. We offered 1% permethrin creme rinse because at the time of the study it was the product most recently introduced onto the Turkish market and was the preferred treatment by local practitioners. The conformity with instructions was at parents' discretion. The single 59 ml pack recommended as suitable for children of this age by the manufacturer may not have been adequate for all those treated. No treatment diaries were kept and no nit combing was performed but each child was examined by investigators for surviving lice or louse nymphs on days 2, 7, 9 and 14 following the first application, using the ‘PDC’ to confirm the effectiveness of the treatments. Although a second application of permethrin creme rinse was not required by the instructions we provided a second treatment pack 7 days after the first treatment so that any nymphs emerging from eggs not killed by the first application could be eliminated.
Statistical analysis
Analyses were conducted using Oxstat II version 1.11 (Microsoft Corp., Redmond, WA, USA) and Epi-Info version 6 (CDC, Atlanta, GA, USA). The χ2 test was used to compare groups for yes/no variables such as presence/absence of infestation and for comparing the efficacy of the two diagnostic methods. Confidence intervals (CIs) for the relative risk (RR) were calculated using the method set out in Altman [Reference Altman6].
RESULTS
There were no significant differences between the numbers of girls and boys either overall or in each age group in either school (Table 1).
Table 1. Comparison of diagnostic methods for head lice and distribution of lice in two schools
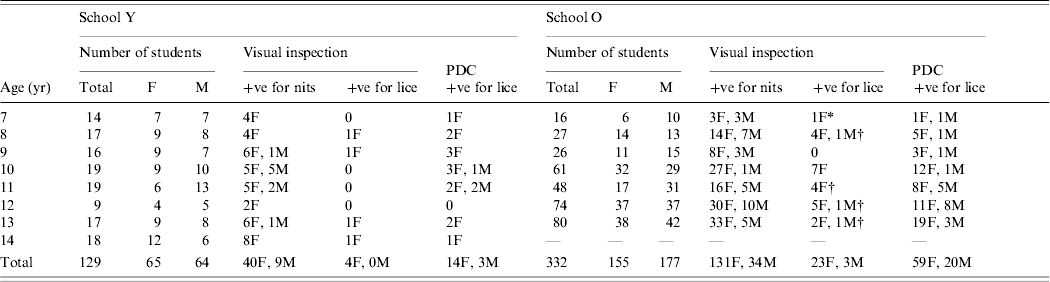
F, Female; M, male; PDC, examined using the ‘PDC’ plastic head louse detection comb.
* One individual without any detectable nits or eggs.
† One individual in each of these groups was not confirmed positive by ‘PDC’.
Visual screening provided evidence of earlier infestations by finding louse eggs and nits in a high proportion of children in both schools. Nits were found on 214/461 (46%) children of whom 171 (80%) were girls (P<0·001). However, few children were found to have lice by this method with only 30 (6·5%) being diagnosed positive, of whom one had no evidence of nits in her hair.
Screening using the ‘PDC’ was significantly (P<0·001) more successful at finding lice and, if all positive diagnoses reported by visual inspection were correct, was 3·2 times more effective (95% CI 2·2–4·7) than visual screening. In School Y, combing diagnosed the infestation in the four children found to be positive by visual screening plus an additional 13 cases, giving a total prevalence of 13%, with a significant difference between the methods (P<0·01). In School O, 79 cases of lice, 24% of the school roll, were detected by combing compared with the 26 cases found by visual inspection (P<0·001). In the two schools 96/461 (20·8%) children were positive for lice.
Combing identified 16 cases considered to have neither nits nor lice using visual screening. In contrast, five children were recorded as having lice present during visual inspection but this was not confirmed by combing. This may have been because the insect was accidentally removed, through misidentification during visual inspection, or because the louse/lice were simply missed by the combing method. Each of these children was followed up by combing on four occasions over the 16 days following the first examination. This allowed us to increase the chance of finding any lice present through repeated examination and also permitted any louse population to increase to more easily detectable numbers if viable eggs were present and hatched during the 2-week period following the initial examination. No lice were found at these checks and it was finally concluded that the original diagnosis was incorrect. This made combing using ‘PDC’ 3·84 times more effective than visual screening (95% CI 2·5–5·9).
Most children found to have lice by either diagnostic method were girls who constituted 73 of the 96 cases (76%) of those identified by combing (P<0·001). Similarly, 27/30 (90%) found to have lice by visual screening were also girls (P<0·001).
We found 43 boys and 171 girls with nits during visual inspection, of whom 10 boys and 66 girls were confirmed to have lice by detection combing (Table 1). The rest of the group, recorded as having nits but no lice at the first examination, was checked 16 days later. At that time only two boys and eight girls (10/138=7·2%), were found to have become positive for lice. Four of these cases had heavy infestations with nits. Overall the risk of converting from having only nits to a positive infestation with lice was significantly greater if a medium to heavy burden of nits was present (RR 9·62, 95% CI 3·2–28·9).
All school children who were offered treatment opted to accept it and were treated 2 days after initial screening (day 2 of the study). Follow-up examinations showed that some louse eggs survived the first application of permethrin and nymphs were found to have emerged from these up to 1 week post-treatment (Table 2). Treatment appeared to be more successful in School Y where all children were louse free 2 days after treatment (day 4 of the study), although three had nymphal lice hatch before the second treatment. However, after the second treatment none were found to have lice. In School O, two children had lice on day 4, and on day 9 a further 21 had lice of various stages. After the second treatment, five children were positive on day 11, and two remained positive on day 16, plus an additional five who had no detectable lice on day 11. One child failed to use the second application of treatment. The treatment success rate in this school was 69/79 (87·3%), i.e. lice free after the second application of permethrin and overall it was 89/99 (89·9%).
Table 2. Effectiveness of permethrin treatment
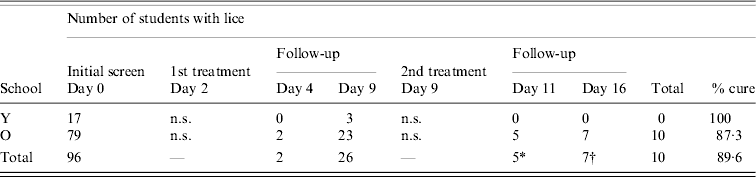
n.s., Not screened
* Three cases positive on day 11 not found to have lice on day 16.
† Five new cases positive day 16.
DISCUSSION
In field surveys of head louse infestation a trade-off is necessary between thoroughness of examination to obtain the most accurate data, the time required to perform the work and the disruption to schooling. We found that our team of six investigators was able to examine thoroughly all 332 school children in the larger of the two schools investigated in less than one school day with no more than 30 min disruption to any one class group. This was more efficient in terms of limiting school disruption than by using a wet combing method [Reference De Maeseneer5].
It has been suggested that diagnosis of head louse infestation by wet combing using conditioner as a lubricant is the most effective method for identifying head louse infestation [Reference De Maeseneer5]. Comparison of two diagnostic combing studies suggests that this is not the case, although any combing is more effective than visual inspection. In the first, dry combing using the Innomed™ comb (Hogil Pharmaceutical Corporation, White Plains, NY, USA), with parallel-sided steel teeth, was reported 4·2 times more effective than visual inspection [Reference Mumçuoglu4]. In the other, wet combing (comb type not specified) appeared to be more effective than visual inspection [Reference De Maeseneer5]. Of those diagnosed positive by wet combing 17/49 (35%) were missed by visual inspection. In comparison 14/46 (30%) participants diagnosed positive by visual screening were not confirmed by wet combing and only one was subsequently found positive after 14 days. An alternative analysis of the study from that of the authors suggested that there was no difference between the two methods [Reference Berger7].
We have shown a significant (P<0·001) advantage of dry combing, using a specially designed plastic head louse detection and removal comb, over the traditional method of visual inspection for identifying active head louse infestations.
We found combing to be 3·8 times more effective than visual inspection compared with the 4·2 times found by Mumçuoglu et al. [Reference Mumçuoglu4]. The differences between the studies were the structure of the combs and possibly the efficiency of the visual inspection techniques employed. We used finger-and-thumb parting of hair over every section of the scalp without gaps, whereas Mumçuoglu and colleagues used applicator sticks to part hair. No comparison of these methods has been conducted so their relative efficiency is untested. Nevertheless both studies demonstrate clearly that detection combing of dry hair is significantly more effective than visual inspection and potentially significantly more effective than wet combing.
We have also confirmed that the presence of louse eggs and nits, without finding live lice, is a poor indicator of active infestation as only 7·6% of nit-infested but apparently louse-free children had developed an active infestation more than 2 weeks after the initial examination.
Other studies of head lice in Turkey have shown variable levels of infestation ranging from 3·4% to 15·8% [Reference Kokturk8–Reference Özçelik, Değerli and Aslan12]. However, in all cases visual inspection was the method used to identify cases; and any stage of lice or nits was deemed evidence of infestation, both of which are likely to have resulted in inaccurate data. However, as we found a considerably increased level of infestation identified by detection combing compared with visual inspection, it is reasonable to conclude that if those investigators had used combing they may have found levels of infestation closer to ours.
Treatment of active cases with 1% permethrin creme rinse cured all cases in one community and 87·3% in the other. Although at least one of the children may have been re-infected by younger siblings at home, it is likely that these were true cases of treatment failure. This may have been due to incomplete use of the product by caregivers, as all treatments were applied at home out of the sight of investigators. If the treatments were applied correctly, it may indicate developing resistance in the population. Although the resistance to this insecticide is well documented elsewhere [Reference Rupes13–Reference Lee16], it apparently did not significantly affect rural areas of Turkey at the time of our study.
The study conducted in Ankara [Reference Tanyuksel9] also used 1% permethrin to treat students with lice. A success rate of 94% was obtained compared to ours of 89·6%, from which we concluded that resistance to permethrin has either not yet developed in Turkish lice or is at a sufficiently low level to affect current treatment regimens. However, since our study was conducted in a rural environment, where the intense use of pediculicides is less likely than in cities, the level of failure could have been due to re-infestation within the extended family.
From this work we have concluded that combing of dry hair, using a specifically designed plastic louse detection comb, is a cheap, rapid, and effective method for accurately screening for head lice in schools and other communities. We also confirmed that it is necessary to find lice in order to diagnose active infestation, and that follow-up over several days if there are large numbers of eggs/nits present can identify low-grade or latent infestations missed by other means. Moreover, at the time of the study, there seemed to be little resistance associated with use of permethrin in this part of Turkey. The data obtained in this study will serve as a baseline for further studies that are planned to compare dry detection combing with wet combing for detection and treatment of head louse infestation.
ACKNOWLEDGEMENTS
We thank all the staff and students at the schools in Yagcilar and Osmancali for their cooperation and hospitality during the performance of this investigation. We also thank Professor Ü. Z. Ok, Vice President of Celal Bayar University, Manisa, for his interest in and support of our work.
DECLARATION OF INTEREST
I.F.B. received travel and accommodation costs from KSL Consulting, Denmark, and has used ‘PDC’ combs in clinical investigations prior to this. KSL is a manufacturer and supplier of louse combs. I.F.B. and K.S.L. have both been consultants to various makers of pharmaceutical products, alternative therapies, and combs for treating louse infestations.






