INTRODUCTION
Darwin (Reference Darwin1859) may have been the first to suggest that differences between closely related species may be the result of adaptation to reducing interspecific competition via selection of different habitats. In a series of articles beginning in the 1960s MacArthur and his colleagues further developed this idea, now most often referred to as resource partitioning or, more specifically, habitat partitioning (MacArthur & Levins, Reference MacArthur and Levins1964). Although early work primarily focused on terrestrial habitats, a recent review (Bowen et al., Reference Bowen, Rocha, Toonen and Karl2013) concluded that the ecological boundaries can be important arenas for evolutionary processes in marine and other ecosystems.
Levesque et al. (Reference Levesque, Juniper and Marcus2003) showed that resource partitioning can occur in Polychaeta. As with all members of the family Serpulidae, Spirobranchus Blainville, 1818 juveniles become sessile and build their own tube. The 37 Spirobranchus species currently recognized (Read et al., Reference Read, Fiege, Bellan, Read and Fauchald2016) are mostly distributed in subtropical and tropical zones, with S. triqueter (Linnaeus, Reference Linnaeus1767) even occurring in the Arctic (Pillai, Reference Pillai2009; Rzhavsky et al., Reference Rzhavsky, Kupriyanova, Sikorski and Dahle2014). The larger species are often recorded inhabiting corals, whereas smaller representatives may occur on almost any solid substrate. A substantial amount of research has been conducted on Spirobranchus giganteus sensu latissimo (Marsden, Reference Marsden1987; Nishi & Nishihira, Reference Nishi and Nishihira1996; Petitjean & Myers, Reference Petitjean and Myers2005; Rowley, Reference Rowley2008), often erroneously reported from all tropical regions as S. giganteus (Pallas, 1766), a species restricted to the Caribbean. The larger species of Spirobranchus are often named ‘Christmas tree worms’ for the bright colours and spiral arrangement of their radioles.
Frank & ten Hove (Reference Frank and ten Hove1992) hypothesized that morphology of branchial crowns may be correlated to different filtering strategies, which may be an indication of resource partitioning. However, despite the wide distribution and striking appearance of members of the genus, ecological processes such as habitat partitioning have not received much attention.
Most of the larger Spirobranchus species are associated with hermatypic corals (see below), their tubes embedded in the coral skeleton. Figure 1 shows different substrates colonized by Spirobranchus in the Gulf of Eilat. Growth rate and angle of the worm's tube are correlated with that of the coral in such a way that the opening of the tube will always stay on top of the surface (Nishi & Nishihira, Reference Nishi and Nishihira1999). Some taxa, such as the Caribbean S. giganteus and the Indo-West Pacific S. corniculatus, are thought to be obligate inhabitants of living corals (Hunte et al., Reference Hunte, Conlin and Marsden1990a; Marsden & Meeuwig, Reference Marsden and Meeuwig1990; Nishi, Reference Nishi1996). Members of the Spirobranchus giganteus complex are abundant on some coral species, rare or even absent from others. Coral preferences may differ between Spirobranchus taxa and thus between biogeographic regions. Dai & Yang (Reference Dai and Yang1995) found that in the coral reefs of Southern Taiwan, S. corniculatus mostly inhabit the corals Porites lutea Milne Edwards & Haime, 1851, P. lobata Dana, 1846, P. lichen Dana, 1846 and Montipora informis Bernard, 1897. In the bank reef off the West coast of Barbados the hexacoral species Diploria strigosa (Dana, 1846), Porites astreoides Lamarck, 1816 and the hydrozoan coral Millepora complanata Lamarck, 1816 are most heavily colonized by S. giganteus while Colpophyllia natans (Houttuyn, 1772), Dendrogyra cylindrus Ehrenberg, 1834, Dichocoenia stokesii Milne Edwards & Haime, 1848, Eusmilia fastigiata (Pallas, 1766) Meandrina meandrites (Linnaeus, 1758) and Mycetophyllia spp. Milne Edwards & Haime, 1848 were not colonized (Conlin, Reference Conlin1988; Hunte et al., Reference Hunte, Conlin and Marsden1990a). Specific searches for associated fauna in potential hosts (not only corals) may result in new association records (Hoeksema & ten Hove, Reference Hoeksema and ten Hove2016; Hoeksema et al., Reference Hoeksema, van Beusekom, ten Hove, Ivanenko, van der Meij and van Moorsel2016).

Fig. 1. Different substrates colonized by Spirobranchus, reef of Eilat, Israel. (A) Spirobranchus gardineri associated with Cyphastrea sp. (B) S. gaymardi associated with Pocillopora sp. (C) Spirobranchus sp. associated with Stylophora sp. (D) S. cruciger associated with Millepora sp. (E) S. corniculatus associated with Acropora sp. (F) Spirobranchus sp. on artificial substrate. Arrow: operculum. (A, D, F) Photos: O. Perry; (B, C, E) photos: A. Hallakoun.
The co-occurrence of worm and coral may be of a mutualistic character. DeVantier et al. (Reference DeVantier, Reichelt and Bradbury1986) showed that the branchiae and especially the operculum of the worm can provide protection to the coral from predators. In some conditions the feeding behaviour may even enhance water flow close to the coral's surface (Strathmann et al., Reference Strathmann, Cameron and Strathmann1984), which might locally prevent bleaching (Ben-Tzvi et al., Reference Ben-Tzvi, Einbinder and Brokovich2006). However, settlement of Spirobranchus taxa on corals is an antagonistic interaction. Corals can be aggressive towards other organisms and compete for resources using sweeper tentacles (Genin & Karp, Reference Genin and Karp1994). Perhaps because of this, some of the more subordinate corals as Porites lutea, P. lobata and P. lichen were found to be most colonized by Spirobranchus corniculatus while the more aggressive corals such as Mycedium elephantotus (Pallas, 1766), Merulina ampliata (Ellis & Solander, 1786) and Galaxea astreata (Lamarck, 1816) were less colonized (Dai, Reference Dai1990; Dai & Yang, Reference Dai and Yang1995). Settling larvae of a species better adapted to cope with coral aggression are more likely to survive. Larval settlement preference has been demonstrated in Spirobranchus (Hunte et al., Reference Hunte, Conlin and Marsden1990a, Marsden & Meeuwig, Reference Marsden and Meeuwig1990) and may be an indicator of host-specific adaptations. An alternative view was proposed by Rowley (Reference Rowley2008), who suggested that S. corniculatus sensu stricto (as S. giganteus) contributes to the success of its hosts in several ways and the relationship should be thought of as mutualism rather than commensalism. Regardless, Hunte et al. (Reference Hunte, Conlin and Marsden1990a) demonstrated that substrate selection by planktonic larvae of S. giganteus was correlated with worm size, such that individuals located on the preferred species of coral reached a larger size. Thus, length can be a surrogate of high performance and provide evidence for adaptive host-specific interactions.
Living hermatypic corals are not the only substrate for the larger Spirobranchus worms. Recently, there have been records that Spirobranchus giganteus s. str. can also live on octocorals, rubble, and substrates such as oil buoys or pillars of a pier (Nygaard, Reference Nygaard2008; Skinner et al., Reference Skinner, Tenório, Penha and Soares2012; Hoeksema et al., Reference Hoeksema, Wah Lau and ten Hove2015). Although purporting to describe S. giganteus, Figure 1D of Skinner et al. (Reference Skinner, Tenório, Penha and Soares2012) depicts S. tetraceros (Schmarda, 1861). Nygaard (Reference Nygaard2008) may have been studying S. polycerus (Schmarda, 1861), a species with spiral branchiae as well and tubes of about 5 mm across (ten Hove, Reference ten Hove1970; Hoeksema & van Moorsel, Reference Hoeksema, van Moorsel and Hoeksema2016). The latter is very common on all kinds of hard substrates in the Antilles, including Bonaire (ten Hove, unpublished data).
The second (mainly) tropical species (but see below), Spirobranchus tetraceros, is an Indo-Pacific invasive in the Mediterranean (Ben-Eliahu & ten Hove, Reference Ben-Eliahu and ten Hove1992), and has been reported from Turkey to inhabit artificial as well as natural hard substrata (Çinar, Reference Çinar2006; Çinar et al., Reference Çinar, Kurt and Dağli2014). Spirobranchus tetraceros was also reported to inhabit artificial substrate and become an extensive fouler in the Suez Canal (Shalla & Holt, Reference Shalla and Holt1999; Selim et al., Reference Selim, Abdel Naby, Gab-Alla and Ghobashy2005). Moreover, S. tetraceros seems to be an opportunistic taxon as it was reported to explosively overgrow corals immediately after a period of stress in the Persian Gulf (Samimi Namin et al., Reference Samimi Namin, Risk, Hoeksema, Zohari and Rezai2010). In view of its exceptionally large distribution, from temperate Australia to tropical regions all over the globe, S. tetraceros most probably is a species-complex. Despite the body of knowledge accumulated on substrate of Spirobranchus spp., for some species, such as S. gardineri Pixell, Reference Pixell1913, hardly any substrate preference data are available. For the typical form (his variant types 1 and 2 in the meantime have both been named S. richardsmithi Pillai, Reference Pillai2009) the genera Millepora Linnaeus, 1758 spp., Psammocora Dana, 1846 spp. and Stylocoeniella Yabe & Sugiyama, 1935 spp. have been mentioned by Smith (Reference Smith1985: 40).
The Gulf of Eilat is the northernmost coral reef, expanding northward from the Red Sea and the Indian Ocean. Eight nominal species of Spirobranchus have been recorded from the Red Sea and its northern Gulf of Eilat, of which seven from both areas (Table 1). The apparent absence of the Indo-West Pacific S. gaymardi from the Red Sea proper while occurring in its most northern gulf is probably due to insufficient collecting effort rather than reflecting a real difference.
Table 1. Spirobranchus species reported from the Red Sea and the Gulf of Eilat.
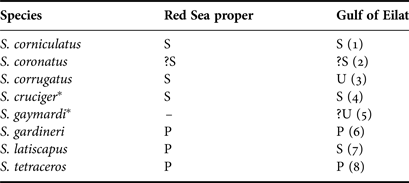
References: (1) Pixell (Reference Pixell1913), Mergner (Reference Mergner1979); (2) Gravier (Reference Gravier1906, as jousseaumei), Amoureux et al. (Reference Amoureux, Rullier and Fishelson1978, Figure 16); (3) Vine & Bailey-Brock (Reference Vine and Bailey-Brock1984, Figure 5B–G), Hassan (Reference Hassan1998); (4) Vine & Bailey-Brock (Reference Vine and Bailey-Brock1984); (5) ten Hove, personal observation; (6) Fauvel (Reference Fauvel1933); (7) Vine & Bailey-Brock (Reference Vine and Bailey-Brock1984); (8) Vine & Bailey-Brock (Reference Vine and Bailey-Brock1984), Hassan (Reference Hassan1998).
Table modified from ten Hove et al. (unpublished).
* Considered to be synonymous with S. corniculatus by Willette et al. (Reference Willette, Iñiguez, Kupriyanova, Starger, Vartman, Toha, Maralit and Barber2015).
P, published, identification confirmed; S, published, but ID has since been synonymized; U, previously unpublished; ?, published, questionable (Not yet verified).
However, species identification remains questionable as some taxa were identified based on too few specimens; other names were regarded to be synonyms by some authors. In order to examine the potential for habitat partitioning in Spirobranchus, our goal was to start resolving conflicting records about which species are found in the Eilat region and what hosts they inhabit. Thus, in this study we surveyed Spirobranchus spp. and their distribution on different substrates in the Gulf of Eilat.
MATERIALS AND METHODS
Collecting and preservation of specimens
The research was performed on the coral reef of Eilat, Gulf of Eilat, Red Sea, 29°33′N 34°57′E, Israel. A total of 189 specimens were collected by scuba from depths of 0.5–12 m (Israel Nature-Parks Authority [INPA] permit number 2014/40533). Seven substrate types were examined for Spirobranchus species (Table 2). Due to INPA limitations and to prevent damage to the reef, worms were sampled mainly from branching and encrusting corals and from artificial substrates such as pillars of a pier without coral association. After taking measurements (see below), a piece of the abdomen of each specimen was preserved in 100% ethanol and stored at −20°C for molecular analysis. The rest of each specimen was fixed in 4% formaldehyde (in SW) for 24 h, rinsed in filtered seawater, transferred to ethanol 70%, and stored at 4°C.
Table 2. Number of specimens of Spirobranchus spp. collected from different habitats.
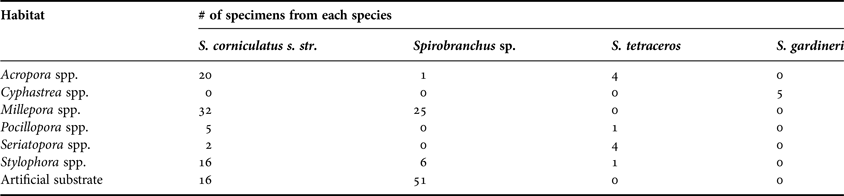
Although we observed worms on massive coral genera such as Porites sp. Link, 1807 and Dipsastraea sp. Blainville, 1830, we did not sample them in order to minimize the damage to slow growing taxa.
Species identification
The Spirobranchus giganteus complex was tentatively resolved by Fiege & ten Hove (Reference Fiege and ten Hove1999, Figure 4), distinguishing 12 separate species mainly on the basis of the morphology of their opercula. However, the morphospecies of the Indo-West Pacific S. corniculatus complex in the strict sense as distinguished by Fiege & ten Hove, S. corniculatus (Grube, 1862), S. gaymardi (Quatrefages, 1865) and S. cruciger (Grube, 1862), are not distinguished by DNA (Willette et al., Reference Willette, Iñiguez, Kupriyanova, Starger, Vartman, Toha, Maralit and Barber2015).
For this study, identification of Spirobranchus taxa is based on the morphology of the operculum (as for instance in Willette et al., Reference Willette, Iñiguez, Kupriyanova, Starger, Vartman, Toha, Maralit and Barber2015; however see below), which is a hard (calcareous) cover sealing the opening of the tube when the worm retracts. The morphology of the operculum can change during growth and as a result its structure in juveniles can resemble the fully grown operculum of another species (ten Hove & Ben-Eliahu, Reference ten Hove and Ben-Eliahu2005). We concentrated our efforts on larger specimens, no longer subject to such ontogenetic changes. However, it is almost impossible to observe and differentiate between Spirobranchus taxa in situ because the operculum also functions as substrate for other organisms obscuring the opercular morphology (Figure 2). Overgrowth of opercula in serpulids is not uncommon, for example it was mentioned by Gambi (Reference Gambi1986) for Ditrupa arietina (O. F. Müller, 1776). Because identification in situ is difficult, and due to confusing and changing insights in the taxonomy of the larger Spirobranchus taxa, many studies followed Fauvel (Reference Fauvel1953) or Day (Reference Day1967) and applied their ‘widespread species’ concept of the nominal species Spirobranchus giganteus to material from Indo-West Pacific origin (Smith, Reference Smith and Hutchings1984; DeVantier et al., Reference DeVantier, Reichelt and Bradbury1986; Nishi & Nishihira, Reference Nishi and Nishihira1999; Floros et al., Reference Floros, Samways and Armstrong2005; Ben-Tzvi et al., Reference Ben-Tzvi, Einbinder and Brokovich2006; Rowley, Reference Rowley2008). However, ten Hove (Reference ten Hove1970), in a first attempt to unravel the ‘giganteus’ complex, drew the attention to the fact that there might be geographically more restricted taxa involved; he distinguished between two, possibly three subspecies: the Caribbean S. giganteus (Pallas, 1776), the Indo-West Pacific S. corniculatus and the Pacific Mid-American S. incrassatus Krøyer in Mörch, 1863. A further attempt was made by Fiege & ten Hove (Reference Fiege and ten Hove1999, especially figure 4), who mentioned 12 taxa which almost all previously had been included in Spirobranchus giganteus sensu latissimo.

Fig. 2. Operculum as substrate: Arrows show operculum with organisms on top. (A–C) operculum under binocular, (D–F) worms in situ. (A) Sponge; (B) Coral; (C) Spirorbis tube; (D–F) Unidentified organisms. Photos: O. Perry.
To facilitate identification, worms were collected and returned to the laboratory. We photographed each specimen using a Canon PowerShot G16 and examined each specimen using a Leica M165 FC binocular microscope and photographed it using a Leica DFC295 digital camera and LAS software. Worms' length was measured from the digital photographs, from base of radioles to end of pygidium, using image analysis ImageJ software (ver. 1.47v NIH). All specimens collected were categorized into groups (morphotypes) according to their opercular morphology, and were either compared to morphotype illustrations by Willette et al. (Reference Willette, Iñiguez, Kupriyanova, Starger, Vartman, Toha, Maralit and Barber2015, figure 2 – however, in this figure two morphotypes have been switched: figure 2a, b show Spirobranchus corniculatus,- figure 2c, d are of S. gaymardi) or attributed to morphologically better defined taxa such as S. tetraceros and S. gardineri. Unfamiliar morphologies which could not be ascribed to one of the above mentioned taxa were recorded as Spirobranchus sp. Corals were identified to genus level by their morphology using a coral fact sheets guide (http://coral.aims.gov.au/factsheet.jsp?speciesCode=0162). It is important to note that while taxonomy of the Spirobranchus corniculatus-complex is still not fully resolved, this paper is focusing on ecological interactions of morphotypes with their substrate.
Molecular work is currently being executed on the worms collected to underpin the taxonomy of the species.
Statistical analyses
We used contingency table and χ2 to test for association between substrate and identified morphotypes. To assess the effects of species identity and substrate type on worm length we used two-way analysis of variance. Preliminary observations revealed an apparent size difference between the two major morphotypes. Thus, in order to control for heritable differences between morphotypes, data were standardized within each species by subtracting the mean length and dividing by the standard deviation, to achieve mean of 0 and standard deviation of 1. All data analyses were carried out with R (R Development Core Team, 2012).
RESULTS
Morphological characterization
Specimens collected were divided into seven opercular morphology groups (Figure 3). Of the six species (eight nominal minus two confirmed synonyms, Spirobranchus corniculatus and S. gaymardi) reported from the Red Sea (Table 1) only five morphotypes (see below) were identified in this survey in the Gulf of Eilat. Among the 189 specimens collected, we identified the following taxa: S. corniculatus, S. cruciger, S. gaymardi (all three are considered part of the S. corniculatus complex s. str., see Fiege & ten Hove, Reference Fiege and ten Hove1999, Figure 4), S. gardineri and S. tetraceros. In addition, two not previously recognized opercular morphologies were observed among the collected samples. The seven groups of opercular morphology can be defined as follows: S. corniculatus (Figure 3A) has an oval (more or less egg-shaped) opercular plate with two laterodorsal spines arising from a short common base. These spines each have a small dorsal tine, a secondary spinule along the bend and are forked at the tip. Spirobranchus gaymardi (Figure 3B) differs from S. corniculatus by the larger dorsal spines and a small medioventral knob to a large forked medioventral spine, all arising from a short common base. The laterodorsal spines have well developed dorsal tines, meeting mid-dorsally. In addition the dorsal spines have one or two secondary spinules along the bend of the spine and are forked at the tip. Spirobranchus cruciger differs from S. gaymardi by the dorsal tines not expanded at their tips and not meeting mid-dorsally (Figure 3C).

Fig. 3. Seven opercular morphology groups of Spirobranchus spp. from Eilat (dorsal views). (A–C) S. corniculatus: (A) morphotype corniculatus s. str.; (B) morphotype gaymardi; (C) morphotype cruciger; (D) S. gardineri; (E) S. tetraceros; (F, G) Spirobranchus sp. Scale bar: 1 mm. Photos: O. Perry.
Spirobranchus gardineri (Figure 3D) has an oval opercular plate with one elongated almost central shaft with two dorsal and one forked midventral spines at the end, all pointing upwards. Spirobranchus tetraceros (Figure 3E) is recognized by an almost circular opercular plate with three pairs of antler-like spines arranged around the middle of the plate; each spine is forked at the tip (moreover, its radioles are arranged in two circles, not spirals; it is the only taxon here with anteriorly fringed peduncular wings, all others have smooth wings). Spirobranchus sp. two new morphotypes (Figure 3F, G) have a circular opercular plate with a pair of large dorsal antler-like spines arising from a short common base, each with a well developed dorsal tine (like those in morphotype gaymardi) well separated, and with two secondary spinules along the bend. There are two stout ventral spines, joined at their base, in some cases forked at their tips. A full description of this taxon is in preparation.
Species–substrate association
Nearly half of all specimens collected belong to the Spirobranchus corniculatus complex s. str. The complex was more abundantly found on corals than on artificial substrate (Table 2), but showed no preference for any particular coral species. Spirobranchus tetraceros and S. gardineri were both uncommon (N = 10, 5.3% and N = 5, 2.7%, respectively) and both were found exclusively in association with corals. Spirobranchus tetraceros was found on Pocillopora Lamarck, 1816 spp., Stylophora Schweigger, 1820 spp., Seriatopora Lamarck, 1816 spp. and Acropora Oken, 1815 spp., whereas S. gardineri was found exclusively on Cyphastrea Milne Edwards & Haime, 1848 spp., a new record in addition to the three genera mentioned by Smith (Reference Smith1985). Spirobranchus sp. was found mainly on artificial substrate (N = 51, 61.5%). Overall, the distribution of Spirobranchus taxa over substrate types differed significantly (χ218 = 295.1, P < 0.001; Figure 4). We only found sufficient numbers of the S. corniculatus complex and the undescribed morphotype (Spirobranchus sp.) for analyses of size. The average length of Spirobranchus sp. and S. corniculatus complex was 14.5 and 21 mm, respectively (Figure 5A).

Fig. 4. Relative abundance of Spirobranchus spp. in different substrates.

Fig. 5. The relationship between substrate and length in Spirobranchus associated with corals and artificial substrate. (A) Average length of Spirobranchus corniculatus complex (grey) and Spirobranchus sp. (black). (B) Standardized lengths of Spirobranchus corniculatus complex (grey) and Spirobranchus sp. (black) differed significantly as a function of substrate.
Spirobranchus sp. was significantly larger when settled on artificial substrate than on corals, whereas individuals of the S. corniculatus complex were larger on corals and smaller on artificial substrate (Figure 5B).
DISCUSSION
Only five of the eight nominal Spirobranchus species previously reported from the Gulf of Eilat were found in this study. Some apparent absences can be explained by misidentification in the past, revisions in taxonomy that have occurred in the intervening years, and difference in sampling effort. Spirobranchus latiscapus, for instance, generally has been reported from dredged material and deeper water, not sampled by us. We cannot overrule the possibility of species disappearance from the area as a result of anthropogenic causes or climate changes (Rilov, Reference Rilov2016). Given the relatively limited collection efforts in this study, it is premature to interpret our results as an indication that a particular species no longer appears in the Gulf of Eilat. Data on substrates used by several of the Spirobranchus spp. are missing or limited.
We found five specimens of S. gardineri in this study, all of them associated with live Cyphastrea. The low abundance of S. tetraceros in this study is slightly surprising, having been reported to be a very abundant Lessepsian migrant in the Suez Canal and Mediterranean (Selim et al., Reference Selim, Abdel Naby, Gab-Alla and Ghobashy2005). However, records from the Red Sea never mention such a massive occurrence (e.g. Ben-Eliahu & ten Hove, Reference Ben-Eliahu and ten Hove2011). Another interesting difference is that the species fouls artificial substrate in the Suez Canal and Alexandria Harbour, but was only found on corals in the present study. Either it has a large ecological plasticity, which is suggested by Samimi Namin et al. (Reference Samimi Namin, Risk, Hoeksema, Zohari and Rezai2010), or we are dealing with a complex of species only to be distinguished with genetics. All three morphotypes of the Spirobranchus corniculatus complex were identified in this study: S. corniculatus, S. cruciger and S. gaymardi. Contrary to previous records that S. corniculatus complex is an obligate symbiont of living corals (Nishi, Reference Nishi1996), this is the first record of S. corniculatus complex s. str. inhabiting an artificial substrate in addition to hermatypic corals. About 20% of these worms were found on artificial substrate in our study (Figure 4). It appears that the association with corals is not obligatory to the S. corniculatus complex s. str. in the Gulf of Eilat, all opercular morphologies belonging to the complex (thus S. corniculatus, cruciger and gaymardi) were found on corals as well as on artificial substrate. This may be attributed to differences in collecting efforts in previous studies or to differentiation in the Red Sea of the S. corniculatus complex. Genetic work (that has already started) may be able to shed light on this seemingly dissimilar behaviour. Two new and previously unrecognized morphologies were found which might represent a single new species. These forms showed a preference for artificial substrate, on which more than 60% had settled.
In S. giganteus s. str., the association with favourable substrate may influence fitness (Hunte et al., Reference Hunte, Marsden and Conlin1990b). In a laboratory experiment, Hunte et al. (Reference Hunte, Marsden and Conlin1990b) showed that worms that were found in their preferred habitat attained larger size. This appears to be supported by our study: worms of two species not only favoured different habitats, but they also apparently attained a larger size in their preferred habitats (S. corniculatus complex is more abundant and larger on corals; Spirobranchus sp. more abundant and larger on artificial substrate). Thus, worms of the S. corniculatus complex are not negatively affected by coral host defences, a finding consistent with the view that the worms may be commensal symbionts (Rowley, Reference Rowley2008). The opposite trend was found for Spirobranchus sp., preferring artificial substrates, making additional research on the relationships between different worm species and their various hosts and habitats necessary.
Spirobranchus worms sampled here showed high levels of plasticity in substrate selection. Worms were found on all six coral genera sampled, as well as on artificial substrate, though two species (S. gardineri, S. tetraceros) were only found on corals. The high level of plasticity in settling on a variety of substrates shown by Spirobranchus corniculatus s.str. as well as probably new species of Spirobranchus in the Gulf of Eilat may also explain the relative abundance of the genus in general.
ACKNOWLEDGEMENTS
The authors dedicate this paper to the memory of the late Dr Nechama Ben-Eliahu, a central figure in the Polychaeta world. We are grateful to her for sharing her vast knowledge with the authors. We would like to show our deep appreciation to Tamar Feldstein (Tel-Aviv University), Dan Perry (Arava Institute for Environmental studies) and Noga Stambler (Bar-Ilan University) for providing helpful comments on the manuscript and scientific guidance. We thank Muriel Dray, Eynav Cohen, Ellie Foran, Ayelet Hallakoun, Assaf Partzelan, Yaniv Shmuel, Ido Shefy, Dror Komet, Jessica Bellworthy, Yoav Balaban, Gabriella Sanka and Irena Kolesnikova (Interuniversity institute for Marine Sciences in Eilat) for assisting in the field and in the lab. Oren Levy and Noa Blecher-Simon (Bar-Ilan University) provided facilities and advice in lab work. Efrat Gavish and Ariel Chipman (Hebrew University of Jerusalem) kindly provided access to the National Natural History Collections and Bert W. Hoeksema (Naturalis Biodiversity Center) to their collections in Leiden. Assaf Zvuloni (The Israel Nature & Parks Authority) assisted with permits. We thank Elena Kupriyanova (Australian Museum Research Institute) for information on field and lab work as well as useful taxonomy discussions.
This research is part of the requirements for a PhD thesis for Orly Perry at Bar Ilan University.
FINANCIAL SUPPORT
The Interuniversity institute for Marine Sciences in Eilat provided logistics, diving and boating services. A Martin Fellowship enabled Orly Perry's visit to Naturalis Biodiversity Center, Leiden.









