An introduction to appetite, obesity and stress
Obesity and appetite regulation as a challenge in current society
The global obesity prevalence is rising. Most alarming is the prevalence of childhood overweight ranging between 10 and 40 % in European countries(Reference Moreno, Pigeot and Ahrens1). On top of that, dieting is mostly only successful for short periods, thus leading to claims that obesity is very resistant to treatment(Reference Oude Luttikhuis, Baur and Jansen2). In an era of food abundance, uncontrolled appetite and overeating are indeed an issue. This is a situation where food is consumed in the absence of hunger; thus, without a homeostatic need but rather as hedonic eating. A combination of more hedonic drive (desire for food) with less control results in uncontrolled eating. Uncontrolled eating can range on a continuum from emotional eating (overeating in response to negative emotions) towards eating impulsivity or disinhibition or external eating (opportunistic eating because food is available) up to binge eating (recurrent episodes of eating too much food because of perceiving lack of control)(Reference Vainik, Neseliler and Konstabel3). As uncontrolled eating fosters eating beyond the saturation point, it can lead to an increased energy intake and over time to overweight and psychological problems. For example, emotional eating rather than lifestyle behaviour (physical activity, smoking, alcohol use, fruit consumption) was associated with higher BMI increase in a prospective study in adults(Reference Koenders and van Strien4). Consequently, understanding the phenomenology of uncontrolled eating can aid in disease prevention (i.e. clinical eating disorders, low well-being, obesity and CVD). Already in youth, these eating behaviours are of public health concern given similar or higher prevalence of stress-eating compared with adults (up to 40 %)(Reference Jaaskelainen, Nevanpera and Remes5, 6) and adolescence being a critical stage for the onset of eating disorders like binge eating(Reference Marzilli, Cerniglia and Cimino7). By targeting youth, bigger prevention effects might be reached over time as dietary choices(Reference Mikkila, Rasanen and Raitakari8), disordered eating behaviours(Reference Neumark-Sztainer, Wall and Larson9) and obesity(Reference Singh, Mulder and Twisk10) track within individuals from childhood/adolescence towards adulthood.
Psychosocial stress: role and prevalence
Although diet and physical activity are still the main drivers, research has broadened its view on additional potential obesity contributing factors. In this context, especially the psychological determinants of obesity have received increasing interest as a driver of energy imbalance(Reference Karasu11, Reference Rehkopf, Laraia and Segal12) with a special focus on chronic psychosocial stress causing unhealthier diet intake and more fat deposition.
Psychological stress occurs when an individual perceives that environmental demands (i.e. stressors) tax or exceed his or her adaptive capacity, resulting in emotional and behavioural disturbances(Reference Lazarus and Folkman13). The stress concept has been used to reflect a very broad mix of situations which might result in contradictory associations with behaviour, biology and health outcomes. A recent perspective concluded that specification is needed of the species, sex, ethnicity, social class and developmental stage of the agent as well as the predictability and controllability of the event and the measures that reflect the presumably stressful event(Reference Kagan14). Indeed, stressor specificity can determine hormonal(Reference Miller, Chen and Zhou15) and dietary(Reference Macht16) changes by stress.
Even during youth, stress has been reported as highly prevalent. In a European sample of 4- to 11-year-old children, 53·4 % lived in familial/social adversities and 40·3 % experienced at least one major negative life event such as a parental divorce(Reference Vanaelst, Huybrechts and De Bourdeaudhuij17). More specifically, 23 % of the 11-year-olds in the international Health Behaviour in School-aged Children study reported being pressured by schoolwork and 12 % reported being bullied at school(Reference Currie, Zanotti and Morgan18).
The problem of chronic psychosocial stress: psychobiology and health effects
The body has a series of different processes which have the effect of maintaining homeostasis. Also during stress, the goal is to maintain stability through changes in the immune system, nervous system and endocrine system for an appropriate amount of time, but then to turn off these reactions immediately afterwards via two main physiological stress systems(Reference Charmandari, Tsigos and Chrousos19). The first stress system is the hypothalamic–pituitary–adrenal axis, with cortisol as the endproduct(Reference Miller, Chen and Zhou15). The second stress system is the autonomic nervous system, with the catecholamines adrenaline and noradrenaline as endproducts, but often heart rate variability is used as a non-invasive biomarker to indirectly measure cardiac parasympathetic and sympathetic activity(20). In contrast to acute stress for which these systems are meant, chronic stress leads to prolonged activation or inefficient management of cortisol and the autonomic system with detrimental physiological consequences(Reference McEwen21, Reference McEwen22). Consequently, chronic stress increases vulnerability to diseases like CVD and inflammation-related disease(Reference Ghike23–Reference Rohleder26), even starting in childhood(Reference Pervanidou and Chrousos27–Reference Danese and McEwen29). The nature and the chronicity of the stressor, as well as the individual’s vulnerability, stress perception and stress coping, are important variables in determining the chronic adverse effects of stress(Reference Miller, Chen and Zhou15). The underlying complex processes and mechanisms are still poorly understood but can help in designing prevention and treatment strategies.
Aim of the present review
The obesity public health problem may be in part driven by chronic stress via uncontrolled eating behaviour and obesogenic dietary choices. As insight in underlying pathways is pivotal, the aim of this review is to briefly summarise the current state of knowledge on the biological underpinnings and to suggest areas for future investigation. After all, research is often very focused (i.e. looking at only one part of one pathway) and monodisciplinary (only focusing on behaviour, psychology, neurology, nutrition or pathology). Especially for newcomers in the field but also for those wanting to think outside the borders of their own research niche, a broad overview is often lacking. As the objective is to present a conceptual framework, no systematic review method has been performed and reviews are preferentially cited where possible. Without the intention of giving an exhaustive list of biomarkers, I want to provide a schematic overview of relevant pathways from stress to uncontrolled eating and obesity. Herein, I progress from simple well-accepted pathways to more complex newer ones. Although simplification is intended, I will underline the complexity by the existence of bidirectional and multifactorial relationships that lead to a vicious circle(Reference Tomiyama30). These links will shed light on how the stress–diet–obesity interaction can be interdisciplinarily examined by integrating several new techniques to further knowledge. Herein, -omics technologies are helpful like metagenomics (measuring genetics of entire communities, here specifically meaning the bacteria in our bodies), epigenomics (measuring complete set of epigenetic modifications) and metabolomics (measuring all metabolites) as they do not target a single component but try to identify patterns in the overall system. Finally, I present saliva as a biological matrix that is easier to collect and allows several of these high-throughput analyses. In this paper, I intend to highlight the importance of an early-life focus in stress–diet–obesity research by citing also childhood-specific literature where existing.
Pathways from psychosocial stress towards appetite and obesity
Psychosocial stress as a cause of obesity: physiology and behaviour
Overall, the literature underlines the importance of the bidirectional associations between mental health and adiposity(Reference Tomiyama30, Reference Gatineau and Dent31). In this review, the main focus will be on the direction from stress towards adiposity as diet plays an important role in this direction. In adults, a comprehensive meta-analysis indeed concluded that work and life stress significantly increase BMI and/or waist longitudinally(Reference Wardle, Chida and Gibson32). In children and adolescents, less research has been performed although this has been booming the last years(Reference Wilson and Sato33). There is evidence from a review that early-life stress is associated with multiple biological and behavioural pathways in children that may increase risk for later obesity(Reference Miller and Lumeng34). A systematic review including twelve longitudinal studies in children/adolescents(Reference Incledon, Wake and Hay35) on clinical depression, perceived stress, anger/anxiety and behaviour concluded that the evidence is low for this relationship in children/adolescents. Another review focused on the effect of both household and individual stressors on children’s overweight parameters(Reference Gundersen, Mahatmya and Garasky36).
Several mechanisms in the effect of stress on adiposity have been described in the literature(Reference Tomiyama30). Mainly mechanisms are divided in physiological/biochemistry and behavioural (lifestyle) pathways (Fig. 1), as will be explained in the next paragraphs. In addition, cognitive characteristics like executive function and self-regulation are important(Reference Aparicio, Canals and Arija37).

Fig. 1. The classic behavioural and physiological pathways in the stress–obesity relationship. Behavioural pathways consist of less physical activity, sleep problems and an unhealthier diet. The underlying physiology is mainly due to increased cortisol levels that stimulate fat storage and change dietary behaviour. Indeed, cortisol influences brain regions essential for dietary behaviour, i.e. the reward and appetite regions, thus many appetite biomarkers are relevant for stress research. VTA, ventral tegmental area; NPY, neuropeptide Y; AgRp, agouti-related protein; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; PYY, peptide tyrosine tyrosine; CCK, cholecystokinin; GLP, glucagon-like peptide.
The direct physiological pathway is dominated by cortisol. This cortisol interacts with lipid metabolism in two ways, as reviewed by Peckett et al. (Reference Peckett, Wright and Riddell38). First, cortisol increases the amount of circulating NEFA by stimulating the lipoprotein lipase enzyme. These NEFA can then be used to accumulate fat in fat cells. Second, cortisol decreases this fat storage. Increased adiposity is caused by hyperplasy (adipogenesis) and in the presence of insulin also by hypertrophy (lipogenesis). In addition, cortisol may influence lipolysis (degradation of adipose tissue TAG to fatty acids): both prolipolytic and antilipolytic activity has been hypothesised, but the mechanisms are still unclear and may depend on duration, dose and location of cortisol exposure. Antilipolytic activity has mainly been observed in high cortisol concentrations and in the abdominal region. The fat storage chiefly occurs in the visceral fat cells since the cortisol receptors have a high density in this region, and thus mainly abdominal obesity is theorised(Reference Bjorntorp39).
Apart from that direct effect on fat storage, stress may indirectly facilitate adiposity through behavioural pathways such as maladaptive coping behaviours leading to an adiposity-stimulating lifestyle(Reference Tomiyama30, Reference Miller and Lumeng34): emotional eating of ‘comfort’ food (rich in sugar and fat), a disordered sleep and a lack of exercise with an increase in screen time.
Psychosocial stress as a cause of uncontrolled eating: biological underpinnings
A meta-analysis concluded that children/adolescents with stress have higher intake of unhealthy food and in the oldest also lower intake of healthy food(Reference Hill, Moss and Sykes-Muskett40). The preferred foods are rich in sugar and fat and have been called ‘comfort food’ since eating functions as a way to cope with stress via distraction and reward feelings(Reference Macht16). Even in paediatric literature, perceived stress and cortisol have been associated with more snacking, sweet food intake and less fruit/vegetable intake(Reference Jenkins, Rew and Sternglanz41–Reference Michels, Sioen and Braet45). These stress-induced dietary changes are often reflected as maladaptive uncontrolled eating behaviour(Reference Macht16). In several studies, children’s and adolescents’ emotions and stress have been associated mainly with emotional eating(Reference Goossens, Braet and Van Vlierberghe46–Reference Nguyen-Rodriguez, McClain and Spruijt-Metz48) and sometimes with increased external eating(Reference Braet and Van Strien47, Reference Hou, Xu and Zhao49). Although eating palatable food can increase positive mood and reduce stress feelings through sensory pleasure(Reference Gibson50), this increase in positive mood is only temporary and is mostly followed by negative feelings like shame and guilt(Reference Gibson50). Thus stress-induced or emotional eating creates a vicious circle. Herein, emotion regulation could be an important skill as a target for obesity prevention/intervention(Reference Aparicio, Canals and Arija37) as the use of adaptive emotion regulation towards negative emotions (like reappraisal, problem-solving and acceptance) is a protective factor(Reference Braet, Theuwis and Van Durme51) minimising unhealthy dietary response(Reference Svaldi, Tuschen-Caffier and Trentowska52) and physiological cortisol response(Reference Perry, Donzella and Parenteau53) after stressor exposure.
A well-known underlying pathway in stress-induced eating is cortisol(Reference Adam and Epel54–Reference Epel, Tomiyama, Dallman, Brownell and Gold57), mainly by hypothalamic actions. After all, hypothalamic nuclei have overlapping functions in appetite regulation, stress response and rewards, while exerting effects on other brain regions like the mesolimbic reward system, cortex and brainstem(Reference al’Absi58). A first hypothalamic nucleus is the paraventricular nucleus regulating cortisol secretion. A second one is the arcuate nucleus acting as the central appetite control centre in which the pro-opiomelanocortin/cocaine- and amphetamine-regulated transcript (POMC/CART) neurons suppress food intake, whereas the neuropeptide Y/agouti-related protein (NPY/AgRP) neurons stimulate appetite. This arcuate nucleus transduces signals further to the other hypothalamic nuclei such as the paraventricular, lateral, dorsomedial and ventromedial nuclei. Certain of these nuclei form the bridge towards the appetite-regulating hypocretinergic (orexins) and endocannabinoid system and even the autonomic nervous system(Reference Abdalla59).
The two main diet-related actions of cortisol are thus increased reward sensitivity (opioid and dopamine system, especially when dieting) and appetite (arcuate nucleus in the hypothalamus), as summarised in Fig. 1. Cortisol is known to up-regulate NPY (an appetite and reward inducer) and dysregulate insulin and leptin (appetite and reward diminishing)(Reference Adam and Epel54). To explain the latter: insulin and leptin decrease appetite and reward but the body becomes resistant due to the dysregulation, thus appetite and reward will be increased(Reference Adam and Epel54). Based on my own longitudinal research, the combination of high stress and hyperleptinaemia might make girls more vulnerable to stress-induced eating(Reference Michels, Sioen and Ruige60). A review indicates a stress-induced change in other hormones that act on the arcuate nucleus such as ghrelin(Reference Labarthe, Fiquet and Hassouna61). In a stressful situation, ghrelin is hypothesised to rise as ghrelin can then lower anxiety feelings (it is anxiolytic). Ghrelin will then also increase hunger and food reward and might thus lead to more emotional eating and a higher obesity risk(Reference Labarthe, Fiquet and Hassouna61). With less evidence on the causal role in emotional eating, also other gastrointestinal appetite-reducing hormones have been related to stress/depression(Reference Lach, Schellekens and Dinan62) and eating disorders(Reference Culbert, Racine and Klump63): peptide tyrosine tyrosine (peptide YY; PYY), cholecystokinin (CCK) and glucagon-like peptide (GLP). Therefore, the next paragraphs will elaborate on the gut–brain axis and its role in appetite.
More recent biological insights in stress–diet–obesity with high-throughput applications: microbiota, epigenetic modifications and metabolites
Gut bacteria
Analysing microbiota in the stress–diet relationship is relevant as: (1) bacteria are related to stress; (2) bacteria are related to diet; and (3) bacteria can interfere in the diet–stress relationship. These three aspects are consecutively discussed in the next three sections.
Gut bacteria communicate with the brain
The human gut contains 1011 bacteria per g intestinal content that play a role in optimal body function. Well-known functions of gut bacteria are digestive such as food fermentation and immunological by creating a defence barrier or controlling inflammatory reactions(Reference Bengmark64). More recently, evidence has appeared that gut bacteria can ‘communicate’ with the brain. This concept is called the ‘microbiota–gut–brain axis’(Reference Moloney, Desbonnet and Clarke65) since microbiota play an active role in this bidirectional gut–brain communication, as frequently reviewed(Reference Moloney, Desbonnet and Clarke65–Reference Aroniadis, Drossman and Simren68). Herein, several pathways exist: neural (autonomic and enteric nervous system), neuro-endocrine (by hormone-producing entero-endocrine cells in the gut epithelium and by SCFA produced by the gut microbiota) and neuro-immune (inflammatory cytokines) pathways(Reference Moloney, Desbonnet and Clarke65). Several brain centres are triggered in this way, but special focus is on the hypothalamus with its arcuate nucleus as energy-regulating centre (see ‘Gut bacteria decide what is on the menu’ section) and the paraventricular nucleus as stress/cortisol-regulating centre; two centres that play a role in the above-mentioned stress-eating or uncontrolled eating pathways.
Evidence for that relationship between stress and microbiota is increasing. The two main physiological stress pathways, i.e. the cortisol(Reference Farzi, Frohlich and Holzer69) as well as the nervus vagus(Reference Bonaz, Bazin and Pellissier70) system, have theoretically and experimentally been linked to gut microbiota. Similarly, many molecules with neuroactive functions such as γ-aminobutyric acid, serotonin, catecholamines, acetylcholine and dopamine have been listed as gut bacteria products(Reference Moloney, Desbonnet and Clarke65). Although several studies have been published on gut microbial changes in clinical depression cases(Reference Jiang, Ling and Zhang71–Reference Lin, Ding and Feng75), results are quite conflicting, for example, α diversity is mostly decreased, sometimes non-significant and sometimes increased. Nevertheless, some studies have demonstrated the causal link. For example, gut microbiota transplantation from patients with depression into rodents successfully induced a depressive phenotype in these animals, demonstrating the powerful influence that the gut microbiota can exert on behaviour(Reference Zheng, Zeng and Zhou73, Reference Kelly, Borre and El Aidy74). In addition, a meta-analysis of probiotic interventions highlighted an overall improvement of psychological reports(Reference McKean, Naug and Nikbakht76). Less observational research has been done in healthy participants and this often has shown limited results: increased lactobacilli during examination periods(Reference Knowles, Nelson and Palombo77), no associations at all with depressive symptoms or perceived stress(Reference Kleiman, Bulik-Sullivan and Glenny78), less emotional arousal with higher Prevotella abundance(Reference Tillisch, Mayer and Gupta79) and genera differences depending on mood but no clear changes depending on specific depressed, anxious or angry mood(Reference Li, Su and Xie80). The instability and immaturity of gut microbiota during childhood and adolescence could increase their susceptibility to environmental insults, such as stress and poor diet, which could result in dysbiosis and potentially have a negative impact on brain functions like stress and appetite regulation(Reference Borre, O’Keeffe and Clarke81). To my knowledge, no such study on the direct stress–bacteria relationship exists in children. More indirectly, a longitudinal study identified maternal prenatal stress as predictor of a child’s gut microbiota(Reference Zijlmans, Korpela and Riksen-Walraven82).
Gut bacteria decide what is on the menu
Since our bacteria are completely dependent on our food for their own metabolism (some prefer fibre, some prefer proteins), it seems logical that they have developed mechanisms to (co-)control our nutritional intake. Indeed, there is more and more evidence that gut bacteria have a role in regulating our appetite, satiety and potentially our food preferences or taste perceptions, as will be described below. Because of its role in energy homeostasis, the gut microbiome has also been associated with the metabolic syndrome(Reference de Clercq, Frissen and Groen83) and childhood obesity(Reference Indiani, Rizzardi and Castelo84).
Our appetite is influenced by hormones and neurotransmitters which can be directly produced by the gut bacteria themselves or whose production is indirectly stimulated by bacteria. Fig. 2 summarises some main pathways. The final target are the two energy-regulating neuron groups POMC/CART and NPY/AgRP within the arcuate nucleus; these neurons are influenced by gut-produced molecules that affect appetite and satiety such as ghrelin, CCK, GLP-1, GLP-2 and PYY(Reference de Clercq, Frissen and Groen83). Indeed, eating disorders are typically linked to changes in these hormonal factors(Reference Culbert, Racine and Klump63). From gut bacteria towards appetite, a first potential indirect pathway is via SCFA. These SCFA are volatile fatty acids like butyrate, acetate, propionate and valerate produced by the gut microbiota as fermentation products from food components such as fibre. These SCFA normally reduce appetite by stimulating the release of appetite-reducing hormones PYY and GLP-1 from the entero-endocrine cells(Reference de Clercq, Frissen and Groen83, Reference Tolhurst, Heffron and Lam85, Reference Larraufie, Martin-Gallausiaux and Lapaque86). Stress-induced gut dysbiosis might cause imbalance in this protective mechanism that in turn can lead to uncontrolled eating. As a second indirect pathway, specific microbes might regulate intestinal endocannabinoid-like compounds such as 2-olyeol-glycerol and oleoyl-ethanolamide which again can stimulate GLP production by entero-endocrine cells(Reference Cani, Plovier and Van Hul87) while gut microbiota dysbiosis seems to decrease GLP-1 sensitivity(Reference Yamane and Inagaki88). Finally, direct effects of the microbiota on the arcuate nucleus have been suggested, for example, an Escherichia coli bacterial strain producing caseinolytic protease B seems to reduce appetite via the arcuate nucleus(Reference Breton, Legrand and Akkermann89). Next to these endocrine parameters, the two other main pathways in the microbiota–gut–brain axis (see ‘Gut bacteria communicate with the brain’ section) might be involved: the nervous system (for example, nervus vagus stimulation) and inflammatory regulation (for example, gut barrier integrity).
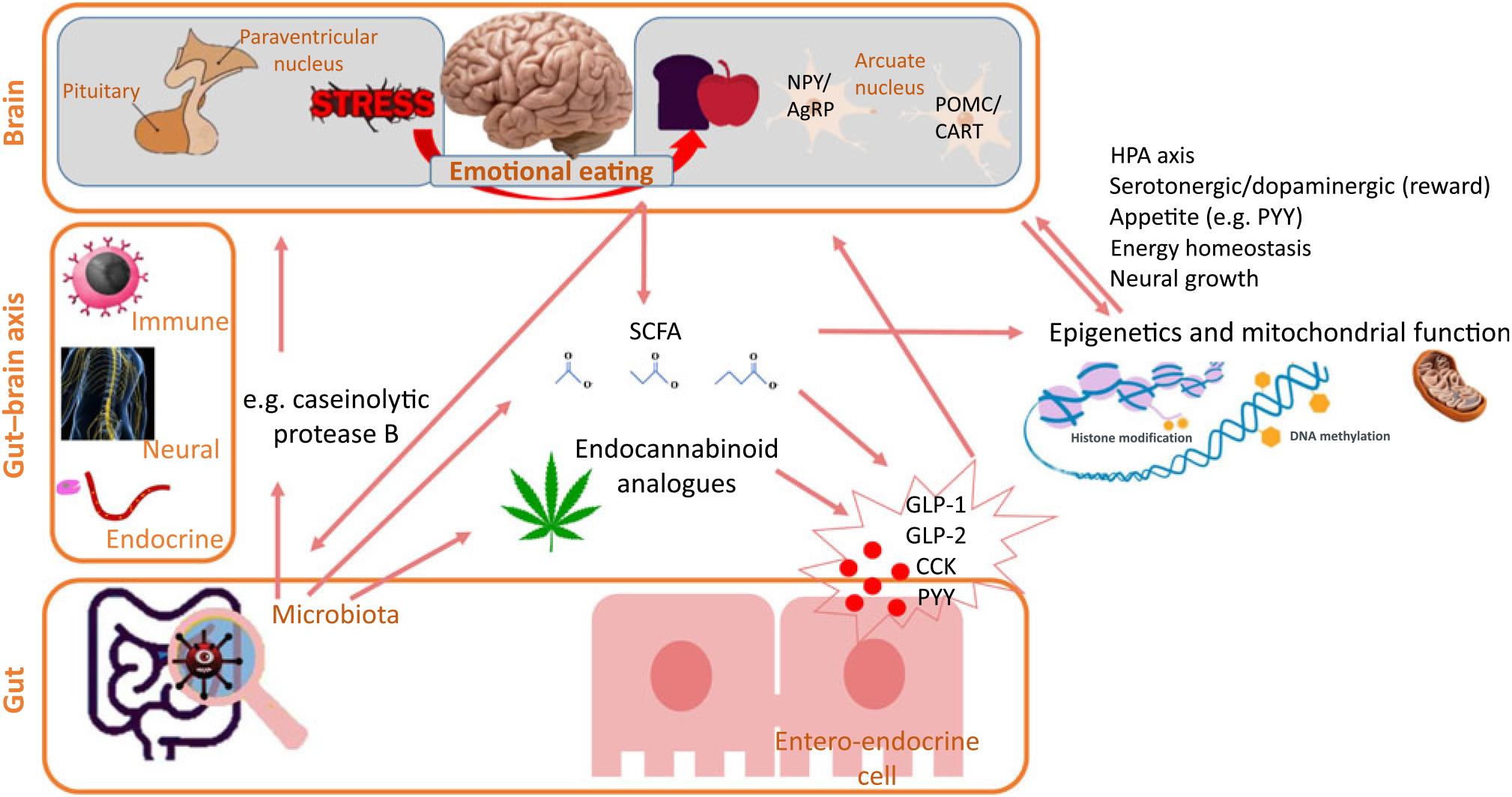
Fig. 2. Some pathways in the microbiota–gut–brain axis from microbiota towards appetite. The gut–brain axis links the gut microbiota with stress and appetite brain centres. The microbiota can directly act upon the appetite brain centres but also indirectly via SCFA and endocannabinoid analogues that regulate entero-endocrine cells’ release of appetite-influencing molecules. An additional indirect pathway towards appetite is that the gut microbiota induces epigenetic changes. It should be considered that most links also work in the other direction, for example, diet can influence epigenetics, gut microbiota composition and SCFA production. NPY, neuropeptide Y; AgRp, agouti-related protein; POMC, pro-opiomelanocortin; CART, cocaine- and amphetamine-regulated transcript; HPA, hypothalamus–pituitary–adrenal; PYY, peptide tyrosine tyrosine; GLP, glucagon-like peptide; CCK, cholecystokinin.
All the above pathways target appetite. For eating behaviour, not only appetite should be targeted but also impulse control and/or changed food preferences and taste perception. For example, food preference for proteins was dependent on intestinal bacteria in an experiment with fruit flies(Reference Leitao-Goncalves, Carvalho-Santos and Francisco90) and many of the mentioned appetite-regulating neuropeptides are summarised in a review on taste sensitivity(Reference Fabian, Beck and Fejerdy91). Direct studies on uncontrolled eating behaviour associated with gut bacteria are missing but, indirectly, gut bacterial changes after bariatric surgery were found to be associated with an observed hedonic eating decline(Reference Sanmiguel, Jacobs and Gupta92).
The stress–bacteria–diet triangle: bidirectionality and implications
Taken together, stress, diet and bacteria form a triangle: gut bacteria are linked to both stress and diet (see the ‘Gut bacteria communicate with the brain’ and ‘Gut bacteria decide what is on the menu’ sections), while diet and stress are also interrelated (see ‘Psychosocial stress as a cause of uncontrolled eating: biological underpinnings’ section). Fig. 3 metaphorically translates this bidirectional link of gut bacteria with stress and diet. These bidirectional links highlight gut bacteria as an interesting target point for research, prevention and treatment as bacterial imbalance has multiple implications. To create a balanced gut bacterial composition/activity, a bacteria-friendly and thus fibre-rich diet should be combined with a relaxed mind. On the other hand, uncontrolled eating and a stressed brain can (at least partially) be prevented by balanced gut bacteria. Thus, gut microbiota can be an intermediate pathway in stress–diet and diet–stress. Nevertheless, no study has shown that the specific gut microbiome changes resulting from stress are in turn directly responsible for dietary and weight changes.

Fig. 3. The stress–bacteria–diet interaction triangle with a comparison with a forest ecosystem. This figure shows that stress, gut bacteria and diet are bidirectionally related to each other. To make the metaphor towards a forest ecosystem, the gut bacteria symbolise the trees in the forest as the ideal situation is a diversity of trees in the forest and a high diversity of bacteria in our gut. The diet is then representing the nutritious soil and stress represents the atmosphere. In the case of the forest, the trees will not survive without an appropriate atmosphere (air/sun/humidity) and nutritious soil while on the other hand, the trees themselves will influence the soil and atmosphere by the autumn leaves that enrich the soil and by the produced oxygen. Thus, bidirectional interactions exist. The same type of interaction can be translated towards the gut microbiota. Concerning food, we know that fibre-rich food can enrich our bacteria and apparently our bacteria might affect our food intake. Concerning our brains, I have summarised in this review the bidirectional gut–brain axis where certain bacteria can influence our stress reactivity while stress might act upon the bacteria. Freely interpreted, it seems these interactions teach us that we should create a relaxed atmosphere and optimal nutrition for our internal forest to obtain a balanced ecosystem of bacteria resulting in low stress and appropriate appetite.
Oral microbiota
The gut is not the only microbial hot spot in our bodies, for example, also the oral cavity has its own microbiota(Reference Acharya, Chan and Kheur93). Yet, little is known about these bacteria, with mainly research in dentistry and increasingly in immune-related diseases(Reference Acharya, Chan and Kheur93). The potential of the oral cavity microbiota has been highlighted in a recent perspective on the gut–brain axis as similar bacterial communities in oral and faecal samples exist(Reference Aroniadis, Drossman and Simren68). Indeed, the influx of oral strains from phylogenetically diverse microbial taxa into the gut microbiome seems extensive in healthy individuals(Reference Schmidt, Hayward and Coelho94). Apart from influencing the gut microbiome, oral microbiota can also directly influence health. Associations with obesity have been observed, potentially due to increasing metabolic efficiency, appetite and insulin resistance(Reference Goodson, Groppo and Halem95). In addition, salivary bacterial genera/families have been associated with fat tasting or overall super-tasting(Reference Besnard, Christensen and Brignot96, Reference Cattaneo, Gargari and Koirala97) amongst others by changes in antioxidant capacity. In stress–obesity research the oral microbiota has not yet been integrated, although for example, in a sample with burn-out salivary Solobacterium moorei levels were higher(Reference Nani, Lima and Marcondes98) and an acute stressor influenced salivary bacterial adherence(Reference Bosch, Turkenburg and Nazmi99).
Epigenetics: relevance for stress, eating behaviour, obesity and gut bacteria
Physiological and behavioural functions are particularly sensitive to the programming effects of environmental factors such as stress and nutrition during early life(Reference Patchev, Rodrigues and Sousa100). One way of programming is epigenetics. Epigenetics can be seen as a measure of metabolic ageing and concerns changes in gene expression that are not due to changes in DNA sequence but, for example, due to DNA methylation or histone modification which then alters transcription and thus expression and bodily functions. An epigenetic clock DNA methylation signature based on seven to 353 sites has outperformed other markers of ageing and is linked to a broad range of health aspects like obesity(Reference Declerck and Vanden Berghe101).
There is increasing evidence that the response to early-life adversity is system/genome-wide and persists into adulthood for example, in relation to childhood abuse(Reference Labonte, Suderman and Maussion102) and even during pregnancy(Reference Provencal and Binder103). Based on a systematic review, stress seems to affect methylation on the cortisol axis itself, on serotonergic/dopaminergic/noradrenergic neurotransmission and neural growth(Reference Bakusic, Schaufeli and Claes104). These pathways are also important in obesity aetiology as they are related to food behaviour. Stress-induced changes in dietary intake (i.e. comfort food) can induce epigenetic effects as nutrition is one of the frequently researched lifestyle factors affecting epigenetics(Reference Park, Friso and Choi105). Even appetite and its related impulses like impulsivity and reward sensitivity are sensitive to epigenetic changes(Reference Archer, Oscar-Berman and Blum106). For example, a review summarised that DNA methylation dysregulation in eating disorder is accompanied by a disturbed dopaminergic and endocannabinoid system(Reference Yilmaz, Hardaway and Bulik107). In rats, it has been shown that a methyl-balanced diet during adolescence might prevent stress-induced binge eating(Reference Schroeder, Jakovcevski and Polacheck108) and can reverse changes in DNA methylation and negative behavioural consequences induced by early-life stress(Reference Weaver, Champagne and Brown109). Since almost no research has been done on stress-induced changes in obesity-related genes, a recent study tried to associate psychosocial factors with gene-level DNA methylation of eighty-seven overweight-associated genes in older adults, although with limited success(Reference Elboudwarej110). Taken together, epigenetic changes by stress, diet and obesity can influence the stress response, eating behaviour and obesity.
Interestingly, gut bacteria are partially involved in this epigenetic programming. Mice lacking gut bacteria possess several transcriptional differences, some related to energy homeostasis and cortisol(Reference Diaz Heijtz, Wang and Anuar111). Indeed, SCFA and certain polyphenol metabolites produced by bacteria are inhibitors of histone deacetylases and can thus induce epigenetic changes(Reference Stilling, Dinan and Cryan112), amongst others influencing appetite through PYY elevations(Reference Larraufie, Martin-Gallausiaux and Lapaque86) (see the left side of Fig. 2). Because of those complex interactions, the bidirectional stress–diet–obesity link can also work transgenerationally (from mother to child) via bacterial and epigenetic changes; for example, maternal lifestyle can make an impact on the neonatal microbiome leading to specific epigenetic signatures that may potentially predispose to the development of late-life obesity(Reference Li113). Nevertheless, such studies on childhood stress should still be tested. As bidirectional links between epigenetic changes and the microbiota exist, integrative studies using both epigenomics and metagenomics are needed(Reference Carbonero114).
The potential of metabolomics as a tool in biological underpinnings
As multiple biological pathways seem to be involved in stress–diet–obesity, a study of all metabolites, i.e. metabolomics, is believed to aid in unravelling the biological underpinnings. Metabolomics is the comprehensive study of the metabolome – the repertoire of small molecules present in cells, tissues and body fluids. The metabolome is regarded as the most revealing real-time quantifiable read-out of the human biochemical state at a system’s level, thus allowing insight in mechanisms and providing predictive, diagnostic or prognostic markers for diverse disease states(Reference Beger, Dunn and Schmidt115). Sometimes interchangeably defined as ‘metabonomics’, the metabolic patterns are dynamic and arise as the product of our own gene-encoded proteins in combination with metabolic products of our microbes and our environment like the food that we eat. Consequently, metabolomics is relevant in the stress–diet–obesity axis as metabolic profiles have been related to gut microbiota(Reference Vernocchi, Del Chierico and Putignani116), stress(Reference Li, Tang and Cheng117), childhood obesity programming(Reference Rauschert, Kirchberg and Marchioro118) and clinical eating disorders(Reference Focker, Timmesfeld and Scherag119). Within stress-related metabolomics, stress profiles are distinguished by neurotransmitters and energy-related metabolites(Reference Li, Tang and Cheng117), which again reflects the stress–obesity relationship. Within obesity-related metabolomics, branched-chain amino acids are often significant and related to gut bacteria(Reference Vernocchi, Del Chierico and Putignani116), although data from adult and childhood obesity research gives sometimes opposite results(Reference Rauschert, Kirchberg and Marchioro118). Another common pathway might be the inflammatory system, as adipose tissue, diet, stress and gut microbiota are associated with inflammation(Reference Grenham, Clarke and Cryan67, Reference Hansel, Hong and Camara120). In addition, mitochondrial metabolites might be relevant to focus on as mitochondria are responsible for cellular energy/signalling and have been reciprocally linked to stress, energy homeostasis, microbiota and gene expression(Reference Picard and Turnbull121–Reference Bajpai, Darra and Agrawal123). Up to now, the study of metabolomics never seems to have combined stress and obesity data or stress–diet data.
The stress–‘omics’ nexus into practice: saliva as a biological sample
In summary, interactions between epigenetic changes, microbiota and metabolic profiles are relevant in the study of stress–diet–obesity (see Fig. 4). Integration of these analyses requires the collection of biological samples. Blood is the classic biological matrix (allowing amongst others metabolomics, proteomics and epigenomics), while faecal samples allow the study of gut bacteria and their related metabolites(Reference Beger, Dunn and Schmidt115, Reference Vernocchi, Del Chierico and Putignani116). Nevertheless, the use of invasive techniques to obtain study material such as blood is not favoured when studying human subjects, especially children. An alternative might be saliva as an easy-to-collect, pain-free biological matrix allowing frequent sampling and parallelling the composition of blood(Reference Yoshizawa, Schafer and Schafer124). Consequently, an increasing amount of laboratory analyses has been performed on saliva(Reference Yoshizawa, Schafer and Schafer124, Reference Wren, Shirtcliff and Drury125), but careful attention to the collection, processing and analysis steps is critical for the implementation of newer applications(Reference Wren, Shirtcliff and Drury125). In fact, saliva might thus offer an interesting biomarker to study stress–diet–obesity including the metagenomic, epigenomic and metabolomic analysis opportunities. Especially in paediatrics, saliva has been proven useful(Reference Pappa, Kousvelari and Vastardis126). Underneath I give a short overview for each of the three -omics techniques in saliva.
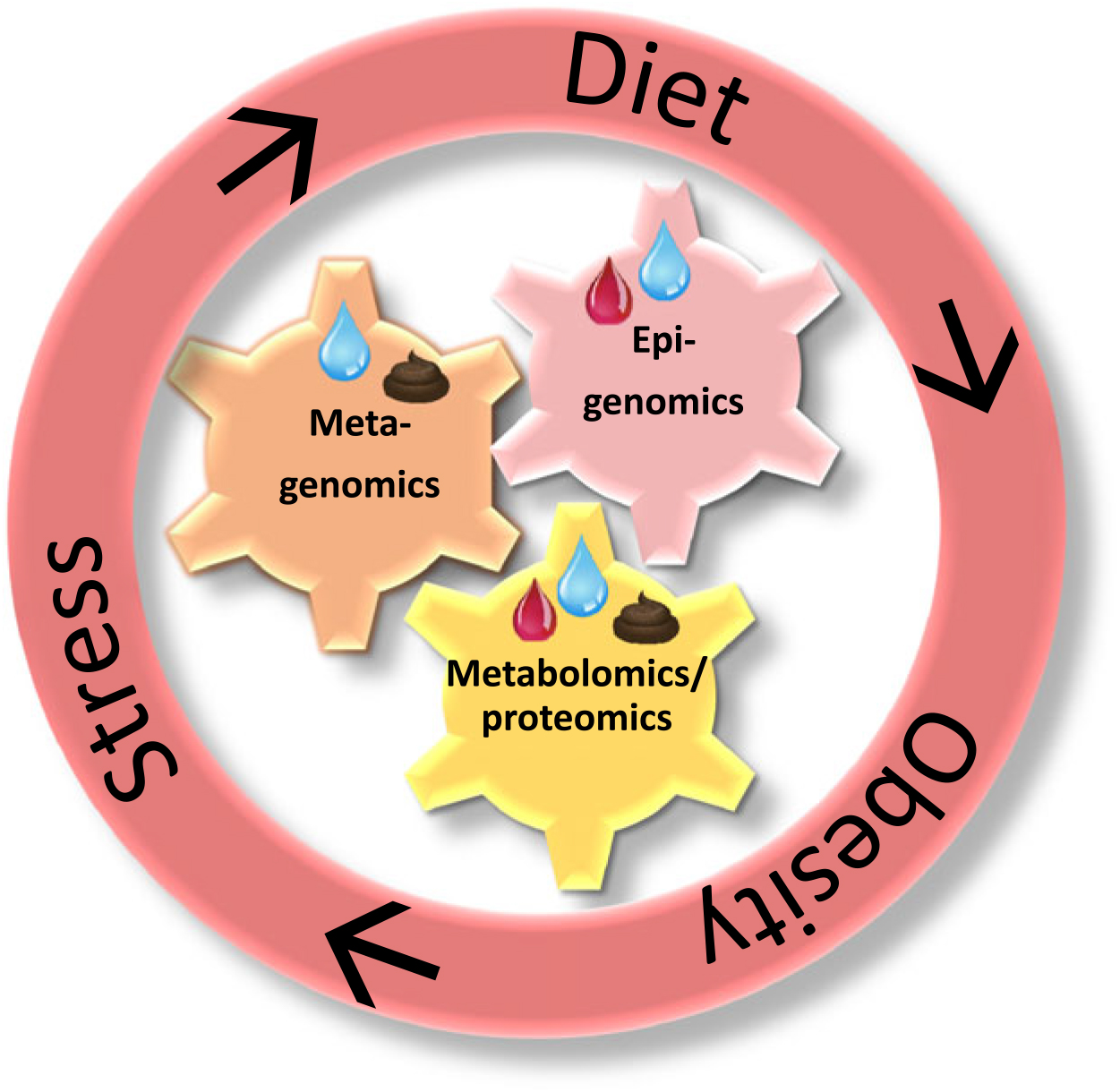
Fig. 4. The interacting -omics fields applicable in the stress–diet–obesity study and the relevant biological matrices herein. Summarising all evidence, the bidirectional stress–diet–obesity link happens via mutually interacting bacterial, epigenetic and metabolic pathways. Integrating metagenomic, epigenomic and metabolomic analyses will further prevention, diagnosis and treatment. Next to stool and blood samples, saliva seems to be a promising biological matrix allowing several -omics analyses in studying the bidirectional stress–obesity relationships. Blue drops represent saliva; red drops represent blood; brown figures represent stool.
First of all, saliva is already routinely used to measure proteins (proteomics) and endocrine parameters. In obese subjects, differences have been reported in salivary cortisol (stress), endocannabinoids (energy balance), inflammatory parameters, antioxidants (diet) and ghrelin (appetite) concentrations(Reference Choromanska, Choromanska and Dabrowska127). Related to eating behaviour, salivary composition has been associated with taste liking, macronutrient intake(Reference Munoz-Gonzalez, Feron and Canon128) and even eating difficulties in children(Reference Morzel, Neyraud and Brignot129). Indeed, many of the above-mentioned appetite hormones are present in saliva(Reference Fabian, Beck and Fejerdy91). Currently, techniques for salivary metabolomics in studying smaller molecules are being fine-tuned(Reference Dame, Aziat and Mandal130).
Second, DNA and RNA isolation from saliva samples is now commercially available to test, for example, RNA expression, microRNA expression, DNA methylation and telomere length(Reference Wren, Shirtcliff and Drury125). In fact, methylation patterns in the brain have been found to be more correlated with salivary DNA methylation than blood methylation(Reference Wren, Shirtcliff and Drury125), thus showing that stress-related research (early-life adversities, depression, etc.) can profit from saliva as a biological sample.
Third, and most innovative, is saliva metagenomics. However, little is known about these bacteria but, as mentioned above, some first associations with stress, diet and obesity have been suggested. Since saliva metabolites are not only of human origin but can also be produced by the oral cavity bacteria, saliva metagenomics should be included in research. For example, academic-induced chronic stress differences in hydrogen sulfide could be explained by salivary microbiota differences(Reference Nani, Lima and Marcondes98).
Limitations to the conceptual framework
As mentioned earlier, it was my goal to highlight some pathways that underlie the stress–diet–obesity association. In my conceptual framework, I highlighted the relevance of several -omics fields such as metagenomics, metabolomics and epigenomics, with a special focus on the use of saliva. Here I list a few limitations in (1) the stress–diet–obesity interactions (2) the promising -omics approaches and (3) the practical use of saliva.
Although stress is often linked to higher/unhealthier dietary intake and weight increase, stress can also cause a weight/fat decrease. It seems that chronic stress leads to weight loss in those individuals who keep their stress response with less energy intake, but it leads to weight gain in those who habituate with their stress response by using comfort food(Reference Peters, Kubera and Hubold131). Comfort food intake depends on the stressor and the individual, for example, dietary intake can be decreased in case of intense emotions and restrained eaters often eat more in response to stress independent of whether it is high-energy food or not(Reference Macht16). Also on the hormonal level, stress can be associated with lower cortisol in certain specific cases(Reference Miller, Chen and Zhou15).
Many of these -omics fields are still developing. Even for the microbiota, strong conclusive evidence on the causal link from stress to specific microbiota and then directly from these microbiota towards diet and obesity is lacking. More proof on the population level is needed, especially the theories towards emotional eating are rather based on animal or preliminary studies. Large European cohorts have reported high levels of inter-individual variation in microbiota composition and suggest that any individual factor would probably have only a very modest effect size, for example, depression could only explain 0·2 % of variance(Reference Falony, Joossens and Vieira-Silva132, Reference Zhernakova, Kurilshikov and Bonder133). Changes in biological parameters due to stress exposure can be difficult to detect because of difficulties in distinguishing different exposures, interactions (for example, between bacteria and between -omic levels) and day-to-day fluctuations. Indeed, a circadian rhythm should be considered, for example, about 15 % of the human plasma metabolome exhibits circadian rhythm(Reference Dallmann, Viola and Tarokh134). Moreover, heterogeneity in methodology is present since these technologies are still developing, costs are high and data integration from different -omics sets poses challenges.
Other limitations exist on sample collection level. For the faecal microbiota, storage conditions of samples can influence the results(Reference Choo, Leong and Rogers135) and a stool sample does not give the same microbiota picture as a rectal swab or rectal mucosa sample(Reference Jones, Zhu and Moan136). Also for saliva, the place of collection is important, with different biochemical and microbiological results when comparing passive drool, stimulated saliva or tongue film samples(Reference Feng, Licandro and Martin137). In fact, each step such as collection, storage, processing, assay and data analysis requires careful consideration of target analyte-dependent issues to prevent undue measurement error(Reference Wren, Shirtcliff and Drury125). During collection also other confounders like medication should be registered.
Conclusion
Our modern lifestyle presents with many psychosocial stressors in a highly palatable food environment and stimulates uncontrolled eating which can lead to increased energy intake and finally overweight. Especially during youth, the long-term effects can be very harmful and intervention/prevention is needed, although less early-life focused research exists. The current psychobiological review tried to offer (a non-exhaustive) insight in the biological pathways from stress to uncontrolled eating and obesity, more specifically the stimulating hormones (lipid metabolism, appetite and motivation/reward related), metagenomic (gut and saliva microbiome), epigenetic and metabolic profiles pinpointing towards the underlying pathways like the over/under-expression of specific genes or the over/under-active function networks such as mitochondrial energy regulation, neurotransmission, metabolism, etc. It is thus clear that stress and diet research should be combined to examine biological pathways of emotional eating in interdisciplinary collaborations (medical doctors, endocrinologists, pharmacologists, neurologists, psychologists, behavioural scientists, microbiologists, molecular biologists, bio-informatics experts, etc.). In my own upcoming research, I hope to distinguish why only some stressed adolescents become obese by examining hormones, metabolites, microbiota and epigenetics. Herein, systems biology, i.e. the simultaneous consideration of different biological levels, is more efficient in building the overall picture and will allow the identification of cross-level interactions like how the microbiota induces epigenetic and metabolic changes. Indeed, integrating epigenomic and metagenomic data into personalised nutrition(Reference Goni, Cuervo and Milagro138) and a systems biology/medicine approach in childhood obesity(Reference Stone, Schetzina and Stuart139) have been recommended. In studying this complex nexus, saliva seems to be an appropriate biological sample. An enhanced focus on modifiable biological mechanisms in this research niche like DNA methylation, microbial endocrine stimulation and neurotransmitter production will offer clues for therapeutic targeting.
Author ORCIDs
Nathalie Michels, 0000-0002-3069-7254
Acknowledgements
N. M. is financially supported by Research Foundation-Flanders (FWO) as a postdoctoral researcher. The present review received no specific grant from any funding agency, commercial or not-for-profit sectors.
There are no conflicts of interest.






