INTRODUCTION
Vibrio vulnificus is the leading cause of seafood-related death in the USA [Reference Hlady, Mullen and Hopkin1, Reference Oliver, Thompson, Austin and Swings2]. The bacterium causes three well described clinical syndromes, primary septicaemia, wound infections, and gastroenteritis. Severe systemic infections are associated with high mortality rates especially in immunocompromised hosts [Reference Blake3, Reference Haq and Dayal4]. In Israel, all disease cases have been characterized by wound infections with or without bloodstream infections [Reference Bisharat5, Reference Zaidenstein6]. Currently, disease cases in Israel are mostly confined to fishermen and fish-market workers (N. Bisharat unpublished data). The causative organism of the disease outbreak in Israel was a new strain of V. vulnificus, named biotype 3 (BT3), which was eventually implicated in all the confirmed infections to date [Reference Bisharat5, Reference Bisharat7]. The implicated fish was St Peter's fish (Tilapia zillii), the main type of fish cultivated in inland fish farms in Israel. Analysis of the lipopolysaccharide (LPS) and outer membrane proteins (OMPs) showed that V. vulnificus BT3 expresses a homogeneous LPS and exhibits highly conserved nucleotide sequences in OMP-encoding genes [Reference Bisharat8].
To date, the only factor that has been clearly associated with virulence of V. vulnificus is the capsular polysaccharide (CPS) [Reference Gulig, Bourdage and Starks9]. Many other factors have been suggested as playing an important role in the pathogenesis of V. vulnificus disease (LPS, acquisition of iron, cytolysin, haemolysin, metalloprotease, RtxA toxin, and pili) [Reference Gulig, Bourdage and Starks9, Reference Strom and Paranjpye10]. LPS has been suggested by some authors as one of the most essential factors (as for most Gram-negative bacteria) for the development of severe V. vulnificus infections such as severe soft tissue damage and septic shock [Reference Oliver, Thompson, Austin and Swings2, Reference Strom and Paranjpye10]. Animal studies showed that injecting purified LPS to rats resulted in shock and death within 1 h [Reference McPherson11] and reversal of its action resulted in complete inhibition of the toxic effects [Reference Elmore12]. While BT1 strains have variable LPS O-polysaccharide side-chains, BT2 serovar E and BT3 have a homogeneous LPS [Reference Bisharat8, Reference Amaro13]. To date no definitive role has been established for the LPS of BT1 strains; however, the O antigen side-chain of BT2 serovar E strains does appear to be an important virulence factor for eels [Reference Amaro14]. Nevertheless, and despite the clear association of V. vulnificus LPS with the classical endotoxic shock syndrome and its role in determining virulence (at least in eels infected with BT2), its role in eliciting an immune response is questionable. Hitherto, analysis of the immune response to LPS has been carried out only in eels infected with BT2, and showed that the immunoprotective potential of LPS was largely insignificant [Reference Amaro15–Reference Esteve-Gassent and Amaro17]. The immune response to V. vulnificus LPS in humans has not been evaluated so far.
Analysis of the disease outbreak in Israel showed that disease severity was variable, being generally milder in fishermen and fish-market workers compared to diseased members of the general public [N. Bisharat, unpublished data]. These differences in disease manifestation may simply be attributed to the health profile of people working in the fish industry (young and healthy males). In this regard, older age and patients with pre-existing liver disease or compromised immune systems are at particularly high risk for severe outcome and fatal septicaemia [Reference Gary Hlady and Klontz18–Reference Levine and Griffin21]. Nevertheless, and given the frequent exposure to fish and fish-farm water it is possible that occupational exposure in fishermen and fish-market workers may have provided some protection against severe or symptomatic infection.
We have utilized the unique epidemiology of the disease in Israel, where all the confirmed infections to date were caused by a single biotype expressing a homogeneous LPS, to study the human immune response to V. vulnificus LPS and test the association between antibodies to LPS and disease susceptibility in fishermen and fish consumers.
METHODS
Study groups, study design and data collection
Study groups
The study groups included members of the general public and fishermen who suffered from laboratory-confirmed V. vulnificus infection and their matched controls. Serum samples were obtained from all participants. The local ethics committee at Ha'Emek Medical Center approved the study and informed consent was obtained from all participants. Over a 2-year period four groups of participants were recruited. The first group consisted of patients (n=20) from the general public who suffered from soft tissue infection (with or without bacteraemia) after handling pond-cultivated fish, where V. vulnificus was grown from blood or wound cultures. The second group consisted of controls (from the general public) matched by age, sex and comorbidities as for the first group, but who had never handled pond-cultivated fish or suffered from any fish-related disease (n=40). The third group consisted of fishermen and fish-market workers who suffered from laboratory-confirmed V. vulnificus infection (n=10). The fourth group consisted of healthy fishermen and fish-market workers (n=40) with the same risk of exposure to V. vulnificus as the infected fishermen. Disease severity was assessed based on the extent of the soft tissue damage and the need for surgical intervention.
Study design
Different approaches in designing the study were used to address the study objectives; a prospective study and a case-control study. The sensitivity, specificity, dynamics and persistence of the serological response to V. vulnificus BT3 LPS was examined in frame of a prospective study. The sensitivity and specificity of the ELISA test was determined using paired sera (acute and convalescent samples) from subjects with laboratory-confirmed infection caused by V. vulnificus BT3. The rate of significant antibody response to the homologous and heterologous V. vulnificus BT3 LPS in these subjects was compared to that measured in sera obtained from subjects with no known previous exposure to V. vulnificus. To study the dynamics and long-term persistence of serum IgG and IgA antibodies to V. vulnificus BT3 LPS, a cohort of new cases infected with V. vulnificus BT3 was recruited and followed up while blood samples were obtained at the acute stage of the disease, 4–6 weeks later, at 6 months, and at 1 year. A curve of IgG and IgA antibody levels to V. vulnificus BT3 LPS by the various time intervals from the laboratory-documented infection was drawn.
Case-control studies
Two case-control studies were conducted to examine the role played by ‘pre-existent’ (acute stage) V. vulnificus LPS antibodies and the risk of developing symptomatic infection and severe disease. In one study, cases were fishermen who developed infection with V. vulnificus BT3 (n=10) while controls (n=40) were fishermen who had similar risk (working in the same fish pond with similar exposure potential to fish or fish-pond water) of exposure to cases but did not develop symptomatic infection. The level of serum IgG and IgA antibodies to V. vulnificus BT3 LPS was measured in cases and controls immediately after the onset of disease in cases. A paired serum sample was obtained from cases and controls during convalescence (4–6 weeks), at 6 months and at 1 year. In a second case-control study, cases were patients from the general public who suffered from laboratory-confirmed V. vulnificus BT3 infection (n=20) after handling pond-cultivated fish. Controls (n=40) were volunteers recruited at primary health clinics in the Ha'Amakim district matched by age, sex and comorbidities to cases but who had never handled pond-cultivated fish or suffered from any fish-related disease. Serum samples were obtained during the acute phase, at 6 weeks, at 6 months, and at 1 year.
Questionnaires were used to obtain data from cases and controls. For cases the data included age, sex, occupation, pre-existing comorbidities, time from exposure to disease onset, site of infection, type of antimicrobial therapy, type of surgical intervention, outcome and residual function of the infected site. For controls the data included age, sex, specific occupation and duration of work at fish ponds, pre-existing comorbidities, type of exposure to fish-pond cultivated fish (fish cultivation, fish processing, fish marketing), history of admission due to a fish-associated illness, and outcome. The local ethics committee at Ha'Emek Medical Center approved the study and informed consent was obtained from all participants.
LPS extraction and ELISA
Crude LPS was extracted from whole cells of selected strains of all three biotypes, ATCC 27562 BT1, CECT 4604 BT2 serovar E, and ATCC BAA-86 BT3. LPS was extracted using the LPS Extraction kit (Intron Biotechnology, Korea) according to the manufacturer's instructions and using basically the water–phenol method of Westphal & Jann [Reference Westphal and Jann22]. Specific serum IgG and IgA antibodies to the homologous (LPS BT3) and heterologous (BT1 and BT2) V. vulnificus were measured in patients and controls by means of ELISA in double dilutions starting with 1:100 dilution for IgG and 1:50 for IgA. Briefly, 100 μl of a solution containing 5 μg LPS/1 ml, a coating buffer (0·05 m carbonate buffer; pH 9·6) was added to each of 96 wells in a microtitration plate (Coaster, Corning, USA), and incubated for 1 h at 37°C. After removal of the coating solution, the plates were incubated for 1 h at 37°C with 0·05 m phosphate-buffered saline (PBS) test buffer supplemented with casein and bovine serum albumin in order to block the remaining unbound plastic sites. The wells were then washed twice in PBS-0·05% Tween-20 washing solution (Dulbecco's PBS, Biological Industries, Israel). Sera were added to the wells in twofold dilutions in PBS test buffer and then incubated overnight at room temperature. After four further washings with PBS-0·05% Tween-20 washing solution, goat anti-human anti-anti-IgG, or anti-IgA, conjugated to alkaline phosphatase (Kirkegaard & Perry Laboratories, USA) and diluted 1:1500 with PBS test buffer was added to the wells and incubated overnight at room temperature. ELISA was completed sequentially by the addition of the enzyme substrate solution containing para-nitrophenylphosphate (1 mg/ml) in diethanolamine buffer at pH 9·8 and 3 m NaOH. Optical density (OD) was read at 405 nm with an automatic ELISA reader (Multiskan EX, Thermo Scientific, USA). Positive and negative control sera were included in every microtitration plate in each of the assays. IgG and IgA antibody levels were expressed in ELISA units calculated by multiplying the ODs with the dilution factor and dividing the product by the number of the dilutions tested. Antibody titres were expressed as geometric mean titres (GMTs) using logarithmic-transformed data of the ELISA titres (a geometric mean is calculated by averaging the logarithms of the test values and then converting the mean to a number, this prevents high values from making the mean unrealistically large). The inter-V. vulnificus biotype specificity of the antibody response was documented by absorption assays in which sera (from three different patients infected with V. vulnificus BT3) with high titres of antibodies to V. vulnificus BT3 were pre-incubated with the homologous- and heterologous-soluble LPS before being tested against V. vulnificus BT3 LPS. A concentration of 50 μg/ml of V. vulnificus BT3 LPS was used as soluble antigen for the absorption studies.
To determine an accurate cut-off value which will document a significant increase in ELISA antibody titres to V. vulnificus BT3 LPS, we used 23 pairs of archived sera obtained in another study from healthy volunteers with no risk of exposure to V. vulnificus at two consecutive time periods (0 and 6 weeks). These sera were tested for anti-BT3 LPS antibodies. The mean ratio (of titres at time 0 and 6 weeks later) was 0·98±0·46 s.d. In a normal distribution 95% of samples will fall between ±2 s.d. (actually ±1·96 s.d.), when adding 2 s.d., the upper value of the mean ratio rose to 1·9. This was the cut-off value which was used throughout the study to detect significant antibody response to V. vulnificus LPS.
Statistical analysis
The data management and statistical analyses were performed using SPSS software (SPSS Inc., USA). GMTs and 95% confidence intervals were determined using logarithmic-transformed data. GMTs of IgG and IgA antibodies to V. vulnificus LPS in the various study groups and at the various times following exposure were compared by Student's t test or ANOVA. Variables such as age, years of occupational exposure, or pre-existing comorbidities constituted potential confounding factors of the possible association between serum antibody levels and the risk of developing symptomatic infection and severe infection with the pathogen. The effect of these variables handled as independent variables on the risk of developing V. vulnificus infection and their possible interaction with the level of LPS specific serum antibodies was assessed by univariate and multivariate analyses. As a criterion for inclusion of variables in the multivariate and logistic regression analysis, only variables with a P value ⩽0·05 obtained in the bivariate analysis were allowed for assessment of potential confounding. Significance was determined at the 0·05 level.
RESULTS AND DISCUSSION
Study group characteristics
During 3 years of subject enrolment and data collection, 20 patients from the general public and 10 fishermen patients with laboratory-confirmed V. vulnificus BT3 infection were recruited to the study. For the first group of patients from the general public 40 controls were matched by age, sex, and other comorbidities. For the second group of patients (fishermen), 40 healthy fishermen served as controls. The clinical characteristics of the two groups of patients and their controls are shown in Table 1.
Table 1. Characteristics of the study groups
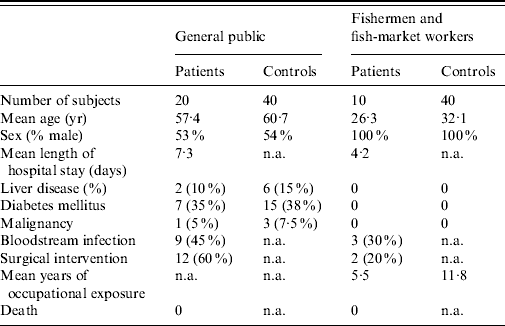
n.a., Not applicable.
The groups enrolled in the study were studied in two patterns, the first, a prospective study where we examined the sensitivity, specificity, and dynamics of the serological response to the LPS of V. vulnificus BT3 in patients and controls. The second, a case-control study was conducted to test the role of antibodies to V. vulnificus BT3 LPS in disease susceptibility in fish farmers and fish consumers.
Sensitivity and specificity of serum antibody response
We found that 60% of diseased fishermen and up to 78% of diseased members of the general public had a significant antibody rise to V. vulnificus BT3 LPS (determined as 1·9-fold rise in titre ratio between IgG and IgG0 at any of the follow-up points). However, in diseased members of the general public admitted with severe disease (severe soft tissue infection with or without bloodstream infection) about 90% had a significant antibody rise.
To document the specificity of the antibody response to V. vulnificus LPS we performed absorption assays (Fig. 1). The assays were performed in several steps, initially, and to evaluate the impact of assay background levels, we used sera from three uninfected individuals and incubated with BT3 LPS (Fig. 1, B). Next, we used sera of three patients with laboratory-confirmed V. vulnificus BT3 disease and incubated with the homologous-soluble LPS (Fig. 1, C) and in parallel with the LPS of BT1 (Fig. 1, D), LPS of BT2 (Fig. 1, E), and then again with the LPS of BT3 (Fig. 1, F). The mixture of serum from patients infected with BT3 (initially absorbed with BT3 LPS) with LPS from BT1 or BT2 did not affect the ELISA reactivity; however, further absorption of the sera with the homologous LPS (BT3) was associated with a significant reduction in the ELISA reactivity. These results indicated that the antibody response to LPS of V. vulnificus BT3 was specific and was not affected by exposure to the heterologous biotypes.
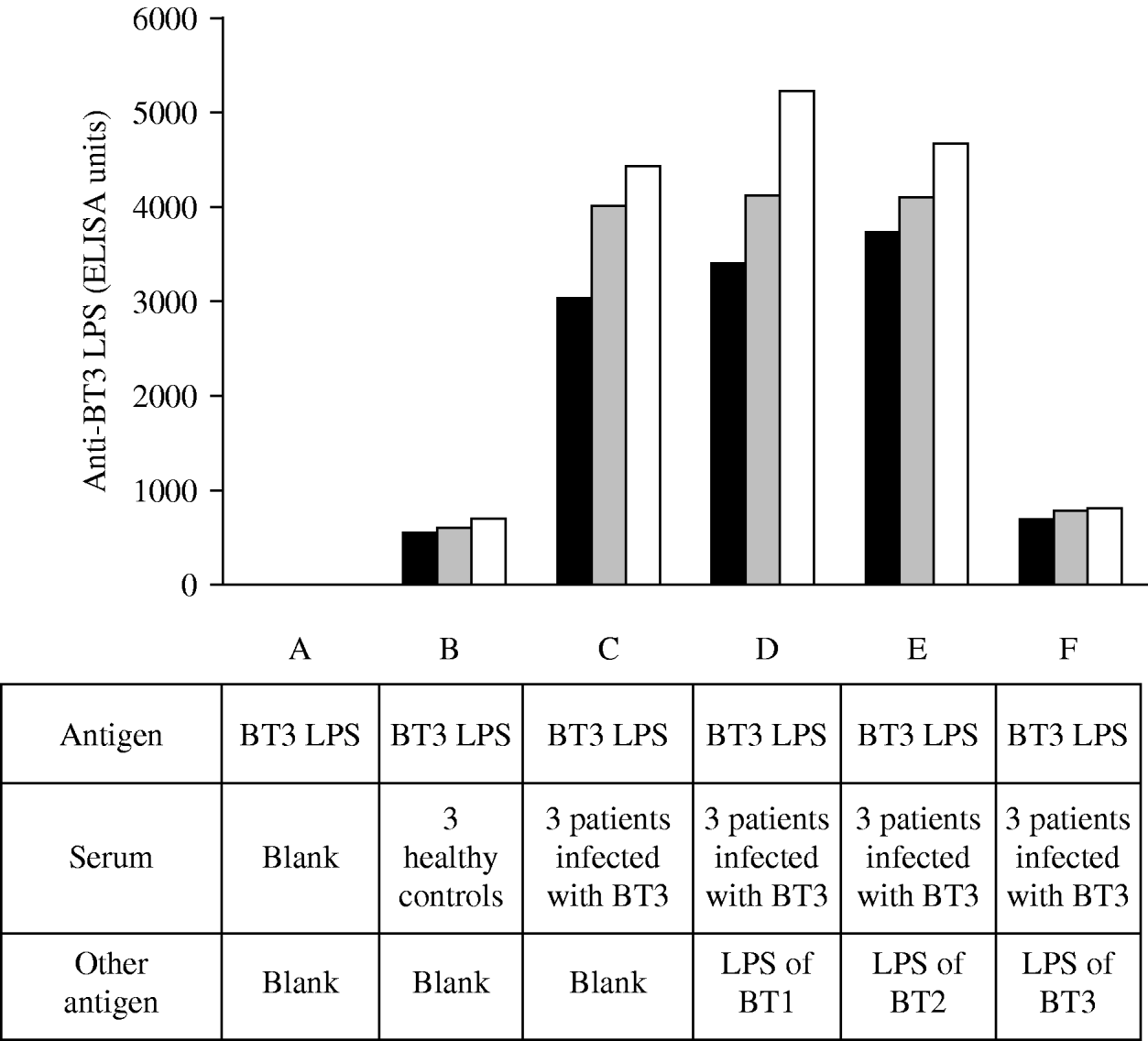
Fig. 1. Absorption assays. Biotype 3 lipopolysaccharide (BT3 LPS) served as an antigen in all the ELISA reactions. A, blank; B, sera of three uninfected healthy controls; C–F, sera of three patients infected with BT3 were pre-incubated with LPS of BT3. Initially no other LPS was added (C), then incubated in parallel with LPS of BT1 (D), with LPS of BT2 (E), and then re-incubated with LPS of BT3 (F). The ELISA reactivity was not affected by the addition of LPS from BT1 or BT2 but decreased upon the re-incubation with LPS of BT3. Each bar represents a single measurement for one individual.
Dynamics of serum antibody response to V. vulnificus BT3 LPS
The dynamics of the antibody response (IgG and IgA) to the LPS of BT3 in patients from the general public, infected fishermen, and healthy close-control fishermen are shown in Figure 2. The GMTs of IgG and IgA from patients (general public and fishermen) showed a statistically significant rise between disease onset and convalescence (P=0·016 and P=0·03, respectively). Subsequently GMTs decreased at 6 months but were still significantly higher than the disease acute stage levels. Yet, in healthy fishermen controls no significant changes were observed in antibody titres over time (P=0·67).
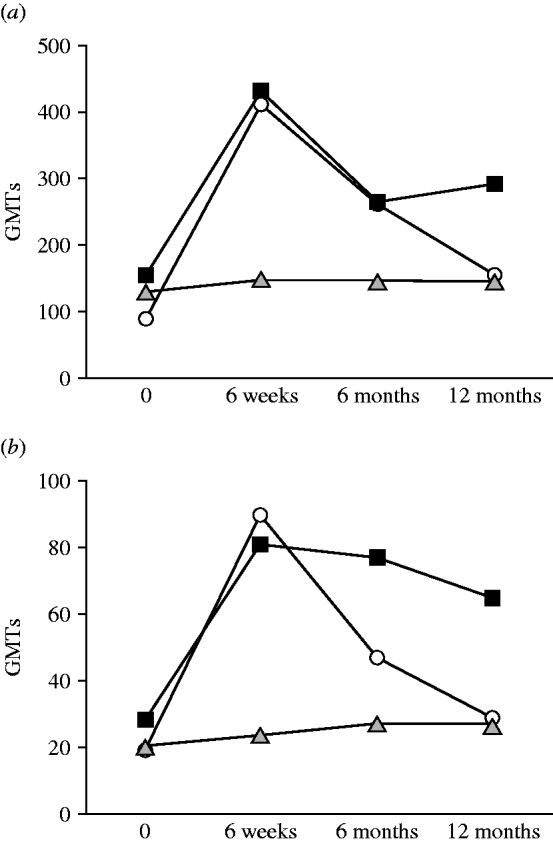
Fig. 2. Dynamics of (a) IgG and (b) IgA antibodies to biotype 3 lipopolysaccharide (BT3 LPS) in patients from the general public (–▪–), infected fishermen (–○–), and healthy fishermen controls (![]() ). GMT, Geometric mean titre.
). GMT, Geometric mean titre.
Occupational exposure and anti-LPS antibodies
The average number of years of occupational exposure for infected fishermen was 5·5 years, compared with 11·8 years for healthy controls (P<0·01). The GMTs of IgG0 and IgA0 (IgG and IgA levels at disease onset for infected fishermen and at the same time frame for controls) are shown in Table 2. Fishermen who developed V. vulnificus BT3-associated diseases had significantly lower levels of IgG0 (but not IgA0) to BT3 LPS than controls. In multivariate analysis including age, years of exposure, levels of IgG0 and IgA0, pre-existing antibodies to V. vulnificus (IgG0) anti-LPS BT3 was the only variable found to be significantly associated with disease occurrence in fishermen.
Table 2. Levels of pre-existent antibodies to lipopolysaccharide of V. vulnificus biotype 3 in fishermen
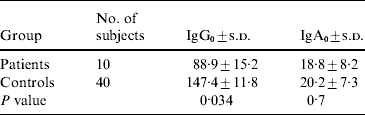
IgG0, IgG at disease onset; IgA0, IgA at disease onset; s.d., standard deviation.
To assess the natural exposure to the three V. vulnificus biotypes, the sera of 10 fishermen infected with V. vulnificus BT3 and 20 matched controls were tested for significant antibody response to LPS of BT1 and BT2 during the year of follow-up (Table 3). The experiments, including the establishment of control ranges, were performed in a similar fashion to that described for BT3 in the Methods section (see ‘LPS extraction and ELISA’). Table 3 shows for every individual the presence or lack of significant antibody rise to the LPS of each of the three biotypes [shown as pluses and minuses, a plus indicates a significant (at least 1·9-fold rise) antibody rise and a minus is a ratio that is <1·9]. Nearly 25% of healthy fishermen were found to have a significant rise in anti-BT1 LPS during the year of follow-up (Table 3). One of the fishermen infected with V. vulnificus BT3 also showed a significant serum antibody response to V. vulnificus BT1 LPS. No significant antibody rises for BT2 LPS were detected in patients or controls.
Table 3. IgG antibody titres between convalescence and disease onsetFootnote * to lipopolysaccharide of three V. vulnificus biotypes in fishermen infected with biotype 3 (BT3) and their matched controls
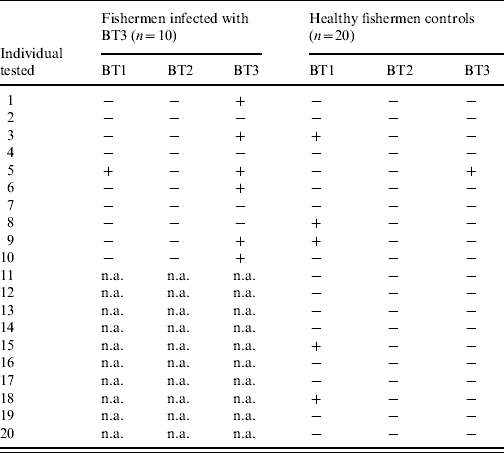
n.a., Not applicable.
The data are shown as pluses and minuses, a plus indicates a significant (⩾1·9-fold rise) antibody rise and a minus is a ratio that is <1·9.
* For patients only, for controls the highest ratio within a year of follow-up was used.
Pre-existing anti-LPS antibodies in patients and controls from the general public
There were no statistically significant differences in levels of pre-existing antibodies to LPS of BT3 in patients from the general public and their controls, GMTs for IgG to BT3 LPS from patients were 153·2 and from controls 122·1 (P=0·18). Interestingly, levels of pre-existing LPS antibodies in patients from the general public were significantly higher than those measured in infected fishermen (IgG0: 153·2 vs. 88·9); however, when controlling for age these differences disappeared (Table 4).
Table 4. Pre-existing antibodies to lipopolysaccharide of V. vulnificus bioptype 3 in patients and controls from the general public
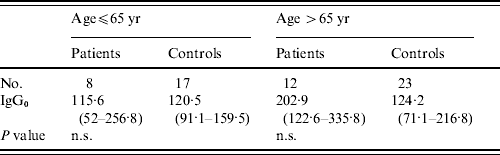
n.s., Not significant.
Values are geometric mean titres of anti-lipopolysaccharide antibodies (95% CI).
The emergence of V. vulnificus disease in Israel was regarded as an epidemiological mystery. Since its first description in 1996 [Reference Bisharat and Raz23], several studies had elaborated the nature and causes of this outbreak. At the species level a genetically distinct [Reference Bisharat7] and highly clonal biotype was found to be the causative organism [Reference Bisharat8]. Human behaviour [Reference Bisharat and Raz23] and global warming [Reference Paz24] may have impacted upon the timing of the disease outbreak. The current study investigated the association between the immune response to V. vulnificus BT3 LPS and disease susceptibility in Israeli fish farmers and fish consumers.
The antibody response to V. vulnificus BT3 LPS was shown to be specific. We found that 60% of diseased fishermen and up to 78% of diseased members of the general public had a significant antibody response to V. vulnificus BT3 LPS. Furthermore, nearly 90% of patients with severe disease had a significant increase in anti-LPS antibodies. This suggests that V. vulnificus LPS is immunogenic and elicits an immune response in most severely infected patients. Nearly 25% of healthy fishermen controls had a significant rise in antibody titres to BT1 LPS. This can reflect more or continuous exposure to BT1 compared to the lack of exposure to BT2 as suggested by ecological studies in Israel (N. Bisharat, unpublished data). Nevertheless, and given the fact that the LPS of BT1 strains are not homogenous, it is entirely possible that fishermen could have been exposed to BT1 strains that had different LPS types than those used for the absorption studies, clearly underestimating the true exposure rates to these two biotypes.
Two case-control studies were conducted in order to test the role played by pre-existing antibodies to V. vulnificus BT3 LPS in disease susceptibility in fishermen and members of the general public. The first study which included 10 fishermen patients and 40 healthy matched controls showed that fishermen patients had worked less time in fish farming and had significantly lower levels of IgG0 to V. vulnificus BT3 LPS than controls. This could imply that continuous or recurrent exposure to V. vulnificus BT3 may have conferred some protection to controls against symptomatic infection. In multivariate analysis, pre-existing levels of IgG anti-LPS BT3 remained the only variable significantly associated with disease occurrence. In the second case-control study which included 20 patients from the general public (fish consumers) and 40 matched controls, and after controlling for age, there was no difference in pre-existing levels of IgG between patients and controls. These interesting observations could suggest that, unlike fishermen, the role of pre-existing anti-LPS antibodies in conferring protection from symptomatic disease might be overwhelmed by other factors such as old age and other comorbidities which are more influential in determining disease susceptibility [Reference Strom and Paranjpye10, Reference Klontz20, Reference Shapiro25]. Nevertheless, the correlation between old age and levels of anti-LPS antibodies is intriguing. Several previous studies have demonstrated the increasing prevalence of antibodies with advancing age [Reference Candore26–Reference Tomer and Shoenfeld28], probably mirroring the increasing comorbidity and disability with advancing age. It is possible that high levels of anti-LPS antibodies in this subgroup are due to age-related non-specific antibodies cross-reacting with the LPS antigen. In fact, a previous work that tested the prevalence of antibodies against CPS of V. vulnificus in patients with confirmed V. vulnificus BT1 infections, shellfish industry workers, and in uninfected persons with minimal occupational exposure, found that antibodies were demonstrable in persons without a previous history of V. vulnificus infection or occupational exposure [Reference Fiore29]. The authors concluded that cross-reacting antibodies to CPS are present in the general population. It may be possible that older age provides a wider window of opportunity to develop such cross-reacting antibodies.
IgA levels in healthy and diseased fishermen throughout the follow-up period were significantly lower than IgG levels; furthermore, pre-existing IgA anti-BT3 LPS antibodies were not associated with protection against severe or symptomatic disease in fishermen. These observations are consistent with current knowledge concerning the limited immunoprotective role of IgA in host defence against non-mucosal and non-enteric bacterial infections [Reference Holmgren, Svennerholm, Mestecky, Bienenstock, Lamm, Strober and McGhee30].
The single work that tested the immunogenic profile of LPS of V. vulnificus was carried out in eels. The use of LPS-based vaccines against vibriosis in eels was not promising and the authors suggested that LPS from V. vulnificus serovar E may not be immunogenic for eels [Reference Collado16]. Conjugation of V. vulnificus LPS to carrier proteins might be helpful in increasing the immunogenicity of potential vaccines in eels. The same approach can be considered for development of V. vulnificus candidate vaccines in humans, especially in elderly people where the specific immune response after natural exposure might be insufficient to provide future protection. This approach has been evaluated for some of the most important human pathogenic Gram-negative bacteria, such as Vibrio cholerae [Reference Gupta31], Shigella sonnei [Reference Cohen32], Escherichia coli O157 [Reference Ahmed33], and recently for Neisseria meningitides [Reference Cox34].
Our study has a few limitations. First, the number of fishermen patients with laboratory-confirmed infection was small, many fishermen who suffered from soft tissue infection after handling pond-cultivated fish but had negative cultures were considered highly probable cases but were excluded from the study. Given the limited number of populations from which to draw subjects, this has affected the number of patients recruited for the study. Second, we included only survivors from the general public. To date, no deaths have been reported in fishermen infected with V. vulnificus in Israel (N. Bisharat, unpublished data). V. vulnificus mortality rates in Israel are fortunately much lower than figures reported from the USA or Southeast Asia (~8% vs. 25–53%) [Reference Zaidenstein6, Reference Klontz20, Reference Mead35, Reference Hsueh36], this discrepancy is mainly attributed to food habits, eating raw or undercooked seafood which is the main source of infection in the USA and Southeast Asia, is extremely uncommon in Israel, thus limiting disease burden and its related mortality. Third, given the possible similarities in some antigenic determinants in the LPS of the three biotypes we cannot rule out some cross-reactivity in anti-LPS antibodies. Nonetheless, and given the homogeneous properties of BT3 LPS and the results of the absorption assays we considered these effects as marginal.
In summary, the data showed that the antibody response to V. vulnificus BT3 LPS is specific. It also showed that higher levels of pre-existing IgG anti-BT3 LPS antibodies might have protected fishermen and fish-market workers against symptomatic infection but not in members of the general public.
ACKNOWLEDGEMENTS
We thank all the participants in the study and especially fishermen and fish-market workers from Bet-She'an and Jordan valley.
DECLARATION OF INTEREST
None.








