INTRODUCTION
Sweetpotato (Ipomoea batatas [L.] Lam.) is a major food crop renowned for its role in food security, nutritional and health benefits and market value in sub-Saharan Africa (Woolfe Reference Woolfe1991; Agili et al. Reference Agili, Nyende, Ngamau and Masinde2012; Arancibia et al. Reference Arancibia, Smith, LaBonte, Main, Smith and Villordon2014). Its storage roots and young leaves are widely consumed by many people in sub-Saharan Africa. Globally, sweetpotato production is ranked sixth among staple crops, after rice (Oryza sativa L.), wheat (Triticum aestivum L.), potato (Solanum tuberosum L.), maize (Zea mays L.) and cassava (Manihot esculenta Crantz.) (CIP 2010). In Mozambique, however, sweetpotato is the third most important food crop after maize and cassava (INIA- IITA/SARRNET 2003). In 2012 alone, sweetpotato production in Mozambique surpassed 900 000 t produced on 120 0000 ha (FAO 2014). Women engage in sweetpotato farming in Mozambique, where it is normally cultivated on small plots of land primarily for family subsistence and for sale of any surplus (INIA-IITA/SARRNET 2003; Agili et al. Reference Agili, Nyende, Ngamau and Masinde2012; Okonya & Kroschel Reference Okonya and Kroschel2014).
Vitamin A deficiency (VAD) is common among women and children in sub-Saharan Africa, South Asia and Southeast Asia; it causes impaired growth, risk of morbidity from common infections and night blindness in children (Birol et al. Reference Birol, Meenakshi, Oparinde, Perez and Tomlins2015). In Mozambique, micronutrient malnutrition is one of the leading health and welfare problems among children and pregnant mothers (WHO 2009). There are 0·71 of children below 5 years of age suffering from VAD (Nutrition Division in Department of Community Health 2003). The Government of Mozambique allocates US$ 116 million/year to fight vitamin A and mineral deficiencies (UNICEF & the Micronutrient Initiative 2004; World Bank 2009). However, the vitamin A supplementation programme did not reach all children below 5 years of age in all provinces of Mozambique (Nutrition Division in Department of Community Health 2003). The rate of vitamin A supplementation was lowest in Niassa Province (0·36) and highest in Maputo City (0·77).
Introducing improved cultivars rich in vitamin A and minerals in farming communities has long-term positive benefits on food and nutrition security. Breeding orange-fleshed sweetpotato (OFSP) cultivars rich in vitamin A helps to reduce deficiencies among the rural poor, who produce and consume sweetpotato in significant quantities and may not have access to other nutrition interventions such as fortification, which mainly target urban populations consuming processed food.
Sweetpotato has a considerable amount of genetic variation for β-carotene (BC) associated with low genotype × environment (G × E) interactions (Manrique & Hermann Reference Manrique and Hermann2000). Selection for breeding clones with elevated BC content appears to be straightforward (Grüneberg et al. Reference Grüneberg, Ma, Mwanga, Carey, Huamani, Diaz, Eyzaguirre, Guaf, Jusuf, Karuniawan, Tjintokohadi, Song, Anil, Hossain, Rahaman, Attaluri, Somé, Afuape, Adofo, Lukonge, Karanja, Ndirigwe, Ssemakul, Agili, Randrianaivoarivony, Chiona, Chipungu, Laurie, Ricardo, Andrade, Rausch Fernandes, Mello, Khan, Labonte, Yencho, Low, Nyongesa, Quinn and Parker2015). A breeding challenge, however, is to combine elevated BC contents with elevated storage root dry matter (DM), yield stability, vine survival for adequate seed production, and local taste and consumer preferences. Typical OFSP cultivars from the American continent – the main source of diversity for sweetpotato – have medium to high BC associated with low-to-medium storage root DM and they are not well adapted to the environments and taste preferences of Southern Africa.
The typical sweetpotato landrace or cultivar in sub-Saharan Africa is white-, cream- or yellow-fleshed with no or very low BC content, high storage root DM and starchy taste (Ewell Reference Ewell2002). A few OFSP cultivars such as Resisto, Jonathan and CIP-199062·1, which were introduced from the American continent, have been tentatively accepted in Mozambique as part of a food-based approach to fight VAD during the last 15 years. Their major limitations are, however, the extremely low vine production of Resisto, especially in drought years; yield instability of Jonathan due to susceptibility to water deficit during early and medium growth stages; and the low root yield of CIP-199062·1 in drought-prone regions as well as its low BC content (Andrade et al. Reference Andrade, Naico, Ricardo and Sandramo2004a , Reference Andrade, Naico, Ricardo and Sandramo b ).
The objectives of the present study were to assess if it is feasible to develop well-adapted OFSP cultivars that combine high yielding ability with high BC and DM content, and to test the usefulness of an accelerated breeding scheme (ABS) in sweetpotato breeding in Southern Africa.
The International Potato Center (CIP), in conjunction with the national sweetpotato breeding programme in Mozambique, engaged in developing OFSP cultivars rich in BC content and better adapted to the diverse agricultural environments of Mozambique. In order to provide the best sweetpotato clones to farmers in the shortest period, an ABS was used (Grüneberg et al. Reference Grüneberg, Manrique, Zhang and Hermann2005). In the ABS, selection of parents, the crossing step, seed collection from crossing blocks and establishment of the seedling nursery are accomplished in the first year. Observation trials commence in the second year by simultaneous planting of all genotypes in the seedling nursery in several environments using non-replicated 1 m2 plots. Selected genotypes with satisfactory root yield, form, taste, flesh colour and other agronomic data are promoted to preliminary and subsequently advanced yield trials during the second year. Multi-location field trials as well as on-farm trials are carried out in the third year across diverse locations for selected genotypes. Following this scheme, all the crucial agronomic data necessary for cultivar release is available by the end of year four. The time taken to release a cultivar is significantly reduced in the ABS compared with conventional methods which require 7–9 years.
MATERIALS AND METHODS
Germplasm development, testing and selection in an accelerated breeding scheme in Mozambique
Two crossing blocks were established in 2006 and 2007 at Umbelúzi Research Station (26°03′S, 32°23′E; 12 m a.s.l.), 35 km south of Maputo, and at Gurúè Research Station (15°19′S, 36°6′E; 1000 m a.s.l.) in Zambézia Province. Each crossing block contained four males and 24 females. A total of 198 592 new clones were generated from both controlled hybridization and from the respective poly-cross nursery at the two locations (Andrade et al. Reference Andrade, Naico, Ricardo, Alvaro, Moniz, Sitoe and Grüneberg2010). New clones from each location were multiplied rapidly and separately, and subjected to a series of trials.
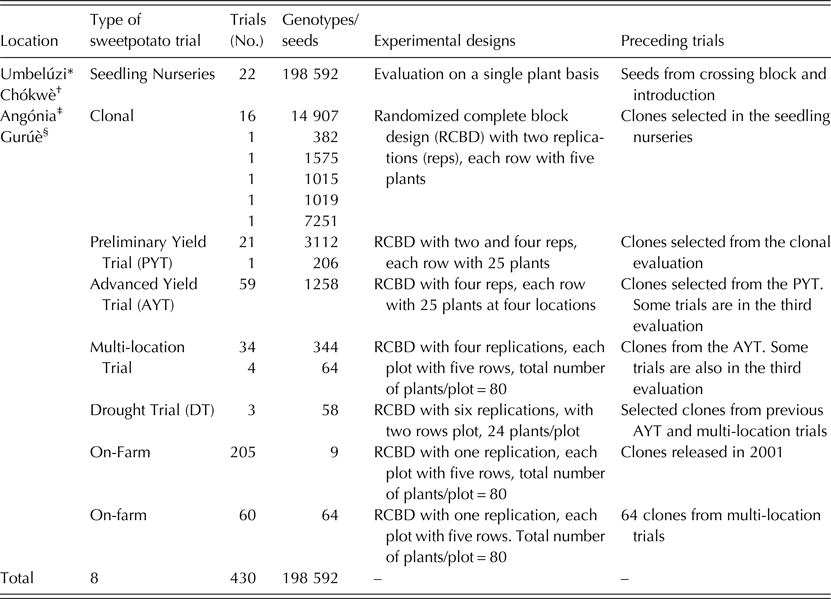
The ABS for clonally propagated crops considers that temporal variation of testing environments equals spatial variation of testing environments in the early stages of a breeding programme (Grüneberg et al. Reference Grüneberg, Manrique, Zhang and Hermann2005). In ABS, all clones derived from true seeds are, therefore, planted simultaneously in several environments, whereas selection in breeding clonally propagated crops is often a process conducted in several steps and years (Fig. 1). The principle of ABS for clonally propagated crops is to accomplish simultaneously the steps that conventional breeding schemes accomplish over several years. Table 1 gives a summary on how the clones were evaluated at each stage. Thereafter, selections of superior clones were made in each trial and selected clones were advanced to the next testing stage. Selection criteria included: storage root yield above 10 t/ha, BC content exceeding 5 mg/100 g DW, DM content >200 g/kg, vine biomass above 10 t/ha, and host plant resistance to pests (including viruses). A total of 64 clones satisfied the selection criteria and they were tested under multi-environment trials (MT) at four sites. Among the 64 clones was Ejumula from Uganda, which had passed several evaluations from previously 19 mega-clones introduced into Mozambique for testing. The principal characteristics of 15 clones evaluated under MTs and nominated for cultivar release in Mozambique are given in Supplementary Table 1 (available from https://www.cambridge.org/core/journals/journal-of-agricultural-science).
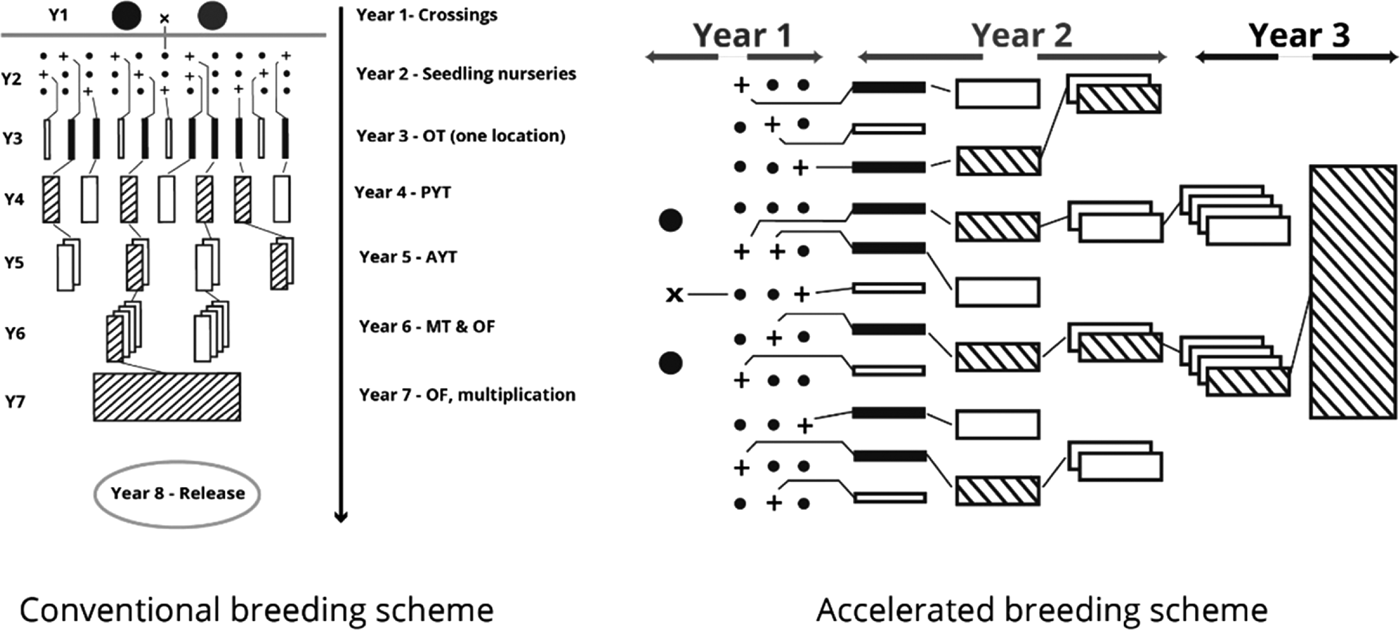
Fig. 1. Conventional (left) and accelerated (right) sweetpotato breeding schemes.
Table 1. Summary of all trials established at Umbelúzi, Chókwè, Angónia and Gurúè Research Stations leading to the release of 15 sweetpotato cultivars in Mozambique
* Supported by United States Agency for International Development (USAID).
† The Bill & Melinda Gates Foundation (BMGF) through the project Sweetpotato Action for Security and Health in Africa (SASHA).
‡ Alliance for a Green Revolution in Africa (AGRA).
§ CGIAR HarvestPlus.
Characteristics of experimental locations
The MTs were conducted at four agricultural research stations of the Instituto de Investigação Agraria de Mozambique (IIAM) (Fig. 2). The research stations are in different mega agro-ecological zones. The main features and weather patterns of the research stations are given in Table 2. Umbelúzi Research Station has minimum temperatures between 11·5 and 22·7 °C, while maximum temperatures fluctuate from 24·7 to 33 °C. It has alluvial stratified soil with texture ranging from sandy loam in the topsoil to sandy at 1·75 m depth. The soils have an organic matter content of 26 g/kg and soil pH varies between 6 and 6·5 (Ripado Reference Ripado1986). Evaporation is extremely high at this location with 2·8–7·2 mm of water loss per day and 1857 mm of water loss per year (Gomes Reference Gomes1996). Chókwè Research Station lies in a semi-arid area and has deep silt clay loams soils whose colours vary from brown to dark grey. Umbelúzi and Chókwè are appropriate sites for drought screening. Gurúè Research Station has a mean temperature of approximately 21·9 °C with high temperatures of up to 32·5 °C in November. Its lowest temperatures of approximately 12·3 °C are experienced in July. The soils are deep clay loam, varying from red to dark brown and are well drained with good natural soil fertility. This site allows virus screening due to high virus incidence. Angónia Research Station is located in a cool zone experiencing annual mean temperatures of approximately 20·9 °C. Its soils are heavy in texture and deep (Andrade et al. Reference Andrade, Naico, Ricardo, Alvaro, Moniz, Sitoe and Grüneberg2010). Angónia Research Station is a good site for testing tolerance to low temperatures.

Fig. 2. Main breeding and testing sites for sweetpotato in Mozambique.
Table 2. Average annual rainfall, altitude, length of dry season of sites used for multi-location trials in Mozambique

Experimental design and agronomic management
A randomized complete block design with four replications was used at each testing site. Row–row spacing was 0·9 m and plant–plant spacing within a row was 0·3 m. Each plot had five rows of 5 m length with 16 plants. The MTs were planted in October 2009 and harvested in March 2010 at the four locations. Trial establishment and crop growth depended on irrigation until December when the rainy season began. Trials were established without any basal or top-dressed fertilizer application. The fields were also kept weed and pest free. Hand hoe weeding was conducted at weed appearance at each location.
Data recording
The following traits were measured in the field:
-
• Virus symptoms (Vir2) were scored 2 months after planting and 1 month before harvesting using a 1–9 scale, where 1 = no symptoms, 5 = moderate and 9 = severe symptoms.
-
• Plant vigour (VV1) was recorded 1 month before harvest on a 1–9 scale, where 1 = not vigourous, 5 = moderate and 9 = very vigourous.
-
• Vine survival (%) (SHI) was recorded at harvest time where the number of surviving plants were recorded and compared with the initial number of vines planted.
At harvest time, the two middle rows of 23 plants were used to measure the following traits:
-
• Total storage root yield (t/ha) (RYTHa) after digging up the roots with hoes and weighing all from the net plot on a balancing scale.
-
• Commercial root yield (t/ha) (RYCHa) using only roots whose weight was above 100 g, and thereafter bulking them on each net plot.
-
• Total vine yield (t/ha) (RVY) by manually cutting the above-ground plant parts on the net plot, bulking them and weighing on a balancing scale.
-
• Total plant biomass (t/ha) was calculated by adding total root and vine yield.
Data recording for the roots also included weevil damage and other injuries, which were measured as follows:
-
• Weevil damage (WED1) using a 1–5 scale, where 1 = no damage, 2 = light damage, 3 = moderate damage, 4 = severe damage and 5 = extremely severe damage.
-
• Other injuries or damage to the roots (DAMR) were recorded using the above scale.
The nutritional quality of each clone was determined in the laboratory. Three roots were randomly selected from each plot and sent to the CIP laboratory in Maputo to assess quality traits. The following traits were measured:
-
• Dry matter content by taking a sample of approximately 100 g after bulking three roots and oven drying at 70 °C for 72 h. BC content using two roots according to the guidelines outlined in Burgos et al. (Reference Burgos, Carpio, Sanchez, Sosa, Porras, Espinoza and Grüneberg2009). The conversion factor is 100 for the expression of results as percentage (%) or 1000 for grams per kilogram (g/kg).
-
• Root cooking taste (COOT1) with the aid of a 1–5 scale, where 1 = very bad, 2 = bad, 3 = average, 4 = good and 5 = excellent.
Data analysis
Analysis of variance (ANOVA) was performed using SAS/STAT Software (SAS 1997). Phenotypic data were initially analysed separately for each site using a one-way ANOVA. Clones which had superior means for all the traits measured at each location were selected for further analysis. The primary trait taken into account for tandem selection was total yield (t/ha) followed by BC content, DM, taste, vine vigour, root weevils and damage as well as virus symptoms. At each location, clones with total storage root yield greater than the trial mean as well as clones with total storage root yield not significantly different from the trial mean were selected for further analysis. Fisher's least significance difference (LSD) at 95% confidence interval was used for mean separation. The selection index (SI) set as: 20% RYTHa + 20% BC + 20% DM + 10% VV1 + 10% RVY + 10% C00T1 + 5%VIR + 5%WED was another important tool to select clones from each site for further analysis. Each trait was weighted in the formula according to its importance in the evaluation and selection of sweetpotato cultivars by researchers and farmers. Principal component analysis (PCA) was conducted to verify whether the eight traits used during selection accounted for most of the variation observed in the dataset.
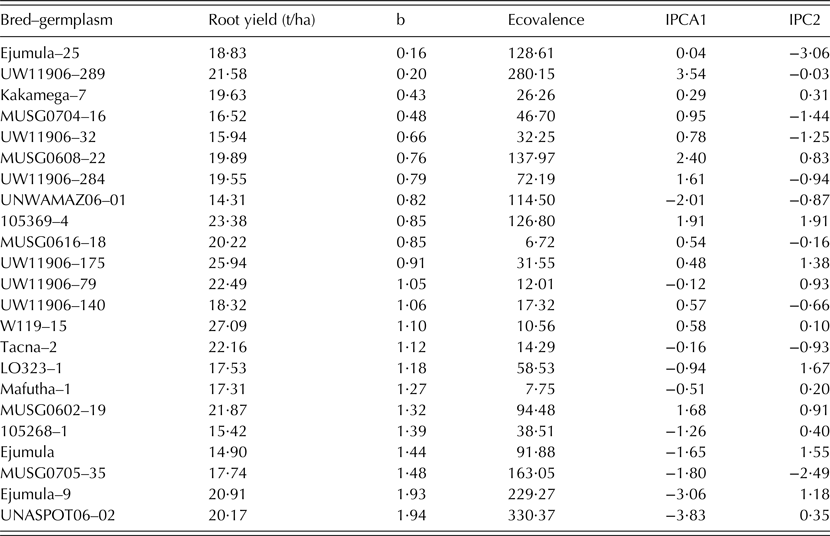
Only 23 clones passed the rigorous selection process and were chosen for further analysis. A combined ANOVA for total storage root yield and other traits was carried out across all four sites. The presence of significant G × E interaction in the combined ANOVA prompted multivariate analysis. Means for total root yield from individual sites were then subjected to the additive main effects multiplicative interaction (AMMI) model (Gauch Reference Gauch1992) to define stability and adaptability of the clones across the testing sites. Stability was also measured by regression (β), and eco-valence (MSInteract; Wricke Reference Wricke1962).
RESULTS
The combined ANOVA for the pooled data of the traits used during selection showed highly significant mean squares for environment (E), genotype (G) and the G × E interaction (Table 3). Environment had highest contribution to the total variation observed for all traits measured. Angónia (20·9 t/ha) and Chókwè (6·3 t/ha) were the best and poorest testing sites, respectively, for total root yield (Table 4). Vine yield was highest at Gurúè (23·3 t/ha) and lowest at Chókwè (15·3 t/ha) closely followed by Umbelúzi (16·7 t/ha)
Table 3. Degrees of freedom (D.F.) sum of squares (S.S.) and mean squares (M.S.) of the combined analysis of variance for root and vine yields (t/ha), dry matter (DM) content (%) and β-carotene (BC, mg/100 g dry weight) of widely adapted sweetpotato clones across four sites in Mozambique

Table 4. Stability parameters (b, regression line; ecovalence; IPCA, interaction principal component of the additive main effect multiplicative interaction model) of root yield for selected sweetpotato bred-germplasm
Virus symptoms were low to medium (<2) for all selected breeding clones except Cecilia and Amelia (Table 5). The released cultivars did not show significant weevil damage, which was low to medium. Esther showed highest vigourous growth but Melinda had the largest total (27·1 t/ha) and marketable (21·1 t/ha) storage root yield across environments among the 15 selected breeding clones. Vine survival was at least 0·69 for all released cultivars, and vine yield was highest in Amelia (31·1 t/ha). Tio Joe and Erica showed the highest BC content (>10 mg/100 g) and above average taste (Table 5). Jane and Amelia (with <10 t/ha across sites) had the best taste among them. The highest multi-trait index (>15) was scored for Delvia, Ininda, Amelia, Namanga and Bela among the 15 released cultivars.
Table 5. Genotypes selected from after multi-site testing at Umbelúzi, Chókwè, Gurúè, Angónia (Mozambique) using the ranking index and the additive multiplicative model interaction (AMMI) analysis of 64 clones during the 2009–2010 cropping season
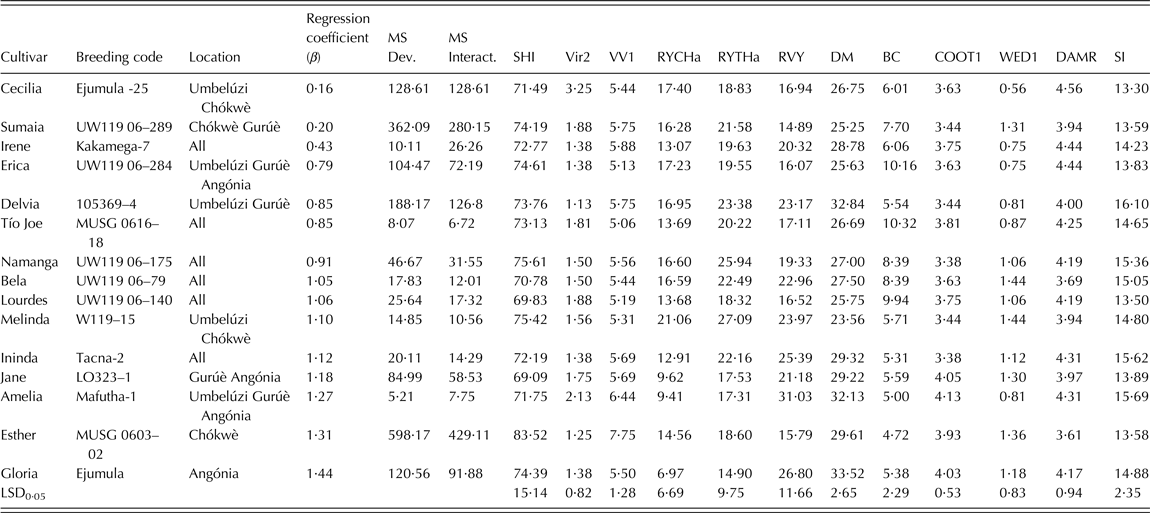
β, coefficient of regression for stability analysis; MS Dev., mean square deviations or deviation from the regression line; MS Interact., mean-square interaction or the eco-valence; SHI, vine survival (%); Vir2, virus symptoms (1 = without symptoms – 9 = extremely severe); VVI, vigour (1 = not vigourous – 9 = very vigourous); RYCHa, marketable storage root yield (t/ha); RYTHa, total storage root yield (t/ha); RVY, total vine yield (t/ha); DM, dry matter (%); BC, β-carotene (mg/100 g fresh root); COOT1, taste (1 = very bad – 5 = excellent); WED1, weevil damage (1 = none – 5 = extremely severe); DAMR, other root injury or damage (1 = none, 5 = extremely severe); SI, Selection index = 20% RYTHa + 20% BC + 20% DM + 10% VV1 + 10% RVY + 10% C00T1 + 5%VIR + 5%WED.
There was a significant G × E crossover interaction for storage root yield among the 23 breeding clones included in MTs (Table 3). About 94·4% of the variation contributed by G × E interaction was explained by the AMMI analysis, whose bi-plot 1 is shown in Fig. 3. Clones 51 (MUSG 0616-18 or Tio Joe), 43 (Kakamega-7 or Irene), 50 (Ejumula-25 or Cecilia) and 27 (UW119 06-140 or Lourdes) lay very close to the origin and were least affected by environmental changes. Ejumula-9 (42) and UNASPOTS 06-02 (30) had positive G × E interaction with Angónia, whereas UNWAMAZ 06-01 (29), 105 268-1 (40), Ejumula or Gloria (59), Mafutha-1 or Amelia (47), LO323-1 or Jane (37) and MUSG 0705-35 (18) had negative G × E interaction with Gurúè and Umbelúzi. Umbelúzi and Gurúè Research Stations were highly and positively correlated to each other. Angónia followed by Chókwè had the highest sum of squares for G × E as noted by the length of the vectors.

Fig. 3. AMMI1 biplot of selected sweetpotato bred-germplasm (blue triangles) evaluated for root yield in four sites (red squares): Umbelúzi, Chókwè, Gurúè and Angónia. Bred-germplasm codes are follows: 4, UW119 06-32; 13, UW119 06-284; 17, MUSG 0704-16; 18, MUSG 070-35; 23, UW119 06-79; 26, UW119 06-175; 27, UW119 06-140; 29, UNWAMAZ 06-01; 30, UNASPOTS 06-02; 34, UW119 06-289; 37, LO323-1; 38, Tacna-2; 40, 105 268-1; 41, 105369-4; 42, Ejumula-9; 43, Kakamega-7; 47, Mafutha-1; 49, W119-15; 50, Ejumula – 25; 51, MUSG 0616-18; 52: MUSG 0608-22; 56, MUSG 0602-19; and 59, Ejumula.
Tio Joe, Amelia, Melinda and Ininda had the lowest eco-valence (<15) for total storage root yield, thus indicating their high adaptability among the released cultivars (Table 5). The dynamic stability based on the regression analysis grouped the released cultivars into three classes. The first important group was that of high yielding cultivars that are suitable for growing across Mozambique (β ∿ 1) and included Lourdes, Namanga, Bela and Melinda. The second group comprised cultivars that could specifically be recommended for production in low potential areas often affected by drought and salinity. These were Cecilia, Sumaia, Irene, Erica, Delvia and Tio Joe. The last group included cultivars that were specifically adapted to high potential areas; i.e. Ininda, Jane, Amelia, Esther and Gloria.
DISCUSSION
The ABS for sweetpotato led to the release of 15 cultivars in Mozambique within a short period. Supplementary Fig. 1 (available from https://www.cambridge.org/core/journals/journal-of-agricultural-science) shows their vines, leaves, flowers and storage roots. The results of the MTs of the advanced breeding clones were similar to previous findings. For example, Henderson et al. (Reference Henderson, Sitch, Macoo, Botão, Pequenino and Whyte1997) found a significant G × E interaction for total storage root yield when conducting multi-site trials during the rainy season in Mozambique. Genotype × environment interaction often poses problems in cultivar selection (Annicchiarico Reference Annicchiarico2002). A crossover G × E interaction, as per the current results, indicates that selection of suitable cultivars under one environment alone is inadequate since this could result in low storage root yield in other environments (Rosielle & Hamblin Reference Rosielle and Hamblin1981). Hence, cultivar releases with the aim of high storage yields across environments are extremely difficult, particularly for the multitude of various agro-ecosystems in Mozambique. ‘Niche breeding’ is, however, expensive considering the population sizes of farmers residing in specific areas. In addition, it is not profitable for clonally propagated crops such as sweetpotato, whose cultivars can easily be shared among farmers soon after their release. The objective of the breeding programme should therefore be to breed high-yielding cultivars with wide adaptation and manage the significant G × E interaction by developing breeding clones with stability and wide adaptability.
Angónia and Chókwè were the best and poorest sites, respectively, for total storage root yield. According to Finlay & Wilkinson (Reference Finlay and Wilkinson1963), the average performance of all genotypes in an environment is taken as a quantitative measure of the quality of the environment. The yield data on each location supported the spatial differences of these locations. Chókwè lies in a semi-arid area with unpredictable rainfall patterns and little precipitation in each growing season, and may therefore serve as a location for drought screening in Mozambique. Angónia has adequate rainfall and temperatures are cooler than in Southern Mozambique during the growing season, making it a suitable site for assessing the yield potential of sweetpotato breeding clones.
Vine yield was highest at Gurúè and lowest at both Chókwè and Umbelúzi. These results agreed with previous research indicating that cool temperatures in Gurúè allow slow plant growth and maximal utilization of heat units, whereas warm environments such as those in Umbelúzi and Chókwè Research Stations encouraged fast growth, thereby leading to low vine productivity. Dual-purpose sweetpotato cultivars could be bred for the Zambézia province, where Gurúè Research Station is located, neighbouring provinces in Mozambique and other regions of Southern Africa with similar climates and agro-ecological contexts.
The AMMI analysis demonstrated that breeding clones close to the origin of the environmental vectors had low G × E interaction. The angle between vectors and environments was proportional to correlation (r) between environments. When the degrees are 0 then r equals 1, while at 90 ° r is 0 and at 180 °, r equals –1. Umbelúzi and Gurúè had a high positive relationship, despite the fact that the two research stations are in different agro-ecological zones. The highly positive correlations between these sites could have resulted from crop husbandry in the trials. Furthermore, the locations are in close proximity to CIP offices and its staff could have influenced trial management.
The length of the vectors in the AMMI analysis is proportional to the size of G × E interaction sum of squares. Angónia followed by Chókwè had the highest G × E interaction sum of squares. These sites had the capacity to discriminate amongst breeding clones of sweetpotato. Selections made in Angónia had a larger chance of success across environments in Mozambique because of the capacity of this site to allow breeding clones to show their actual performance. Angónia Research Station can be used for screening breeding germplasm at early stages.
Tio Joe (MUSG 0616-18), Lourdes (UW119 06-140), Namanga (UW119 06-175), Bela (UW119 06-79), Melinda (W119-15) and Ininda (Tacna-2) combined average stability with little deviation from the regression line and had relatively small eco-valence. According to Finlay & Wilkinson (Reference Finlay and Wilkinson1963), a regression slope (β) equal to 1 indicates average stability across environments, while β above 1 shows above average stability for genotypes suitable for high potential areas and β below 1 signals below average stability for genotypes that are appropriate for low potential areas. Eco-valence used the size of G × E interaction sum of squares as a measure of stability. Both stability analysis methods were useful in finding the structure of the G × E interaction and estimating robust parameters that allow identifying released cultivars suitable for distinct environments across Mozambique. It is also important to note that none of the released cultivars were poorly adapted to all environments (i.e., b ∿ 1 and low storage root yield) because of the rigorous selection for total storage root yield prior to dissecting the G × E interaction and conducting stability analysis.
The ABS followed in the present study significantly accelerated the release of sweetpotato cultivars in Mozambique. However, the breeding team did not do an economic analysis to quantify the benefits associated with the ABS besides reducing the time of cultivar release by half. In addition, due to travel logistics and accessibility, trials were not established in northern Mozambique. It was difficult to make recommendations for a specific cultivar in this region. However, a new project in northern Mozambique did adoption studies for the released cultivars in 2013 and 2014. Cultivar performance was good and consumer acceptance levels were high.
In summary, the ABS allowed development of 15 cultivars that were released and recommended for production in different areas of Mozambique. These released cultivars combined high levels of BC, acceptance by consumers and high storage root yield in various sweetpotato producing areas of Mozambique. These cultivars are at different multiplication stages across Mozambique and are promoted by various partners and governmental departments. In addition to cultivation for family consumption, some of the released cultivars could also provide a base for breeding new cultivars with wide adaptability in Mozambique and other regions of Southern Africa with similar contexts.
The United States Agency for International Development (USAID), the Rockefeller Foundation, CGIAR HarvestPlus, the Alliance for a Green Revolution in Africa (AGRA), and the Bill & Melinda Gates Foundation (BMGF) – through the project Sweetpotato Action for Security and Health in Africa (SASHA) – provided grants that enabled the breeding research-for-development across Mozambique and also the laboratory analysis of quality traits. Special thanks also go to staff who participated in the planting of the trials and data recording at the research stations in Mozambique.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S002185961600099X











