The metabolic syndrome (MetS) constitutes a cluster of cardiovascular risk factors, which double the risk for CHD and increase the risk for type 2 diabetes mellitus (T2DM) 5-fold( Reference O’Neill and O’Driscoll 1 ). Although the MetS is a growing epidemic worldwide, the prevalence reaches 25–50 % in some urban areas of South Asia, and the prevalence of individual risk factors is even greater, with obesity and low HDL-cholesterol reaching 68 and 81 %, respectively( Reference Pandit, Goswami and Ghosh 2 ). These risk factors are also growing in prevalence among children and young adults( Reference Pandit, Goswami and Ghosh 2 ).
The risk of the MetS can be reduced through diet and lifestyle changes( Reference Zivkovic, German and Sanyal 3 , Reference Ambrosini, Emmett and Northstone 4 ). In particular, approaches including weight loss( Reference Ambrosini, Emmett and Northstone 4 , Reference Yamaoka and Tango 5 ), increasing whole-grain sources of carbohydrates( Reference Kristensen, Toubro and Jensen 6 , Reference Hollænder, Ross and Kristensen 7 ) and increasing dairy products intake( Reference Louie, Flood and Rangan 8 ) have a demonstrable effect on components of the MetS. However, the majority of studies on the MetS have been carried out in older, Western populations or Southern Asians who have migrated to Western countries( Reference Ambrosini, Emmett and Northstone 4 – Reference Babio, Sorlí and Bulló 9 ).
There are differences in the presentation of the MetS in Southern Asians, who are at higher risk of MetS risk factors such as increased waist circumference (WC) and hyperglycaemia at a given BMI along with lower HDL-cholesterol and higher levels of small, dense LDL-cholesterol compared with their Caucasian counterparts( Reference Pandit, Goswami and Ghosh 2 ). Diet and lifestyle factors may also differ – for example, Indians obtain a higher percentage of their energy content from carbohydrates compared with Europeans, potentially increasing the risk of hypertriacylglycerolaemia( Reference Radhika, Sathya and Ganesan 10 ). Lower intakes of MUFA, n-3 PUFA and fibre and higher intakes of total fat, SFA and trans-fat have also been reported( Reference Misra, Khurana and Isharwal 11 ).
The effect of small changes in diet on metabolic risk factors is subtle and occurs over time. Therefore, prospective studies are needed to account for confounding variables such as weight change and longitudinal trends in dietary intake. Furthermore, it is important to note that a-one-size-fits all approach may not be appropriate given the heterogeneous presentation of the MetS. For example, a recent study found that a 10 % increase in the consumption of rice was associated with lower weight gain, reduced risk of hypertension, but an increased risk of hyperglycaemia( Reference Shi, Taylor and Hu 12 ), indicating that diets could be tailored to the prevalent risk factors. Studies with large sample sizes are needed to examine the effects of specific nutrients on components of the MetS( Reference Shi, Taylor and Hu 12 – Reference Sierra-Johnson, Undén and Linestrand 14 ).
The DIABRISK-SL study( Reference Wijesuriya, Gulliford and Vasantharajah 15 ) is a randomised controlled parallel group clinical trial performed at a single centre in Colombo, Sri Lanka, to examine the effects of a lifestyle intervention on the primary composite cardio-metabolic end points of new-onset T2DM, impaired glucose tolerance, impaired fasting glycaemia, new-onset hypertension and albuminuria, following 5 years of intervention. In this study, we explore the dietary factors that mediate the beneficial effects on the primary outcomes.
Methods
The design of the trial is described in detail elsewhere( Reference Wijesuriya, Gulliford and Vasantharajah 15 ). In brief, 4683 Sri Lankan urban young males and females aged 5–40 years at high risk of the MetS were randomised to an intensive lifestyle-modification group or to a less-intensive control group. The representative sample was recruited following screening of 23 298 individuals aged 5–40 years in schools, workplaces, universities and community organisations across Columbo, Sri Lanka. Clinical and dietary data were collected at baseline and annually up to the final follow-up. In this study, we utilised only the data of the 2637 subjects who completed the 5-year study and for whom complete dietary data were available. All participants provided written informed consent, and the study was given ethics approval by the Sri Lanka Medical Association Ethical Review Committee (ERC 07-010). Permission from the Ministry of Education was obtained for this study and was conducted under the Good Clinical Practice Guidelines and according to the principles expressed in the Declaration of Helsinki for clinical research.
Participants
Inclusion criteria were aged 5–40 years with two or more of the following risk factors for cardio-metabolic disease: first-degree family history of T2DM, physical inactivity, elevated BMI and raised WC. These risk factors were defined as follows: physical inactivity (<30 min continuous exercise for <3 d/week); WC (in subjects between 5 and 17 years defined as ≥91th percentile; 18–40 years: females ≥80 cm and males ≥90 cm); and raised BMI (in subjects aged 5–18 years defined as a BMI value greater than internationally standardised age- and sex-specific percentile cut-offs and between 18 and 40 years as BMI≥23 kg/m2)( Reference Wijesuriya, Gulliford and Vasantharajah 15 ). Exclusion criteria included subjects with no or only one identifiable risk factor; subjects with diagnosed end points (T2DM, CVD, hypertension and renal disease); or subjects on any form of medication used for the treatment of diabetes, hypertension, renal disease or dyslipidaemia either at screening or during the study. Subjects were also excluded if they had any active communicable or non-communicative diseases such as cancer, asthma or other forms of chronic lung disease, depression, tuberculosis or were or became pregnant either at screening or during the study.
Intensive v. control group
Subjects were randomised to an intensive lifestyle intervention group in which they had three monthly telephone and/or face-to-face contacts to assess progress and reiterate goals. The total number of one-to-one contacts was four per year. In the control group, the same advice was given, but with the face-to-face contacts being annual. For the purposes of this analysis, the intervention and control groups were combined.
Dietary goals
Overall, lifestyle advice was based on the Indian Diabetes Prevention Program( Reference Ramachandran, Snehalatha and Mary 16 ): (1) individual advice to balance food intake and physical activity and to achieve or maintain appropriate body weight; (2) avoidance of simple sugars and refined carbohydrates; (3) reduce total fat intake (not to exceed 20 g/d) and restrict the use of SFA; and (4) include more fibre-rich foods such as whole grains, legumes, vegetables and fruits( Reference Ramachandran, Snehalatha and Mary 16 ). The advice was based on the needs of the subjects. For example, in adults with a raised BMI or WC, advice was given to achieve 5 % weight loss. For children, the aim was to limit weight gain. Subjects were also advised on the importance of regular meals and to avoid delaying or missing meals.
Details on physical activity are described in detail elsewhere( Reference Shi, Taylor and Hu 12 ).
In order to standardise both provision of dietary advice and collection of dietary data, an exchange system was designed to replace energy-dense, high-fat, refined foods (unrecommended items) with low-energy dense, fibre-rich food sources (recommended items) (online Supplementary material) to correspond with the four dietary aims. With the use of the exchange model, participants were guided towards replacing the non-recommended with recommended items. Although the advice given was individualised, the focus was largely on replacing polished starches with unpolished starches, starchy vegetables with-non starch vegetables, high-fat meats and dairy products with low-fat meats and dairy products and reducing the consumption of sugar-sweetened beverages.
A FFQ was then adapted from the exchange model to determine the number of servings of each food group to allow annual dietary data collection. Serving sizes used were rice serving spoons and tablespoons, and the food recall was completed by a trained peer-to-peer educator with the participant. This exchange model approach was used as it facilitated the provision of effective dietary advice across a population by non-medically or nutritionally trained individuals from local communities, and would therefore be straightforward to translate to similar settings.
The intervention also addressed meal timings such as reducing snacks and consuming regular meals, based on traditional Sri Lankan meals and customs. The number of meals skipped and snacks consumed per day was also recorded by the educator.
Peer-to-peer educators
The intervention was delivered by peer-to-peer educators recruited from the same communities as the study participants. More than 50 % of the participants were <18 years of age, and this type of community-driven intervention is an effective approach for promoting lifestyle changes( Reference Twiss, Dickinson and Duma 17 ). All educators received initial and ongoing training by a team of local and international educators, assessed by the International Diabetes Federation, and supervised by academics from King’s College London. A training manual was provided; during weekly meetings throughout the study period, cases were discussed and agreed, and each educator was required to make a weekly presentation on a relevant topic to the principal investigator and head of research. This ensured that correct advice was being imparted to the participants.
Outcomes
The clinical outcomes are defined in a description of the trial published elsewhere( Reference Wijesuriya, Gulliford and Vasantharajah 15 ). In brief, the MetS was defined by the International Diabetes Federation( Reference Sierra-Johnson, Undén and Linestrand 14 ). At baseline and each year of the trial (Y1, Y2, Y3 and Y4), the following outcomes were measured: weight, WC, BMI, fasting plasma glucose (FPG), fasting TAG, fasting HDL-cholesterol and LDL-cholesterol, and blood pressure. At Y3, but not Y4 (final visit), an oral glucose tolerance test (OGTT) was performed. For changes in 2-h plasma glucose (2hPG) as the dependent variable, all other variables entered in the regression equation were also taken from Y3. Physical activity was measured by the International Physical Activity Questionnaire, and dietary intake was self-reported using the culturally appropriate food FFQ.
Statistics
Exploratory analysis using correlations, independent t tests and one-way ANOVA was carried out to determine variables of interest and inform the regression analyses. Multiple regression analysis was performed to examine the effect of the independent variables of dietary factors on the dependent variables of change in clinical outcomes from baseline to Y4. As the primary advice was to replace non-recommended items with recommended food items, for the independent variables, we created ratios of total recommended items:non-recommended items for each year of data collection, which were averaged from baseline to the final Y4 follow-up. We also created ratios of unpolished:polished rice and low-fat:high-fat food items, which were also averaged over 5 years to account for any changes in diet over the course of the study. We also created a variable for total unpolished:polished starches to include bread, and all associations were the same as for unpolished:polished rice, and thus the data are not presented here. In addition, based on the current literature on dairy products (total, low-fat only and full-fat only)( Reference Louie, Flood and Rangan 8 ) and soluble fibres (dahl and pulses)( Reference Dall’Alba, Silva and Antonio 18 ), we also created two groups for these, again averaged over the study period. We also created averages of weekly intake of specific food groups over the 5-year study period, again based on existing literature – namely, red meat( Reference Babio, Sorlí and Bulló 9 ), sugar-sweetened beverages( Reference Malik, Popkin and Bray 19 ) and processed meat( Reference Song, Manson and Buring 20 ). The dependent variables were the changes from baseline to Y4 follow-up in FPG, HDL-cholesterol, LDL-cholesterol, TAG, WC, blood pressure and weight. Regression with change in weight and WC as outcomes of interest included baseline BMI, the baseline value for dependent variables of interest, sex, age, ethnicity, alcohol intake, smoking status and physical activity (total metabolic equivalents per week averaged over 5 years), and the two major dietary components – unpolished:unpolished rice and non-fat:high-fat food products. The regression models for all other outcome variables were additionally adjusted for change in body weight. An OGTT was performed at Y3, not Y4, so that the effect of dietary variables on 2hPG could be assessed using ratio/food group intake and physical activity averaged over 4 years and weight change upto 4 years, instead of 5 years. Normality tests were performed for all dependent variables and non-normal variables were transformed before analysis; data were checked for homoscedasticity and collinearity of independent variables. Dummy variables were created for categorical independent variables. Standardised β values are reported to enable comparison of dietary factors with clinical outcomes. Statistical analyses were performed using SPSS statistical software for Windows, version 14.0 (SPSS). A P value of<0·05 was considered to be significant.
Results
Study participants
Baseline demographic characteristics and food intake data of all participants with complete dietary data (n 2637) are shown in Table 1 separated by age (> or <18 years).
Table 1 Baseline demographic characteristics and food intake data (Mean values and standard deviations for demographic variables; medians and interquartile ranges (IQR) for food intake data, which were not normally distributed)Footnote *

M, male; F, female; FPG, fasting plasma glucose; 2hPG, 2-h plasma glucose; TC, total cholesterol.
* Where median and IQR are <1 serving/week, data are not shown.
Weight and waist change
All food patterns were significantly related to weight change when examined by the t test. Participants who ate meals outside home (P<0·0001), did not have regular meals (P<0·0001), skipped breakfast (P<0·0001), lunch (P<0·001) or dinner (P<0·001), and had ≥1 snacks/d (P<0·0001) had significantly higher weight gain at the Y4 follow-up. WC at Y4 was also significantly higher in those who ate meals outside the home (P<0·0001), those who did not eat regular meals (P<0·001), those who skipped breakfast (P<0·0001) and those who had ≥1 snacks/d (P<0·001) (Fig. 1). However, when controlled for age, sex, BMI, physical activity, alcohol intake, baseline weight or WC, only having regular meals was significantly related to weight change (β=−0·037, P=0·032) and WC (β=−0·038, P=0·011).

Fig. 1 Changes in weight (a) and waist circumference (b) at 5 years by whether or not meals are eaten out of the home (external meals), whether three regular meals are eaten per d (regular meals), whether breakfast (b/fast), lunch or dinner are delayed or skipped (skip b/fast, skip lunch, skip dinner) and whether ≥1 snacks are consumed per d (Snacks). Differences determined by independent t test. Values are means, with their standard errors represented by vertical bars. Snacks, regular snacks; N, no; WC, waist circumference; Y, yes. * P<0·05, *** P<0·001, **** P<0·0001.
There was no effect of any food or food group on weight change and WC at the Y4 follow-up after controlling for confounders.
Biochemical outcomes
Fasting plasma glucose
The ratio of total recommended:non-recommended items (β=−0·049, P=0·023) and unpolished:polished rice (β=−0·084, P=0·007) was inversely related to change in FPG at Y4 after controlling for age, sex, BMI, physical activity, alcohol intake, baseline FPG and weight change (Table 2) (Fig. 2(a)). Red meat was also positively related to FPG (β=0·05, P=0·018), but processed meat as a separate category was not. Not having regular meals was also associated with a higher FPG at the Y4 follow-up (β=0·049, P=0·01) (Table 2).

Fig. 2 Change in fasting plasma glucose (a) and HDL-cholesterol (b) among participants consuming a ratio of recommended:non-recommended items and a ratio of unpolished:polished rice of more or less than 1. Differences determined by independent t test. Values are means, with their standard errors represented by vertical bars. FPG, fasting plasma glucose; Rec, recommended. * P<0·05, *** P<0·001.
Table 2 β-Coefficients for the effect of food patterns, groups and specific foods on fasting plasma glucose (FPG) and 2-h plasma glucose (2hPG)
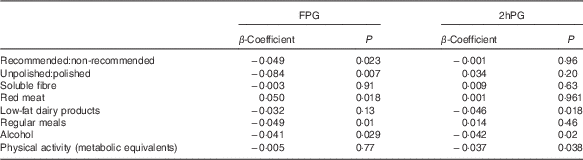
2-h Plasma glucose
The total amount of recommended:non-recommended food items was not related to 2hPG at Y4, nor was unpolished:polished rice and low-fat:high-fat diet. However, low-fat dairy products (β=−0·046, P=0·018), but not total or high-fat dairy products (β=0·003, P=0·853; β=−0·010, P=0·601), was inversely related to 2hPG at the Y4 follow-up (Table 2). Physical activity was also negatively related to 2hPG (Table 2).
HDL-cholesterol
The ratio of total recommended:non-recommended items (β=0·044, P=0·032) and unpolished:polished rice (β=0·066, P=0·031) was positively related to HDL-cholesterol concentrations (Table 3) (Fig. 2(b)). There was no relationship between any other dietary variable including pulses or meal patterns and HDL-cholesterol concentrations.
Table 3 β-Coefficients for the effect of the ratio of unpolished rice:white rice on clinical and biochemical outcomes
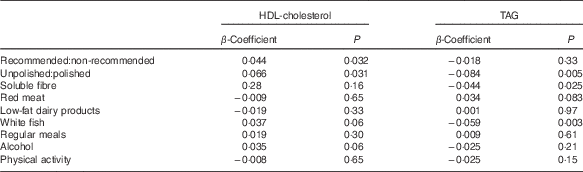
TAG
The ratio of unpolished:polished rice (β=−0·084, P=0·005), white fish intake (β=−0·059, P=0·003) and soluble fibre intake (β=−0·044, P=0·025) were also negatively related to TAG concentrations (Table 3).
Blood pressure
After adjusting for age, sex, BMI, physical activity, alcohol intake and baseline blood pressure, there was no relationship between meal timings and blood pressure. Total dairy products demonstrated a borderline association with diastolic (β=−0·031, P=0·052) blood pressure. When low-fat dairy products were analysed separately, the relationship was significant (β=−0·046, P=0·004). There was no relationship between any other dietary variables including fruits and vegetables, low-fat products in general and unpolished:polished rice and blood pressure.
Total and LDL-cholesterol
There was no relationship between any dietary variable and total or LDL-cholesterol.
Relationship between meal timings and food group intake
Participants who skipped meals had a significantly lower ratio of recommended:non-recommended food items (P<0·001), low:high-fat products (P<0·0001) and unpolished:polished rice (P<0·0001) compared with those who did not skip meals. Participants who snacked had a lower ratio of recommended:non-recommended food items (P<0·0001) and low:fat products (P<0·0001). Participants who ate meals out of home had a lower ratio of recommended:non-recommended food items (P<0·0001) and unpolished:polished rice (P<0·0001).
Discussion
In this 5-year interventional trial in a young population at high risk of CVD, it has been shown that replacing white rice with unpolished rice may be a particularly effective individual dietary change to reduce risk factors for T2DM and CVD. Other foods and food groups that appear to exert their risk reduction effects via specific metabolic risk factors are also highlighted.
White rice consumption has been linked to T2DM risk in several cohort studies( Reference Nanri, Mizoue and Noda 21 , Reference Sun, Spiegelman and van Dam 22 ). The relative risk is higher in Southern Asians per serving( Reference Sun, Spiegelman and van Dam 22 ) and Southern Asians consume more white rice than their Western counterparts( Reference Misra, Khurana and Isharwal 11 , Reference Hu, Pan and Malik 23 ). Rice immediately harvested comprises the bran, germ and endosperm. White – or polished – rice is produced by milling which removes the hull, bran (primarily non-cellulosic polysaccharides, cellulose and lignin) and most of the wheatgerm( Reference Slavin 24 ). Unpolished rice retains beneficial fatty acids including oleic and linoleic acid( Reference Slavin 24 ), protein, bran and some vitamins( Reference Slavin 24 ) and is higher in fibre and has a lower glycaemic index than white rice( Reference Hu, Pan and Malik 23 , Reference Slavin 24 ).
Total dietary fibre, cereal fibre and low glycaemic index foods have been repeatedly linked to improved glycaemic control and lower risk of T2DM in a variety of studies( 25 – Reference Schulze, Schulz and Heidemann 29 ). In general, whole grains, cereals or insoluble fibres are associated with lower T2DM risk in cohort studies( Reference Weickert and Pfeiffer 28 ), whereas soluble fibres reduce postprandial glucose concentrations in the controlled setting( Reference Weickert and Pfeiffer 28 ). Our data suggest that sources of whole-grain fibre (mainly insoluble) may exert their T2DM-preventive effects by specifically altering fasting glucose concentrations, as the association was strong and not altered by adjusting for multiple confounders, and no association was found between unpolished rice or starches on 2hPG concentrations in any model. There is limited epidemiological data on this question as the majority of cohort studies use T2DM incidence as the dependent variable, not FPG or 2hPG concentrations( 25 , Reference Schulze, Liu and Rimm 26 , Reference Schulze, Schulz and Heidemann 29 ), but controlled trials and cohort studies that measure FPG and 2hPG with appropriate adjustment have shown a reduction in fasting but not postprandial glucose( Reference Karlström, Vessby and Asp 30 – Reference Bakker, Hoogeveen and Nijpels 32 ). Fasting glucose concentrations are rising at a rate of 0·07 mmol/l per decade across the population( Reference Danaei, Finucane and Lu 33 ); our finding that replacement of white rice with unpolished rice significantly reduces FPG has important public health implications in countries such as Sri Lanka where rice is a staple food.
Dietary fibre has also been linked to reduced weight and WC in a number of cohort and controlled studies( Reference Abrams, Griffin and Hawthorne 34 – Reference Liber and Szajewska 36 ), with the mechanism posited to be by a bulking effect, lowering energy density and/or the glycaemic index of the diet( Reference Weickert and Pfeiffer 28 ). Products of dietary fibre fermentation have also been shown to increase the production of hormones, which decrease appetite( Reference Chandalia, Garg and Lutjohann 27 , Reference Danaei, Finucane and Lu 33 , Reference Ludwig, Pereira and Kroenke 35 ). For example, inulin supplementation attenuates weight gain in overweight children over 1 year( Reference Abrams, Griffin and Hawthorne 34 ). Although it has been thought that soluble fibres are generally fermentable, while insoluble fibres are not( Reference Weickert and Pfeiffer 28 ), other authors have shown that long-term, cereal fibres such as cellulose are fermented and associated with increases in glucagon-like peptide-1 (GLP-1) and a reduction in appetite( Reference Freeland, Wilson and Wolever 37 ). As our regression models were adjusted for changes in weight, our data therefore suggest that substitution of white rice with unpolished rice has a two-pronged effect on T2DM risk, first by limiting weight gain and second by its independent effects on FPG.
No effect was observed by replacing total high-fat food items with their low-fat varieties on any of the outcome measures studied. The role of fat in risk of T2DM and CVD is complex, with different fat sources, classes and separate fatty acids possibly having distinct and opposing effects on T2DM risk( Reference Morio, Fardet and Legrand 38 ). Dairy fat in particular has been linked to a reduction in risk( Reference Louie, Flood and Rangan 8 , Reference Morio, Fardet and Legrand 38 ), although our finding showed that low-fat dairy products but not total dairy products were associated with a reduction in 2hPG, suggesting that the erstwhile nutrients of dairy products such as Ca and milk proteins may be mediating this beneficial effect( Reference Pasin and Comerford 39 ).
An association between red meat and FPG was found, but not with 2hPG. This is supported by previous cohort studies( Reference Malik, Popkin and Bray 19 ), with data also suggesting that red meat intake is specifically associated with fasting glucose and risk of impaired fasting glucose( Reference Fretts, Follis and Nettleton 40 , Reference Heikkilä, Schwab and Krachler 41 ). Although no association between processed meats and any outcome was found, in contrast to a body of work implicating processed meat in CHD and T2DM( Reference Micha, Michas and Mozaffarian 42 ), this may reflect the limited consumption of such foods in the Sri Lankan diet.
The lipid profile is an important component in the development of CVD, and Southern Asians have lower HDL-cholesterol compared with their Caucasian counterparts( Reference Pandit, Goswami and Ghosh 2 ). Although the relationship between whole grains, glucose concentrations and TAG levels are well documented( Reference Weickert and Pfeiffer 28 ), there is little suggestion in the literature that dietary fibre influences HDL-cholesterol concentrations. In the present study, replacing white rice with unpolished rice was associated with increases in HDL-cholesterol, and the relationship remained significant after adjusting for multiple factors including factors known to alter HDL-cholesterol such as alcohol intake, smoking status and physical activity. Interestingly, both consumption of white rice and glycaemic load of the diet negatively associate with HDL-cholesterol across a number of populations( Reference Radhika, Ganesan and Sathya 43 , Reference Mosdøl, Witte and Frost 44 ). Therefore, it appears that adding whole grain to the diet is important for glycaemic control, weight management and reduction of TAG. In addition, its displacement of high glycaemic index, highly refined rice may be equally important for the benefit on HDL-cholesterol.
There is a growing body of data demonstrating the importance of meal timings on CVD risk factors( Reference Pot, Hardy and Stephen 13 , Reference Sierra-Johnson, Undén and Linestrand 14 ). Although our finding that regular meals are negatively associated with weight and WC is limited by the lack of control for energy intake, it is supported by data from large cross-sectional studies in the UK( Reference Pot, Hardy and Stephen 13 ) and Sweden( Reference Sierra-Johnson, Undén and Linestrand 14 ) and by prospective studies in children( Reference Franko, Striegel-Moore and Thompson 45 ) and adults( Reference Pot, Hardy and Stephen 46 ). After controlling for weight change, irregular meals was also positively associated with FPG, which may be due to the effect of irregular meals on circadian secretion of hormones including GLP-1 and insulin( Reference Pot, Hardy and Stephen 46 ). Advice to consume regular meals was easy to translate across this population and could be tested independently in a controlled setting.
Some interesting associations between dietary factors and specific components of the MetS were observed. It is now clear that the pre-diabetic period is characterised by two separate conditions – impaired fasting glucose and impaired glucose tolerance – each with its own distinct underlying pathophysiology( Reference Abdul-Ghani, Tripathy and DeFronzo 47 ). Our findings highlight the distinct roles nutrients and meal patterning may play in the development of T2DM, and suggest that regular meals, a reduction in red meat and increased bran intake may specifically affect fasting glucose dysregulation, whereas physical activity and low-fat dairy products may target peripheral insulin resistance, which characterises impaired glucose tolerance( Reference Abdul-Ghani, Tripathy and DeFronzo 47 ).
Some strengths of this study while acknowledging its limitations are highlighted. First, food intake data were collected every year during this 5-year study, enabling the calculation of average intake over time, and the reduction of within-person measurement errors( Reference Hu, Pan and Malik 23 ). The long follow-up period also enabled the examination of the effect on clinical factors over time. However, because the FFQ – which themselves have limitations – were adapted to the target population, it was not validated. Although portion sizes were indicated by the participants using serving spoons, this method could have introduced error. The analyses cannot therefore be reliably adjusted for energy intake. Nevertheless, the primary findings of this study have been controlled for weight change over 5 years, which is arguably a more reliable estimate of energy intake and energy balance. In Sri Lankan cuisine, soya, sunflower, palm and coconut oils are used for cooking and have known effects on CVD risk factors( Reference Medagama, Fernando and Widanapathirana 48 , Reference Tennakoon, Kumar and Meyer 49 ). Although fried foods were controlled for in our analyses, the FFQ did not include information on the different dietary oils or fats used in cooking and it is possible that differences in these sources mediated the relationships seen. This is particularly relevant as coconut oil is commonly consumed as an added factor in Sri Lanka, and is implicated in both promoting or limiting CVD progression( Reference Eyres, Eyres and Chisholm 50 , Reference Pietraszek, Hermansen and Pedersen 51 ). We acknowledge our exchange model included some foods such as egg yolks and nuts in the non-recommended category, even though they are thought to have neutral or beneficial effects on T2DM or CVD risk in most people. However, its primary design was to reduce the energy density of the diet in a way that was simple to understand and translate for community-recruited educators who did not have a background in nutrition. Finally, these analyses used pooled data from the intervention and control group of a primary prevention trial. Thus, although the original study was controlled, this current analysis cannot infer causation, and the data were not adjusted for multiple comparisons.
In conclusion, a pragmatic dietary intervention can significantly reduce risk factors for the MetS in a high-risk population. Replacement of white with unpolished rice may be a particularly effective dietary advice for this and similar populations.
Acknowledgements
The authors acknowledge the contributions of Dr Thilinie Jayasekera, Talya Fernando, Dr Nuwan Ranawaka for their help with this work. The authors thank Dr Varsha, Dr Mahesh (MV Diabetes Centre, WHO collaborating centre for research education and training in diabetes, Chennai, India) and Dr Arambepola (University of Colombo Sri Lanka) for their advice and input as members of the DIABRISK-SL independent study advisory committee. The authors also wish to thank all the participants of the study.
This project is supported by a BRIDGES Grant from the International Diabetes Federation. BRIDGES, an International Diabetes Federation project, is supported by an educational grant from Lilly Diabetes and a grant awarded from the Diabetes Association of Sri Lanka. The funder had no role in the design, analysis or writing of this article.
J. K., G. V., L. G. and M. W. conceived the study and its design and were involved in implementation of the study. N. G. led the dietary analysis plan, carried out all data analysis and wrote the manuscript with feedback from the other authors. L. V., J. K., M. G., G. V., L. G. and M. W. were involved in coordination of the study. All the authors read and approved the final manuscript. N. G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors declare no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114516002476








