Introduction
The standard management of gastric carcinoma requires trimodal treatment. However, the role of adjuvant radiotherapy (RT) has become questionable after the recent CRITICS (Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer) trial. Reference Cats, Jansen and van Grieken1 The currently proposed line of management is perioperative chemotherapy followed by definitive surgery and adjuvant chemotherapy. Reference Cats, Jansen and van Grieken1 The standard surgical procedure is radical gastrectomy (total/subtotal) with D2 lymphadenectomy. Reference Brenkman, Haverkamp, Ruurda and van Hillegersberg2 In developing countries, many patients do not receive perioperative therapy. Furthermore, adequate surgery (i.e., D2 lymph node dissection) is not routinely practiced at all centres because of limited resources, lack of access to expert cancer care and financial constraints. Reference Ibrahim and Gilbert3 In such situations, adjuvant chemoradiation therapy (CTRT) plays a vital role in reducing locoregional recurrence and systemic metastasis. While contouring for RT in gastric cancer, various structures are considered as organs at risk (OARs). The QUANTEC (Quantitative analysis of normal tissue effects in the clinic) guidelines recommend certain dose limitations in RT planning. Reference Bentzen, Constine and Deasy4 Various OARs delineated in gastric cancer RT include the liver, kidneys, spinal cord, small bowel and large bowel.
It has always been presumed that the spleen does not need to be contoured, as it is not considered to be an organ of significance. However, recent studies have provided evidence to the contrary. The spleen plays a major role in the adaptive immune response and clears pathogens from the circulation. Reference Cesta5 Evolving evidence supports the development of hyposplenism after abdominal RT. RT resulting in hyposplensim has been shown to later lead to late sepsis, pneumonia, extended hospital stay, sepsis-related mortality and increased financial burden to the patient. Reference Trip, Sikorska and van Sandick6 Thus, the spleen needs to be considered as an OAR during RT for gastric cancer.
This study aimed to evaluate the RT dose administered to the spleen during adjuvant CTRT for gastric cancer resulting in significant haematological toxicity. With this study, we hope to address the current literature gap regarding dose restriction to the spleen during adjuvant RT for gastric cancers.
Materials and Methods
All patients with histologically proven nonmetastatic gastric adenocarcinoma who underwent adjuvant CTRT between January 2017 and December 2021 were considered for this study. Patients who underwent splenectomy as part of definitive surgery were excluded from the study. In addition, patients who received perioperative chemotherapy were excluded, as this would have been a confounding factor in the development of haematological toxicity. Patient records were accessed from the Medical Records Department (MRD) of our hospital after obtaining clearance from the ethics committee board. Data pertaining to patient demographics (age, sex, comorbidities, performance status, signs and symptoms), pathological tumour characteristics (pT stage and pN stage) and treatment details (type of surgery, radiation and chemotherapy doses and associated toxicities) were accessed.
Details of Radiotherapy
The patients were placed in a supine position with their arms over the chest. A thermoplastic mask was used for immobilisation. Contrast-enhanced planning computed tomography (CT) was preferred to delineate the lymph nodes. The images were delineated according to the guidelines by Gundersen and Tepper et al. Reference Tepper and Gunderson7 The planning target volume (PTV) dose was 45 Gy in 25 fractions with five fractions each week and one fraction every day. The PTV included the residual stomach, tumour bed and adjacent nodal stations. Planning was conducted using either three-dimensional conformal RT (3D CRT) or intensity-modulated RT (IMRT). Twenty-five patients were planned using the 3D CRT technique and nine were planned using the IMRT technique. The patients were also advised to receive concurrent chemotherapy in the form of oral capecitabine 825 mg/m2 twice daily on all days of RT. The spleen was contoured retrospectively on the CT images acquired during the planning phase of RT. The mean dose delivered to the spleen (Dmean) was retrospectively obtained.
Assessments
The patients were evaluated weekly with routine complete blood counts (CBC), which included haemoglobin, total white blood cell (WBC) count, absolute neutrophil count (ANC) and platelet counts. Haematological toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5. Concurrent chemotherapy was withheld for patients who reported grade 3 or 4 haematological toxicities. The patients were assessed after a gap of 4 weeks with routine complete blood count to evaluate recovery of counts. These patients were reconsidered for adjuvant chemotherapy if the counts had normalised.
Statistical Analysis
Statistical analyses were performed using the IBM SPSS Statistics version 25·0. Continuous variables were analysed for mean, median and standard deviation, and comparisons were made using the Student’s t-test for mean and Mann–Whitney U test for median. Categorical data are described using proportions, and comparisons were performed using the chi-square test. Univariate and multivariate analyses were performed to identify the possible factors affecting the development of leukopenia and thrombocytopenia. Receiver operating characteristic (ROC) curve analysis was performed to determine the RT cut-off doses for different grades of haematological toxicities.
Results
A total of 74 patients who had received adjuvant CTRT after gastrectomy for carcinoma of the stomach between January 2017 and December 2021 were evaluated. Of these, 24 patients received neoadjuvant chemotherapy and hence were excluded from the study. Furthermore, 10 patients defaulted during RT, and data of six patients were not retrievable. Thus, 34 patients were included in the final analysis.
Patient demographics are summarised in Table 1. The median age of the patients was 60 years (range, 39–79 years). Of the total patients, 27 (79%) were men and seven (21%) were women; 50% of the patients had comorbidities, such as hypertension and diabetes mellitus. All patients had an Eastern Cooperative Oncology Group performance status score of 0 or 1. The most common symptom at presentation was abdominal pain (88%), followed by loss of appetite (79%), vomiting (59%) and weight loss (50%).
Table 1. Patient characteristics

Abbreviations: ECOG, Eastern Cooperative Oncology Group.
Tumour characteristics are summarised in Tables 2 and 3. The most common site of the primary tumour was the distal stomach (56%), whereas the proximal and middle third of the stomach were observed in 21% and 23% of the cases, respectively.
Table 2. Tumour characteristics

Table 3. Tumour and nodal staging (AJCC 8th edition)

Abbreviations: AJCC, American Joint Committee on Cancer; T, tumour, N, nodal.
The treatment details are summarised in Table 4. Approximately 77% of the patients underwent subtotal gastrectomy, while the rest underwent total gastrectomy. A minimum D1 lymph node dissection was performed in 94% of the patients. Of these, 62% (n = 11) also underwent D2 lymph node dissection. Four patients (12%) showed positive splenic nodes in the final histopathological report. The mean spleen volume was calculated as 186·65 cc (range 90–303 cc). The mean RT dose to the spleen was calculated as 35·35 Gy (range 20–42 Gy).
Table 4. Treatment details

The development of various grades of haematological toxicities is summarised in Table 5. Approximately 82% of the patients developed grade 2 leukopenia by the third to fourth week of starting RT and 67% further progressed to develop grade 3 leukopenia. Five (15%) of these patients eventually developed grade 4 leukopenia. RT was stopped in six (18%) patients for a duration of 1 week.
Table 5. Toxicity grading for leukopenia and thrombocytopenia during RT-CTCAE V 5
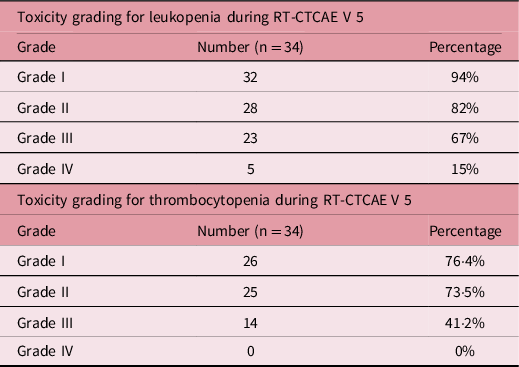
Abbreviations: CTCAE V 5, Common Terminology Criteria for Adverse Events Version 5.
Approximately 73% of the patients developed grade 2 thrombocytopenia by the fourth week of RT. Approximately 41% of the patients progressed to grade 3 thrombocytopenia. No case of grade 4 thrombocytopenia was reported in this study.
Seven patients (20·5%) were not fit for concurrent chemotherapy either because of the development of grade 3 leukopenia, grade 2/3 thrombocytopenia, or a combination of both; hence, only RT was administered to such patients.
The development of thrombocytopenia and leukopenia on univariate analysis correlated with the location of primary gastric cancer. Grade 1–3 thrombocytopenia and grade 1–3 leukopenia were more frequent in the distal gastric cancer primaries than in the middle and proximal primaries (Table 6). Age, sex, gastric cancer stage and spleen volume did not appear to influence the development of haematological toxicities.
Table 6. Univariate analysis for factors affecting leukopenia and thrombocytopenia

The extent of splenic radiation correlated with the development of grade 3/4 leukopenia and grade 3 thrombocytopenia. A positive correlation was observed between the splenic dose of RT and the grade of leukopenia (odds ratio [OR], 1·90; 95% CI 1·17–3·07; p = 0·009) (Figure 1).

Figure 1. A graph showing the correlation between mean splenic dose of RT (Gy) with grades of leukopenia.
In addition, a positive correlation was noted between the splenic RT dose and grade of thrombocytopenia (OR, 1·64; 95% CI 1·12–2·40, p = 0·01) (Figure 2).

Figure 2. Graph showing correlation between mean splenic dose of RT (Gy) with grades of thrombocytopenia.
The addition of concurrent chemotherapy did not correlate with the development of haematological toxicities in univariate analysis.
The ROC curve analysis for splenic dose of RT resulting in various grades of leukopenia and thrombocytopenia is summarised in Table 7. It revealed that the RT dose cut-off value for Grade 3 leukopenia was 35·5 Gy at the highest Youden index of 0·788, with a sensitivity of 96% and specificity of 83% (area under the ROC curve [AUC], 0·905; CI 0·802–1·000; p ≤ 0·001) (Figure 3).
Table 7. Splenic radiotherapy cut-off values obtained by the receiver operating characteristics analysis


Figure 3. Receiver operating characteristics (ROC) curve showing the area under the curve for radiotherapy dose received to the spleen with respect to development of leucopenia.
The cut-off value for RT dose for grade 3 thrombocytopenia was 36·5 Gy at the highest Youden index of 0·557, with a sensitivity of 85·7% and specificity of 70% (AUC, 0·863; CI 0·740–0·985, p < 0·001) (Figure 4).

Figure 4. Receiver operating characteristics (ROC) curve showing the area under the curve for radiotherapy dose received to the spleen with respect to development of thrombocytopenia.
Discussion
Radiation-induced hyposplenism was first described in 1980 by Dailey et al. in a case report of a 24-year-old woman with Hodgkin’s lymphoma. Reference Dailey, Coleman and Kaplan8 The patient’s autopsy report showed conclusive evidence of splenic atrophy, which was probably the antecedent cause of sepsis resulting in her death 12 years after RT. In a subsequent study, Coleman et al. described the effect of RT on the spleen in patients with lymphoma. Reference Coleman, McDougall, Dailey, Ager, Bush and Kaplan9 They suggested that 40 Gy of RT results in hyposplenism 4–5 years later. Despite these concerns in the 1980s, the spleen was not considered as an OAR. However, recent emerging data have put the spleen at the forefront.
In a November 2021 publication by the Royal College of Radiologists (RCR), the authors concluded that a spleen Dmean dose of 10 Gy can reduce the size of the spleen. 10 Dmean > 10 Gy is associated with a higher risk of sepsis and mortality, and Dmean > 40 Gy would result in a very high risk of severe late sepsis and sepsis-related mortality. These guidelines are based on two retrospective studies of gastric and paediatric cancers. Reference Trip, Sikorska and van Sandick6,Reference Weil, Madenci and Liu11
Trip et al. evaluated 46 patients who underwent adjuvant chemoradiotherapy for gastric cancer. Reference Trip, Sikorska and van Sandick6 They reported a mean splenic dose of 40 Gy (32–46 Gy). Among the 46 patients, 11 had 13 episodes of pneumonias and three fatal sepsis during a median follow-up of 67 months. They concluded that a high dose to the spleen during RT for gastric cancer results in hyposplenism, eventually leading to the development of pneumonia and fatal sepsis. This mirrors the findings of our study, where Dmean doses >35·5 Gy resulted in severe leukopenia.
Weil et al. conducted a retrospective analysis of 20,264 survivors of childhood cancer. Reference Weil, Madenci and Liu11 Among them, 46% had received RT and 0·6% developed infection-related late mortality. Children receiving RT doses >20 Gy were six times at a higher risk of developing infection-related late mortality than those receiving < 20 Gy RT. Based on this, the recent Societe Internationale d’Oncologie Pediatrique (SIOP) Europe Radiation Oncology Working Group recommended a mean dose of 10 Gy to the spleen in paediatric patients receiving RT to the abdomen. They also concluded that antibiotic prophylaxis or vaccination should be considered in children receiving > 10 Gy RT dose to the spleen. Reference Arunagiri, Kelly and Dunlea12
Van Rossum et al. reported that patients who underwent CTRT for oesophageal cancers and developed severe leukopenia had a poor prognosis. Reference van Rossum, Deng and Routman13
Alexandru et al. suggested that an increase of 1 Gy in Dmean RT to the spleen results in an absolute decrease in lymphocyte count by 1%. Reference Alexandru, Rodica, Dragos-Eugen and Mihai-Teodor14
Nearly 70% of our patients developed grade 2 leukopenia by 3–4 weeks after starting RT. The rates of grade 2 and 3 leukopenia were 70% and 38%, respectively. Patients who did not receive concurrent chemotherapy developed grade 2 leukopenia. Hence, the development of leukopenia cannot be solely attributed to the administration of capecitabine. Similarly, Phua et al. reported a very low rate of leukopenia (2·2%) with capecitabine usage. Reference Phua, Wong and Tan15
All the aforementioned studies focused on the development of leukopenia. However, the development of thrombocytopenia is an equally threatening haematological toxicity. Weinmann et al. reported a severe pancytopenia rate of 10–30% in patients with chronic lymphocytic leukemia who underwent splenic irradiation. Reference Weinmann, Becker, Einsele and Bamberg16 Zoarsky et al. reported anaemia (28%), leukopenia (21%), thrombocytopenia (30%) and pancytopenia (8%) in patients receiving a total dose range of 15–30 Gy for hypersplenism. Reference Zaorsky, Williams and Barta17 In our study, grade 2 thrombocytopenia was observed in 30% of the patients and grade 3 in 15%.
Furthermore, the distal and middle gastric primaries had a higher incidence of leukopenia and thrombocytopenia than proximal gastric primaries. This may be because the splenic nodes were dissected for a proximal primary tumour, and this area was spared in adjuvant RT. This may have resulted in the lower toxicity for such primaries.
This study had some shortcomings. First, the retrospective design of the study prevents generalisation of the finding. Therefore, more robust prospective data are needed to corroborate the findings. As this was a retrospective analysis, we did not evaluate the onset of pneumonia or sepsis in patients with leukopenia. This finding could have further reiterated our results. Second, we did not conduct serial assessments of spleen volume during RT. This would have provided an estimation of volume loss and thus would have helped predict the possibility of haematological toxicities. Long-term follow-up data are needed to further validate if RT to the spleen results in permanent hyposplenism. Thus, this study was hypothesis-generating, and the findings need to be further verified by prospective trials in the future.
Conclusion
The spleen is a vital organ of the immune system and should be considered an OAR during RT. There was a significant risk of developing haematological toxicities when the spleen received a mean dose of more than 35·5 Gy. Although this study was limited to patients with gastric cancer, the results can be generalised to all patients receiving abdominal radiation. More advanced RT planning techniques, such as IMRT, should be incorporated as a routine while planning abdominal RT involving the spleen to optimise the treatment dose. Further, we propose exploring the usage of prophylactic vaccinations in patients receiving Dmean > 35·5 Gy to the spleen.
Acknowledgements
None.
Conflict of Interest
None.
Financial Support
None.
Ethics Approval and Consent to Participate
Institutional Ethics committee approval obtained. Consented for participation
Consent for Publication
All the authors consent for publication.
Availability of Data and Material
Yes.
Authors’ Contributions-
UV – concept, data collection and analysis, manuscript writing
PSS – data analysis, result interpretation and manuscript writing
KS – data analysis and result interpretation
SLS – data analysis and statistics
AS – manuscript writing














