INTRODUCTION
Hepatitis C virus (HCV) has been spreading in people who inject drugs (PWID) in Australia and worldwide since the 1970s or earlier [Reference Rodger1]. Australian research from the mid-2000s found high HCV incidences [30·8/100 person-years (py) [Reference Maher2]; 15·5/100 py in HCV-naive PWID and 46·8/100 py] in previously infected PWID [Reference Grebely3] and an extremely high incidence (133/100 py) in people who had been injecting for <12 months [Reference Maher2]. While initiatives that encourage bloodborne virus testing and reduced sharing of injecting equipment in PWID have existed in Australia and elsewhere since the 1980s [Reference Bell4], only recently have studies begun to show reductions in HCV incidence in PWID. Analysis of serial cross-sectional studies of Australian PWID attending needle-and-syringe programmes (NSPs) showed incidence was declining in those naive to HCV, from 30·8/100 py in 2003 to 4·0/100 py in 2009 [Reference Iversen5].
The reasons for this apparent reduction in HCV incidence are not clear. In the Australian state of Victoria, decreased availability of high-purity heroin in the early 2000s [Reference Dietze and Fitzgerald6] and the high uptake of opioid substitution therapy (OST) [7] and improved NSP coverage [8] are possible explanations [Reference Higgs and Maher9]. Other researchers have presented compelling evidence that OST protects against transmission of HCV [Reference Iversen5, Reference White10–Reference Turner13]. Nolan et al. [Reference Nolan11] found methadone maintenance therapy (MMT) was protective against HCV seroconversion, reporting a dose-response protective effect of increasing exposure to MMT in over 1000 Canadian participants. Similarly, in Australia, White et al. [Reference White10] found OST was independently protective against HCV infection in PWID who reported opioids as the main drug injected. In a pooled analysis of UK data from 2001 to 2009, Turner et al. found significant reductions in HCV incidence associated with OST use and high NSP coverage [Reference Turner13].
We sought to provide updated estimates of HCV incidence and investigate the effect of OST and needle-sharing on the HCV epidemic in Melbourne, Australia using data from an ongoing prospective cohort study of PWID. Our specific aims were to (1) report the incidence of new HCV infection and reinfection between 2009 and 2014 in this cohort; (2) assess whether self-reported OST use is protective against new HCV infection or reinfection; and (3) measure the association between needle-sharing behaviour and HCV (new infection and reinfection) incidence.
METHODS
Our research was conducted in Melbourne, Australia's second-largest city, as part of the ongoing Melbourne Injecting Drug Users Cohort Study (MIX). MIX was designed to increase our understanding of the natural history of injecting drug use and identify risk and protective factors for PWIDs’ ill-health and health service utilization, focusing particularly on young, out-of-treatment PWID [Reference Horyniak14].
Recruitment
PWID reporting regular heroin or methamphetamine injection in the past 6 months were recruited in urban Melbourne through respondent-driven sampling (RDS), street outreach and snowball sampling. Eligibility criteria included being aged >18 years and providing a valid Medicare (Australia's universal healthcare system) number and contact details for data linkage (not described in this article). Two further eligibility criteria that aimed to recruit participants who were young (aged <31 years) and not prescribed OST were withdrawn during early recruitment owing to the ageing PWID population in Melbourne and fluctuating drug-market conditions.
Field researchers completed baseline recruitment (after informed consent) between November 2008 and March 2010 in one outer-urban and two inner-urban areas of Melbourne with illicit drug street markets using a combination of RDS (a modified chain-referral sampling technique used for the recruitment of hard-to-reach populations [Reference Heckathorn15]), street outreach, and snowball sampling. For RDS, up to five PWID from each recruitment site who were known to study researchers through participation in previous studies or through agency referral and who met the study eligibility criteria acted as ‘seeds’. Following interview, each seed received a set of uniquely numbered recruitment coupons and was invited to recruit up to three peers into the study. In street outreach and snowball sampling, PWID responded to word-of-mouth advertising and flyers posted in relevant community agencies, and field researchers who regularly attended each of the recruitment locations enrolled those deemed eligible. These participants were then asked to invite their contacts to participate in the study [Reference Aitken16].
Data collection
We attempt to collect blood samples from our participants annually for HCV antibody (anti-HCV) and HCV RNA testing, and associated behavioural and service-use data are collected [Reference Horyniak14] using interviewer-administered questionnaires on hand-held personal digital assistants. Data are downloaded into a database constructed using Questionnaire Design System versions 2·4–2·6. The questionnaire covers demographic and social characteristics, drug-use characteristics and drug-market access, health and social functioning and health service use. Interviews are conducted either in public spaces or in our mobile study van, and average 39 min in length. Participants were reimbursed AUD30 (about US$28 in August 2014) for their time and out-of-pocket expenses in accordance with accepted practice [Reference Fry17], and another AUD10 for each RDS coupon returned that results in an eligible interview, plus a further AUD10 per blood sample.
Blood sample analysis
The Victorian Infectious Disease Reference Laboratory screens blood samples for anti-HCV using the Murex anti-HCV v. 4.0 (Murex Biotech, South Africa) and anti-HCV-positive specimens were confirmed using the Bio-Rad Monolisa anti-HCV plus V2 assay (France). Irrespective of anti-HCV status, samples are tested for HCV RNA by the COBAS AMPLICOR HCV test v. 2.0 (Roche Molecular Systems, USA).
Incidence measurement
Participants were considered incident cases if their status changed from anti-HCV negative to anti-HCV positive during the study (usually, but not always, with a corresponding observed change in HCV RNA status). Participants were considered reinfected if their status changed from anti-HCV positive and HCV RNA negative to HCV RNA positive.
We estimated crude HCV incidences (naive, reinfection, combined) using the dates of eligible participants’ first ‘negative’ (anti-HCV negative and RNA negative for naive, anti-HCV positive and RNA negative for reinfections) test and the midpoint between their last ‘negative’ and first ‘positive’ test observation. Analyses were based on study participants with valid test results from at least two blood samples.
Statistical analysis
Given the interval-censored nature of time to HCV infection in our data, we undertook discrete-time survival analyses of HCV incidence (naive and reinfection) to compare the effects of the binary variables ‘needle-sharing’ (injecting with someone else's syringe/needle in the past month) and ‘OST status’ (self-reported OST programme in past month) on rate of infection. Using person-year/observation record data, we used generalized linear modelling to estimate rate differences, specifying a binomial distribution and complementary log-log link function. Models were offset for elapsed time between respondent interviews to account for differences in exposure between interviews, and dummy indicators for each interview were included in models to account for temporal variation in the baseline hazard. These models produce exponentiated coefficients approximating hazard ratios (HRs) when the proportional hazards assumptions hold in continuous time and survival time is interval-censored [Reference Allison18].
We compared HRs for needle-sharing across HCV-naive and reinfection populations using seemingly unrelated estimation [Reference Weesie19] with post-hoc Wald tests. Seemingly unrelated estimation can be used to test cross-model hypotheses, essentially reducing estimates from separate models to a single parameter vector and generating a between-model covariance matrix on which the cross-model test is based. The procedure uses robust variance estimation [Reference Huber20], which corrects standard errors to account for individuals providing both naive infection and reinfection observations. Given the relatively small numbers of HCV-naive and reinfection events observed, we restricted survival analyses to unadjusted comparisons of risk difference to avoid overfitting models. All statistical analyses were undertaken using Stata v. 13.0 [21].
Needle-sharing and OST status factors were modelled in survival analyses as time-dependent exposures and were lagged one interview observation, providing a model estimating the effects of participant risk/protective behaviour preceding HCV infection or censoring.
Ethical standards
The Victorian Department of Health and Monash University Human Research Ethics Committees approved the MIX study. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Of 757 MIX participants, 552 had provided blood samples to February 2015, and of those 461 (84%) had provided at least one follow-up sample and were eligible for incidence analyses. Table 1 shows selected baseline characteristics of PWID by eligible (multiple) and single blood sample populations. Participants who provided multiple blood samples were more likely to report current OST than those who provided only one sample.
Table 1. Baseline characteristics of PWID with multiple blood samples and single samples (excluded)

PWID, People who inject drugs.
Some variables have missing data.
* P < 0·01.
Of the 461 multi-test participants, 217 (47%) were consistently HCV Ab and HCV RNA positive and were therefore excluded from incidence analyses. Thirteen (3%) participants were excluded from incidence analyses due to indeterminate blood results. Two hundred and thirty-one participants (50%) were eligible for incidence analyses: 95 (246 observations) showed no evidence of HCV exposure at their baseline blood test in the study (HCV Ab and RNA negative) and 139 (316 observations) showed evidence of previous (prior to baseline) HCV exposure but no infection (HCV Ab positive and RNA negative). Three participants were at risk of both naive HCV infection and HCV reinfection during the study.
Nineteen (20%) of 95 previously unexposed participants were infected across 250·2 py and 39 (28%) of 139 previously exposed participants were reinfected across 314·5 py. Key demographic and other characteristics are summarized in Table 2.
Table 2. Baseline characteristics of HCV-uninfected, previously exposed and chronically infected participants
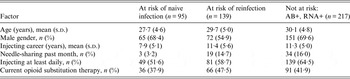
Some variables have missing data.
HCV incidence
Incidence of naive HCV infection was 7·6/100 py [95% confidence interval (CI) 4·8–11·9], reinfection 12·4/100 py (95% CI 9·1–17·0) and either form of HCV infection 9·7/100 py (95% CI 7·4–12·6).
HCV incidence and needle-sharing
Discrete-time survival analysis of naive HCV infection showed that PWID who reported needle-sharing at the previous interview were nearly five times more likely to become newly HCV infected than those who reported no sharing (HR 4·9, 95% CI 1·3–17·7, P = 0·016). This relationship was not statistically significant for HCV reinfection (HR 1·85, 95% CI 0·79–4·3, P = 0·153). Using seemingly unrelated estimation, cross-model comparison of the effect of prior needle/syringe sharing suggested that the observed difference in hazard between naive and reinfected at-risk populations was sample specific [Wald χ 2(1) = 1·46, P = 0·228].
HCV incidence and current OST
Discrete time-survival analysis showed past month use of OST was not-significantly associated with either naive HCV infection (HR 0·85, 95% CI 0·34–2·1, P = 0·735) or HCV reinfection (HR 0·9, 95% CI 0·45–1·79, P = 0·769).
Discussion
Our HCV-naive and reinfection incidence rates are much lower than those measured in our group's study of HCV transmission in a similar cohort recruited from the same locations between 2005 and 2007 [Reference Aitken22]. As such, this study strengthens the evidence [Reference Iversen5, Reference White10] that HCV incidence in Australian PWID has declined over the past decade or more. Our study also reaffirms our previous finding that, descriptively, HCV reinfection incidence is greater than the rate of naive infection [Reference Aitken22], although this is not universally the case in longitudinal cohorts of PWID [Reference Grebely3, Reference McDonald23]. However, these data suggest a substantially greater decline in HCV reinfection (compared to naive infection) in PWID in Melbourne.
HCV incidence and needle-sharing
We found that prior needle-sharing was strongly associated with subsequent new HCV infection incidence, but not with reinfection incidence. Cross-model comparison suggested this difference in the effect of sharing was sample specific and did not indicate a real difference in needle-sharing behaviour in the population of PWID that might explain different rates of naive HCV infection and reinfection. Nevertheless, the strong association of needle-sharing and naive infection reinforces the importance of providing PWID with sterile injecting equipment to avoid needle-sharing and reduce their risk of HCV infection or reinfection.
HCV incidence and OST
Previous evidence for OST's ability to protect against HCV infection is mixed, although most recent research supports a protective effect. Hagan et al.’s [Reference Hagan24] meta-analysis found that MMT did not reduce the risk of HCV infection. In contrast, White et al.’s community-based prospective cohort study of PWID in Sydney [Reference White10] found OST was independently protective against HCV infection, as did Nolan et al.’s much larger Canadian study [Reference Nolan11]. Our own data, collected in a different Australian jurisdiction but involving numbers of incident cases and person-years of observation similar to those in White et al.’s study, do not corroborate their results or the results of Nolan et al. Nevertheless, as Des Jarlais [Reference Des Jarlais25] pointed out, MMT provision is not a simple, stable concept, meaning variation in its effects on the health of recipients across different national and programmatic settings is inevitable.
Limitations
Our relatively small number of incident events meant we were unable to evaluate the independent effects of needle/syringe sharing and OST use on HCV infection adjusted for other factors (e.g. risk behaviours, age, gender) that may confound these associations. Furthermore, the small numbers yielded relatively large standard errors around incidence estimates, making inference about a true population rate for PWID less precise. Single-test participants (excluded from analysis) were significantly less likely to be receiving OST than multi-test participants, but differed little on all other measurements. Moreover, as both needle/syringe sharing and OST use measures were temporally defined in terms of past-month exposure only, our analyses were unable to estimate the effects of differing levels of prior sharing/use with respect to HCV incidence.
CONCLUSION
Our study supports previous evidence of greatly reduced HCV incidence in Australian PWID. Needle-sharing was significantly associated with higher incidence of naive HCV infection but not of reinfection; OST had a non-significant protective effect. Needle-sharing remains the crucial behaviour that harm reduction services must target to maintain the apparent decline in the Australian HCV epidemic.
ACKNOWLEDGEMENTS
We gratefully acknowledge our participants’ willingness to contribute to the research and the tireless work of the MIX research assistants in recruiting and interviewing participants.
MIX is funded through National Health and Medical Research Council (NHMRC) project grant 545 891, untied philanthropic contributions and the Centre for Research Excellence on Injecting Drug Use (CREIDU). The authors gratefully acknowledge the Victorian Operational Infrastructure Support Program's support for the Burnet Institute, and support for one research assistant from the Invergowrie Foundation. The National Drug Research Institute at Curtin University is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvement Grants Fund. P.M.D. and M.A.S. are recipients of NHMRC Senior Research and Career Development Fellowships respectively. P.G.H. is supported by a Curtin University Research Fellowship.
DECLARATION OF INTEREST
None.





