INTRODUCTION
There is a consensus among ecologists and environmental researchers that stringent management measures are needed in order to combat environmental degradation, which includes pollution, habitat fragmentation, climate change and spread of disease (Siddig et al. Reference Siddig, Ellison, Ochs, Villar-Leemand and Laub2016). Among various management procedures, biomonitoring has been suggested as the best procedure for the assessment of environmental condition at small or at the large scale (Spellerberg, Reference Spellerberg2005; Siddig et al. Reference Siddig, Ellison, Ochs, Villar-Leemand and Laub2016). Although different biological organisms have been utilized for the biomonitoring purpose, parasite–host systems have emerged as the new indicator system from last three decades and various environmental parasitologists have used them for impact assessment studies. The main focus of using parasite–host sentinel system has been to evaluate infection dynamics of parasites, alteration in diversity and accumulation capability in response to various stressors. For example, Palm (Reference Palm and Mehlhorn2011) successfully showed how parasite diversity of tropical grouper (Epinephelus fuscoguttatus) can be used as an indicator of environmental change in Indonesian waters, whereas Rückert et al. (Reference Rückert, Hagen, Yuniar and Palm2009) showed how parasite descriptors can be used to reflect environmental condition in Segara Anakan lagoon (Indonesia).
The interaction between different environmental pollutants and host–parasite system has been a central theme of research in last three decades, and there has been a great progress in understanding various aspects of host–parasite interaction in relation to various stresses. There are various possibilities when host, parasite and stressful environment interact with each other. The parasitism may show increase or decrease depending upon the type of stressor involved in the interaction. For example, parasitic infections upsurge under eutrophic water conditions, whereas parasitism might decrease under highly eutrophic condition, thus indicating the deleterious effect of enhanced eutrophication on the parasite infection (Zargar et al. Reference Zargar, Yousuf, Chishti and Ahmed2012c ). Generally, past research has shown mixed response against various stressors, with some showing positive effect, while others showing negative response. However, eutrophications (nutrient enrichment of water bodies) are considered to be suitable for the transmission and progression of many fish parasites (Blanar et al. Reference Blanar, Kelly, Munkittrick, Houlahan, Deborah, MacLatchy and Marcogliese2009; Zargar et al. Reference Zargar, Yousuf, Chishti, Ahmed, Bashir and Ahmed2012b ). Another important aspect is that the alteration in parasitic infection under stressful condition can either reduce health of fish or may have other negative effects. For example, the increased level of parasitism may further reduce the health condition of fish, which has been already weakened by contamination exposure. It is therefore important to understand such interaction associated with health status of endangered species (Morley et al. Reference Morley, Costa and Lewis2010). In addition, there is need to understand the overarching factors influencing parasite–host interactions under highly stressed aquatic ecosystem.
Among fish cestodes, Caryophyllaeid tapeworms have been central of focus due to their simple seasonality (Williams and Jones, Reference Williams and Jones1994) and their potential to act as pathogen of both farmed and wild fish (Kennedy, Reference Kennedy, Pike and Lewis1994; Oros et al. Reference Oros, Hanzelová and Scholz2004). Furthermore, investigations on the phylogeographical and molecular aspects of Caryophyllaeid cestode (Atractolytocestus huronensis) have been suggested to be necessary for the elucidation of invasive nature and other speciation mechanisms. However, very few attempts have been made to ascertain the relationship between Caryophyllaeid tapeworm and health condition of fish under highly stressed aquatic ecosystems. It is pertinent to mention that there is scanty literature where researchers have attempted to assess the within-lake variation of parasitic infection. However, Karvonen et al. (Reference Karvonen, Cheng and Valtonen2005) attempted to analyse the spatial and temporal variation in factors affecting the parasite fauna of perch (Perca fluviatilis) at different locations of the same lake and observed that the geographical distance within the same lake affects the similarity of parasites. The intra-lake variation in physicochemical features has been found to have an influence on the infection level of monogenean gill parasite in highly stressed lake (Zargar et al. Reference Zargar, Yousuf, Chishti and Ahmed2012c ). In addition, seasonal dynamics, total length and weight of the host have been found to influence the infection of Asian tapeworm in the hypereutrophic lake (Zargar et al. Reference Zargar, Chishti, Yousuf and Ahmed2012a ). However, none of the study has used integrative approach to elucidate the predictors responsible for the progression of infection and degradation of health status of fish under enhanced stressed conditions. Against this backdrop, we investigated the predictors of A. oreini infection in three fish species of genus Schizothorax across intra-lake eutrophic gradient and the effect of infection on the health traits of fish.
The main aims of this study were: (1) to analyse the effect of intra-lake pollution gradient on the infection pattern of the A. oreini; (2) to investigate how water quality affects the copepod density and consequently alters the infection pattern; and (3) to assess the indirect effect on the health status of fish as reflected by condition based indices. It was predicted that Adenoscolex oreini might show alteration in the infection level across intra eutrophic gradient and among fish species due to deterioration of water quality, planktonic community and host features. In addition we supposed that the copepod density, seasonality of infection and water quality are intimately related with each other and influence the fish health status.
MATERIALS AND METHODS
Study area
The Kashmir Valley is positioned between the northwest and southeast of the Himalaya (33°01′–35°00′N lat.; 73° 48′–75°30′E long.) at an elevation of ⩾1500 m. The Anchar Lake (34°01′N, 74°02′E), a hypereutrophic lentic water body located in the northwest side of Srinagar city, was selected for the present investigation (Table 1 and Fig. 1). The lake has been considered as monbasined with surface area of 6·6 km2.
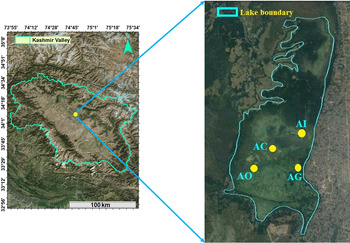
Fig. 1. Map of the study sites in the Anchar lake.
Table 1. Brief description of investigated lake of northwest Himalayan Region

a Khanday et al. (Reference Khanday, Yousuf, Reshi, Rashid, Jehangir and Romshoo2016).
b Shah et al. (Reference Shah, Yousuf, Chisti and Ahmad2013).
c Zargar et al. (Reference Zargar, Yousuf, Chishti and Ahmed2012c ).
The temperature in the month of January (coldest month) and July (warmest) ranges between −2 to 3 °C and 34–35 °C, respectively. The lake is fed by river Sind and also by number of springs present in its basin itself and along its periphery. The major portion of the lake is occupied by different types of submerged and free-floating macrophytes. The lake possesses about 1·69 km2 as open water and rest of the lake has now changed into marshland (Khanday et al. Reference Khanday, Yousuf, Reshi, Rashid, Jehangir and Romshoo2016). In addition to influx of household wastage from human habitations, the lake has been further reduced due to illegal encroachments by local population. The maximum length, breadth and depth are 3·55, 0·65 km and 3·5 m, respectively. Four sites were selected for the present study – Anchar Inlet (AI, 34°08′–89°07′N and 74°47–69°2; Elevation, 1596), Anchar Centre (AC, 34°8–72°5 and 74°47′–43°7; Elevation 1594), Anchar Ghat (AG, 34°8′–75° and 74°47′–63°4′; Elevation 1599), Anchar Outlet (AI, 34°08′–43°8′ and 74°46′–42°8′, Elevation, 1589).
Water sampling
The water quality of the Lake was carried out as per the international standards (CSIR, 1974; Mackereth et al. Reference Mackereth, Heron and Talling1978; APHA, 2005). The analysis of some of the parameters including water temperature, transparency and conductivity were conducted on the spot and rest of the physicochemical features were assessed in the laboratory. For estimation of dissolved oxygen (DO), water samples were fixed on the sampling spot and were then analysed in the lab as per azide modification of the Winkler method (APHA, 2005). The other measurements of water quality were assessed by using the methods/equipments as prescribed by CSIR (1974), Mackereth et al. (Reference Mackereth, Heron and Talling1978) and CSIR (1974). The water temperature, transparency, pH and conductivity were measured by using the Celsius mercury thermometer calibrated up to 0·1 °C, Secchi disc, digital pH meter (Microprocessor pH System-1011E) and Systronics model 104 conductivity meter, respectively. Total hardness of the samples was analysed by the EDTA method (CSIR, 1974), while as total alkalinity and chloride content of water samples was estimated by using Mackereth et al. (Reference Mackereth, Heron and Talling1978). Ammonia-nitrogen (NH3-N) and nitrate-nitrogen (NO3-N) was estimated by using the phenate method (APHA, 2005) and salicylate method (CSIR, 1974).
Host, copepod and parasite collection
In total, 417 specimens including 142 individuals of Schizothorax niger, 138 individuals of Schizothorax curvifrons and 137 individuals of Schizothorax esocinus were examined. Fish samples, caught by fishermen by using traditional gears (nets), were carried to laboratory for further investigation, where morphometric indices such as total length (TL), standard length (SL), and body weight (BW) and sex for each fish specimen was estimated. The length–weight (L–W) relationship, condition factor (Fulton's index) and gonadosomatic index (GSI) were determined according to the following equations:
-
Length-weight relationship: W = aL b, (Le Cren's, Reference Le Cren1951)
-
Condition factor/Fulton′s index: K = W/L 3 × 105, (De Vlaming et al. Reference De Vlaming, Grossman and Chapman1982)
-
GSI = Gonad weight/body weight × 100. (De Vlaming et al. Reference De Vlaming, Grossman and Chapman1982)
Copepods were obtained from four study sites from the Anchar Lake by plankton net of 64 µm mesh size. The plankton copepods were quantified by sieveing 10 L of water through planktonic net with the help of Van Dorn water sampler at each sampling spot upto 100 m. The collected content was transferred to collection vial where it was fixed in buffered 4% formalin solution. The sample was carried to laboratory where it was concentrated to a known volume and shaken gently. The quantitative estimation of copepod was done using Sedgwick-Rafter cell with the dimensions of 50 × 20 × 1 mm3, following the method given in APHA (2005). Counting of planktonic copepods was carried out by taking 1 mL of collected sample in a Sedgwick-Rafter cell and the entire content were counted. The collected species were identified with the help of standard taxonomic works (Edmondson et al. Reference Edmondson, Anderson and Peterson1956) and Battish (Reference Battish1992). The copepod densities (as individuals per litre) were estimated as per Wanganeo and Wanganeo (Reference Wanganeo and Wanganeo2006) with some modification.
where, C = the number of copepods recorded; A = the area of field of microscope; D = the depth of field (SRC depth) in mm; E = the number of fields counted
The fish samples were examined for the presence of A. oreini by using the method of Ergens and Lom (Reference Ergens and Lom1970). The collected caryophylledian tapeworms were immediately killed in as flash containing saline at 60–80 °C. The tapeworms were then shaken for 20–30 s. The fixation of the scolex was carried out separately by placing it on a clean glass slide and then placing a clean cover slip over it (Carnoy's fixative; cf. Weesner, Reference Weesner1968). The remaining body of cetode was fixed by keeping it between two slides for 5–10 min, while as comparatively thicker samples were kept in carnoy's fixative for longer duration. The specimens were finally transferred from carnoy's fixative to the 70% alcohol in order to remove excess of fixative. All the collected specimens were then fixed in the mixture of alcohol and glycerol (70% alcohol with 5% glycerol).
Data analysis
The infection descriptors [prevalence, mean intensity (MI) and mean abundance (MA)] were estimated by Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) and Margolis et al. (Reference Margolis, Esch, Holmes, Kuris and Schad1982). Inter-species and within-lake differences in prevalence were statistically tested by the Chi-square (χ 2) test. Differences in MI and MA across sampling sites and host species were tested by one-way ANOVA. Seasonal difference in prevalence was assessed by the χ 2 test, whereas MI and MA were statistically tested by one-way ANOVA. Correlation analysis was carried out between different water quality parameters and prevalence (%) and between tapeworm abundance and plankton abundance. The χ 2 test was used to test the effect of body length, sex and condition factor on the prevalence of A. oreini. Generalized Linear Model (GLM) was used to show the effect of site, month and length on the intensity of A. oreini and copepod abundance. Multiple comparison test [Tukey HSD (honest significant differences)] was carried out wherever needed after it was clear that there is overall significant difference. The regression statistics and curves were used for the comparison of L–W relationship among different sites and the slopes of log-transformed data [log (X + 1) was used to deal with zero and negative values] related to parasitic burden by Pearson's correlation. To test for an effect of MI on health indicators as reflected by GSI and condition factor, infected fish were tested for differences in slopes and intercepts.
RESULTS
Physicochemical features of water
The overall variation of various physicochemical features along with average (±s.d.) is presented in Table 1. From the data it is evident that physicochemical features varied significantly (P < 0·05) across study sites. The results showed that this lake has attained highly eutrophic condition. Previous researchers have found that heavy nutrient loading from the municipal drains, household and agricultural waste has drastically deteriorated the water quality of this lake and therefore it has been categorised as the Hypereutrophic lake (Pandit and Yousuf, Reference Pandit and Yousuf2002). The data from the present study also confirm their findings. In addition, the physicochemical features also showed intra-lake eutrophic gradient. The site close to human habitation showed significantly (P < 0·05) low water quality as compared with site near inlet (AI), which depicted pristine conditions. As per the International standards, AI, Anchar Centre and Anchar Ghat were categorized as least eutrophic, eutrophic and hypertrophic sites, respectively. Anchar outlet showed same trophic condition as that of Anchar Centre (Table 1). Overall, the lake shows signs of accelerated rate of cultural eutrophication due to the enhanced stress and strain of anthropogenic interference.
Variation of A. oreini infection among different hosts
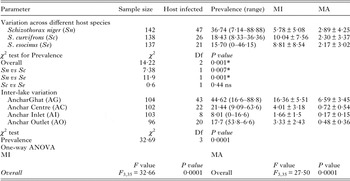
Significant variation in the A. oreini infection across different fish species was observed during the present study with highest prevalence recorded in S. niger (% = 36·74, χ 2 = 14·22, P = 0·0001) and least in S. esocinus (% = 15·7). The prevalence among three different fish species when compared statistically revealed significant differences between S. niger vs S. curvifrons (P = 0·007) and S. niger vs S. esocinus (P = 0·0001), while insignificant variation was observed between S. curvifrons vs S. esocinus (P = 0·44) (Table 2).
Table 2. Intra-lake variation of physicochemical features of water in Anchar Lake
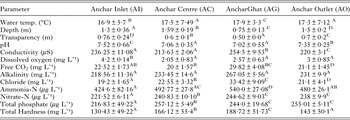
Similar superscript within the same row designates the insignificant differences
Intra-lake variation of A. oreini infection
The overall prevalence of A. oreini in three species was significantly higher in Anchar Ghat (AG: % = 44·62, χ 2 = 32·69, P = 0·0001) compared with Anchar Centre (AC: % = 21·44) and Anchar Inlet (AI: % = 8·01). The hypereutrophic site, i.e. AG showed significantly (P = 0·0001) greater MI (16·36 ± 5·51) and MA (6·59 ± 3·45; Table 3). The hypertrophic site further showed significant variation in MI and MA when compared with eutrophic and least eutrophic sites (AG vs AC, P = 0·0001; AG vs AI, P = 0·0001; AC vs AI, P = 0·4; AO vs AG, P = 0·0001), whereas eutrophic and least eutrophic sites showed insignificant variation (AG vs AC, P = 0·0001; AG vs AI, P = 0·0001; AC vs AI, P = 0·0001; AC vs AI, P = 0·83 and AO vs AG, P = 0·0001).
Table 3. Variation of infection with reference to host species and study site. Comparative statistical analysis (χ 2and one-way ANOVA) is also given
Seasonal variation of infection and copepod density
The infection indices (Prevalence, MI and MA) showed clear monthly and seasonal pattern with the maximum infection level in summer months and least in winter months. All three fish species showed significant (P < 0·05) seasonality in prevalence across different seasons. MI and MA also showed similar trend across different months and seasons, except in S. esocinus, which showed insignificant difference between different seasons (Table 4). All the infection indices (Prevalence, MI and MA) peaked from May to August and showed decline from that onwards.
Table 4. Seasonal alteration of A. oreini in three fish species and copepod density in Anchar lake

Correlation between water quality, prevalence of cestode and copepod density
The percentage (prevalence) of infection showed significant positive correlation (for prevalence in Schizothrorax niger, rs = 0·85, P < 0·01; For S. curvifrons, rs = 0·78, P < 0·01; for S. esocinus, rs = 0·69, P < 0·05) with the water temperature in all three fish species (Fig. 2). Only two species of copepods were observed during the present study. The density of Cyclops scutifer depicted significant correlation with water temperature (rs = 0·78, P < 0·01) and with prevalence of infection (rs = 0·69, P < 0·05 & rs = 0·64, P < 0·05), whilst Eucyclops agilis showed insignificant correlation with water temperature (rs = 0·49, P > 0·05) and with prevalence of infection (rs = 0·55, P > 0·05).
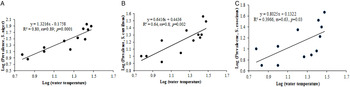
Fig. 2. Scatter plot between water temperature and prevalence: (A) Schizothorax niger, (B) S. curvifrons, (C) S. esocinus.
Influence of host length and sex on A. oreini
Different length groups showed insignificant variation (P > 0·05) in cestode infection. The prevalence insignificantly increased (P > 0·05) from lower length group (% = 15) to higher length group in S. curvifrons (% = 35, χ 2 = 1·57, P = 0·66), whereas it showed first increased prevalence in S. niger and then prevalence decreased (χ 2 = 3·33, P = 0·34). The case with S. esocinus, however, was different because it showed same trend in prevalence in first three length groups and then it increased (χ 2 = 3·75, P = 0·29).
Sex of host significantly (χ 2 = 8·77, P = 0·003) influenced prevalence in S. niger as infection level was high in females (Prevalence = 50%) than in males (Prevalence = 25·71%). Similar trend was observed in S. curvifrons with greater infection observed in females (26·66%, χ 2 = 3·48, P = 0·062, tendency towards significance). The prevalence, however, showed insignificant variation in infection (χ 2 = 0·001, P = 0·97).
Generalized Linear Model analysis
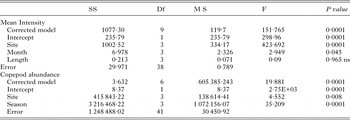
GLM analysis clearly shows that the site (F = 423·69, P = 0·001 for MI; F = 4·44, P = 0·008 for copepod abundance) and the month (F = 2·95, P = 0·045 for MI; F = 35·2, P = 0·0001) are the important factors, which influence the cestode infection (Table 5).
Table 5. Generalized Linear Model showing the effect of site, month and length on the intensity of A. oreini and abundance of copepod
Effect of A. oreini on the health status of fish
GSI was significantly low (P < 0·05) in the parasitized fish at all sampling sites and also showed variation across the site with significantly high GSI at AI (Table 6). The qualitative health indicator, i.e. condition factor (k) was significantly higher in uninfected fishes compared with infected fishes in S. niger and S. curvifrons (t-test = 5·6, P < 0·0001 for S. niger and t-test = 3·82, P < 0·003), whereas condition factor (k) was insignificantly high in uninfected fishes compared with infected fishes in S. esocinus (t test = 0·74, P = 0·47). Linear regressions showed that fish health as reflected by GSI was significantly (P < 0·05) predicted by infection intensity (Table 6 and Fig. 2). The coefficient (slope) from the L–W regressions of infected fishes when compared among different study sites showed significant variation (F 3,8 = 4·9, P = 0·03, Table 7, Fig. 3). In addition, the slope from the L–W regression was negatively related with parasite burden (P < 0·05, Table 8; Fig. 4).
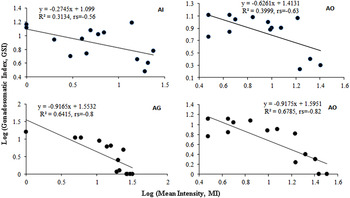
Fig. 3. Regression models showing the correlation between log (mean intensity) and log (GSI) in S. niger at Anchar Inlet (AI), Anchar Centre (AC), Anchar Ghat (AG) and Anchar Outlet (AO).
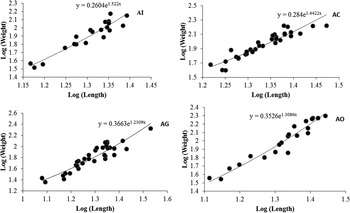
Fig. 4. Regression models showing the correlation between Log (Mean Intensity) and log (weight) in infected fish (pooled) at Anchar Inlet (AI), Anchar Centre (AC), Anchar Ghat (AG) and Anchar Outlet (AO).
Table 6. Variation of Gonadasomic index and condition factor in uninfected and infected fish in two native fish across four study sites
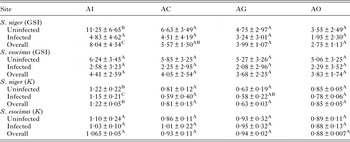
Different superscript in the same row shows significant differences
Table 7. Regression statistics of length–weight relationship (uninfected and infected fish) across different study sites
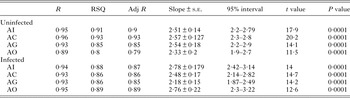
Table 8. Correlation between parasite burden and slope of regression (length–weight relationship)
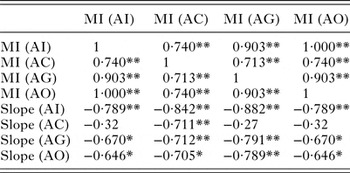
**Correlation is significant at the 0·01 level (2-tailed).
*Correlation is significant at the 0·05 level (2-tailed).
DISCUSSION
Variation of A. oreini infection among three native fish species
The study revealed significant variation of the infection level of A. oreini across different species of genus Schizothorax possibly due to varied type of feeding behaviour/food preferences in hosts. The role of feeding behaviour on the occurrence of parasitism in fish is well documented in the literature. The role of foraging behaviour, for example, has been reported as an important determinant of parasite communities (Knudsen et al. Reference Knudsen, Curtis and Kristoffersen2004; Valtonen et al. Reference Valtonen, Marcogliese and Julkunen2010). The feeding behaviour of different species of genus Schizothorax, which have been studies in past, revealed that these fishes are herbivores, and their diet is mainly composed of phytoplankton and rest contained aquatic invertebrates (Jan and Das, Reference Jan and Das1970). The variation in the uptake of intermediate hosts by the individuals of different species may also account for species-specific variation in the cestode infection. In addition, the influence of anthropogenic eutrophication on the food availability could also affect the dynamics of the infection in different fish species (Budria and Candolin, Reference Budria and Candolin2015).
The habitat preference and restriction of lacustrine fish, S. niger in lentic water bodies favour more cestode infection, whereas better physiological and immunological condition of the S. esocinus may result in low prevalence of tapeworm. The past studies have revealed that parasitic resistance due to specific and non-specific immune responses also influences the infection pattern (Karvonen et al. Reference Karvonen, Paukku, Valtonen and Hudson2003). However, it is early to say whether there exists any relationship between infection pattern of three native species and immune response. Moreover, an immunological study with regard to A. oreini of fish has not been carried out on large scale and the lack of information on this aspect calls for further investigation.
The effect of environmental change in the Anchar Lake may be also responsible for the above variation due to direct impact of deteriorated environment on host and parasite through altered physiological requirements (Brunner and Eizaguirre, Reference Brunner and Eizaguirre2016). The alteration in host behaviour (such as mate preference) (Budria and Candolin, Reference Budria and Candolin2015) and impairment in the host defences due to insufficient feeding behaviour have been also reported to be influenced by eutrophication (Brunner et al. Reference Brunner, Schmid-Hempel and Barribeau2014).
Intra-lake variation of A. oreini infection
The higher prevalence at the hypereutrophic site (Anchar Ghat) is due to the effect of highly stressful condition that weakens the immune system of host present there, and thus fish becomes safe haven for the Caryophyllied tapeworm to colonize. The enhanced eutrophic condition at this site may favour intermediate hosts as it has been stated that some invertebrates are favoured or show increased abundance under eutrophic conditions and consequently change the parasite history (McKenzie and Townsend, Reference McKenzie and Townsend2007). The high intensity of infection at highly eutrophic site in the present study could be due to inflow of municipal as well as other effluents released from the SKIMS, the only tertiary-care hospital in Kashmir (Bhat et al. Reference Bhat, Rather and Pandit2001). Though municipal effluents have shown increase in prevalence in the myxozoan parasites of Notropis hudsonis (Marcogliese et al. Reference Marcogliese, Gendron and Cone2009) there is scanty study on cestodes in general and Caryophyllaeid tapeworms in particular which show any relationship between municipal effluent and infection parameters. The results further revealed insignificant variation in the cestode infection between eutrophic and least eutrophic sites, which depicts that host in these locations are immunologically resistant to cestode infection. In previous studies, within-lake dynamics in fish parasites have shown considerable variation in the infection levels across different study sites. Karvonen et al. (Reference Karvonen, Cheng and Valtonen2005) explained that the similarity of the parasite fauna is affected by reproductive behaviour, especially spawning period of host population and further demonstrated that geographical distance affected helminths across locations negatively.
Monthly and seasonal variations of infection
The results clearly showed seasonality in the infection level and further depicted that warmer months are suitable for the progression of A. oreini, which is likely due to the reduced generation time, resulting in the increased abundance of tapeworms in a shorter period. Previous studies have shown the importance of water temperature on different life stages of parasites vis-a-vis growth and progression (Brouder and Hoffnagle, Reference Brouder and Hoffnagle1997).
It is also clear from the empirical data that summer months are the most suitable for the occurrence of A. oreini, which is due to feasible environmental conditions for this particular parasite. As per climatic records of Kashmir valley, the July month (mean maximum temperature 32 °C) is the hottest of the all months and it can be safely argued that A. oreini is adapted in higher range of temperature. The maturation of the A. oreini is seasonal and recruitment of the parasite has been reported in autumn (Dhar and Peerzada, Reference Dhar and Peerzada1989). The seasonality of A. oreini as reflected by the present study deviate from the past studies as it has been reported that some Caryophyllaeid tapeworms have two peaks of infection (one in spring and another in autumn, Milbrink, Reference Milbrink1975). The lack of seasonality has been also attributed to the survival ability of Caryophyllaeus laticeps larvae in all months (Kulakovskaya, Reference Kulakovskaya1964). Furthermore, it has been earlier reported by Kennedy (Reference Kennedy1969) that highest infection for C. Laticeps was observed during April and May and declined in July. Inconsistencies prevailing in different studies with regard to monthly and seasonal variation of caryophylledians could be due to variation in the availability of infective stage (Kennedy, Reference Kennedy1969). In addition, fish hormone levels are also predicted to influence the maturation of the infective stage of caryophylledians (Williams and Jones, Reference Williams and Jones1994).
The MI and abundance was significantly higher during summer and lower in winter in all the three fish species showing that seasonality pattern is almost similar. The upsurge of intermediate hosts during the summer months may be primary cause for the highest prevalence of A. oreini infection in our study, which is well supported by the findings of Bertasso and Avenant-Oldewage (Reference Bertasso and Avenant-Oldewage2005).
Correlation between water quality, prevalence of cestode and copepod density
The A. oreini showed significant variation in infection level in all the three fish species with respect to water quality. It is further evident from the results that infection level of A. oreini increased with the increase of temperature, which coincides with the increased abundance of the intermediate host at the peak of eutrophic conditions (Beer and German, Reference Beer and German1993). Similar types of observations were observed by Zargar et al. (Reference Zargar, Chishti, Yousuf and Ahmed2012a ), while working on the effect of water quality on the Asian tape worm in the Anchar Lake. As is evident from the data and from earlier reports (Zargar et al. Reference Zargar, Chishti, Yousuf and Ahmed2012a ) that eutrophication level in Anchar Lake reaches to peak level during summer months; such conditions are feasible for the growth and progression of the intermediate hosts and finally results in the larval transmission (Sandland and Goater, Reference Sandland and Goater2000). The temporal and spatial pattern of parasites including helminths has been found to be affected by various anthropogenic stresses (Zargar et al. Reference Zargar, Yousuf, Chishti, Ahmed, Bashir and Ahmed2012b ; Shah et al. Reference Shah, Yousuf, Chisti and Ahmad2013; Budria and Candolin, Reference Budria and Candolin2014, Reference Budria and Candolin2015). Recent study has shown that complex interactions between water temperature and biotic factors determine the occurrence of parasite fauna, including trematodes and cestodes (Karvonen et al. Reference Karvonen, Bjarni, Kristjansson, Skulason, Lanki, Rellstab and Jokela2013).
The result depicts that copepod density, especially C. scutifer significantly correlated with the infection of A. oreini and water quality. Planktonic copepods have showed their association with the cestode infection in past. Marcogliese and Esch (Reference Marcogliese and Esch1989a , Reference Marcogliese and Esch b ), for example, revealed that planktonic cyclopoid species proved susceptible to the Asian tapeworm, while as benthic copepods were not susceptible to the infection. The low species diversity of copepods in the present study is in conformity with the recent study that indicate that copepod fauna decrease in lentic water bodies of Kashmir due to increase in the nutrient enrichment (Ping et al. Reference Ping, Yan, Man and Takamura1996; Bhat, Reference Bhat2012). A study carried out by Bhat (Reference Bhat2012) have revealed decrease in copepod species richness from seven recorded in 1980s to four in Dal Lake, which is currently under eutrophic condition. The current study is, however, inconsistent with the findings of Malley et al. (Reference Malley, Chang, Findlay and Linsey1988) who revealed that cyclopoids are able to withstand stressful conditions. The availability of planktonic communities show association with trophic state of water bodies and are important for the progression of the life cycle of cestodes. While oligotrophic condition of lakes is favourable for the occurrence of copepods, eutrophic state reduces the population of copepods (Molzen, Reference Molzen2005). There is paucity of information with regard to the ecology and association of the natural intermediate host of A. oreini with the water quality, and therefore further studies are suggested in future.
Influence of host length and sex on A. oreini
The total length was insignificantly related with the infection of A. oreini. Various studies have shown length to be an important factor, which influence helminth occurrence in general and tapeworms in particular. It has been also demonstrated that various factors, especially ageing and surface area of fish have an impact on the helminth accumulation in fish (Koskivaara, Reference Koskivaara1992; Brouder, Reference Brouder1999; Tekin-Ozan et al. Reference Tekin-Ozan, Kir and Barlas2008).
Results showed more infection burden (MI) of A. oreini in female hosts of S. niger and S. curvifrons compared with their male counter parts, which could be due to the host hormone level, feeding behaviour and favourable environment for colonization. The study on the Caryophyllaied tapeworm, C. laticeps by Kennedy (Reference Kennedy1968) stated that tapeworm infection was more in females and confirmed that host hormone levels play a key role in the seasonality of tapeworm. It can be speculated that the testosterone immune-suppression, corticosteroid-based immune suppression and differences between the size and behaviour of the sexes may be reason behind the greater infection level in females than in males (Thomas, Reference Thomas2003).
The insignificant effect of sex on the S. esocinus shows that water quality and seasonality have more effect on the A. oreini than host sex. The feeding habits in female and male may mask the effect of sex on the intensity of A. oreini. Unequal distribution of parasitic infection between male and female have been reported in various studies and is still matter of debate among the parasitologists (Williams and Jones, Reference Williams and Jones1994).
Effect of A. oreini on the health traits of fish
The data from the present study indicate low GSI, which is the reflection of possible impacts of infection on the reproduction investment of fish. Comparatively high GSI at AI in S. niger both in infected and uninfected fish shows that low stress (here eutrophication) favours reproductive processes positively, whereas enhanced stress may have detrimental effect on reproductive index (Schreck, Reference Schreck2010). It has been reported that contaminants affect fish reproduction in several ways (Bieniarz et al. Reference Bieniarz, Epler and Sokolowska-Mikolajczyk1997). It is pertinent to mention that the GSI represents the measure of reproductive potential of fish. The GSI in the present investigation was low as compared with the previous studies, which are possibly due to deterioration of environmental factors of the lake over the past few decades. The significant decrease in reproductive trait across study sites and among different fish species suggests physical and biological transport of the contaminants in the lake.
The condition factor was significantly higher in uninfected hosts as compared with infected hosts in S. niger and S. curvifrons. The infection can decrease the immunity as well as growth of fish, which finally leads to decrease in health condition of a fish (Khan and Thulin, Reference Khan and Thulin1991; Poulin, Reference Poulin1992). Although, few reports have demonstrated the enhanced growth and greater condition coefficient due to parasitic burden (Megan and Bean, Reference Megan and Bean2008; Ondrackova et al. Reference Ondrackova, Davidova, Blazek, Gelnar and Jurajda2009), majority are of the opinion that feeding habits and type of food may enhance the condition coefficient (Ondrackova et al. Reference Ondrackova, Davidova, Blazek, Gelnar and Jurajda2009; Polacik et al. Reference Polacik, Janac, Jurajda, Adamek, Ondrackova, Trichkova and Vassilev2009). It has been argued that healthy and well-conditioned fish have capability to acquire more infection because they accumulate more food and remain in those habits where exchange of parasite is possible (Thomas, Reference Thomas2003). Enhanced somatic growth in host can be also influenced by the release of factors through the stimulation host's immune and endrocrinological system (Thomas, Reference Thomas2003). The significant variation in slope (from the L–W regression equation) of infected fishes shows that A. oreini infection and pollution have marked relationship with size and growth of fish and leads to stunted growth thereby having the negative effect on L–W relationship (Esiest, Reference Esiest2013). Although literature shows that pollution and parasitism has an impact on L–W relationship, there are other factors, including endocrine system, which regulate morphometric indices.
CONCLUSIONS
It can concluded from the results that infection pattern of tapeworm is determined by water quality, host species, copepod density and host biological feature. The results indicate that there exists a significant correlation between copepod density especially C. scutifer with the infection parameters, suggesting that the copepods in conjunction with lake features play an important role in determining the infection pattern of A. oreini. The results further reveal that the effect of altered water quality and parasite affect the health status of native fish as reflected by GSI and condition factor. This investigation will entice more research into additional aspects of different Caryophyllaeid tapeworms and will be useful for fisheries resource managers to prevent the damage posed by this caryophyllied infection on endemic fish fauna, which is currently under threat.
ACKNOWLEDGEMENTS
We acknowledge and appreciate the help and encouragement received from Professor Mohammad Aslam, Professor and Director, Research and Development, Central University of Kashmir. We also appreciate the kind support in the form laboratory facility from the former Director CORD, Professor Bashir Ahmad Ganai.
FINANCIAL SUPPORT
The first author, Ummer Rashid Zargar, received financial support from the Department of Science and Technology (SERB-DST), Govt. of India, in the form of a Fast Track Young Scientist Fellowship (Order No: YSS/2014/000845).














