Introduction
Global biodiversity has decreased at an alarming rate over the past 40 years (Butchart et al. Reference Butchart, Walpole, Collen, van Strien, Scharlemann, Almond and Bruno2010, Hoffmann et al. Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart and Cox2010) with the main declines occurring primarily in tropical areas where most threatened vertebrates are found (Hoffmann et al. Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart and Cox2010). In South-East Asia, extinction risk has increased markedly (Hoffmann et al. Reference Hoffmann, Hilton-Taylor, Angulo, Böhm, Brooks, Butchart and Cox2010, Duckworth et al. Reference Duckworth, Batters, Belant, Bennett, Brunner, Burton and Harris2012) due to anthropogenic activities, habitat loss and overexploitation (Bennett Reference Bennett2002, Cardillo et al. Reference Cardillo, Mace, Gittleman and Purvis2006). These pressures continue to increase (Butchart et al. Reference Butchart, Walpole, Collen, van Strien, Scharlemann, Almond and Bruno2010) as the rate of deforestation in the region is highest among tropical regions (Achard et al. Reference Achard, Eva, Stibig, Mayaux, Gallego, Richards and Malingreau2002, Sodhi and Brook Reference Sodhi and Brook2006), mainly affecting lowland areas (Sodhi et al. Reference Sodhi, Posa, Lee, Bickford, Koh and Brook2010). As a consequence, recent predictions estimate that nearly 50% of the regional mammal populations and 32% of bird populations will be extinct by the end of this century (Brook et al. Reference Brook, Sodhi and Ng2003), and at least half of this proportion could represent global extinctions, and the number could be higher due to other threats such as climate change and invasive species (Brook et al. Reference Brook, Sodhi and Ng2003, Laurance Reference Laurance2006). Besides habitat loss, mainly resulting from illegal logging and agricultural expansion for large-scale commercial products (Ziegler et al. Reference Ziegler, Fox and Xu2009), overexploitation of animal species for food, medicine, pest control, trophies, tonics, etc. has also contributed significantly to this rapid decline (Corlett Reference Corlett2007, Duckworth et al. Reference Duckworth, Batters, Belant, Bennett, Brunner, Burton and Harris2012).
Deforestation, and consequent habitat fragmentation, lead to reductions in the amount of suitable habitat for forest-dependent species (Forman Reference Forman1995), increasing edge effects for wildlife (Beier et al. Reference Beier, Van Drielen and Kankam2002), and decreasing abundance, as well as increasing risk of extinction (Laurance and Bierregaard Reference Laurance and Bierregaard1997). Animals occupying degraded forests may face difficulties such as physical environmental change (Saunders et al. Reference Saunders, Hobbs and Margules1991, Murcia Reference Murcia1995, Gascon et al. Reference Gascon, Williamson and da Fonseca2000), shortage of food and shelter, and introduced species (Schwitzer et al. Reference Schwitzer, Glatt, Nekaris and Ganzhorn2011). To survive, various species with different natural history traits have different abilities to respond to these threats (Henle et al. Reference Henle, Davies, Kleyer, Margules and Settele2004, Cardillo et al. Reference Cardillo, Mace, Gittleman and Purvis2006). For example, some species adapt well to logged forest (Brodie et al. Reference Brodie, Giordano and Ambu2015) but others which rely heavily on intact primary forests are absent from altered habitat and small patches of primary habitat (Barlow et al. Reference Barlow, Mestre, Gardner and Peres2007, Danielsen et al. Reference Danielsen, Beukema, Burgess, Parish, Bruehl, Donald and Struebig2009). The response of galliform species to these impacts varies across the group. Most galliforms cannot persist after conversion of forest to other land-use types (e.g. agriculture) but they can tolerate degraded habitats such as secondary or logged forests, and farmland edges (Brickle et al. Reference Brickle, Duckworth, Tordoff, Poole, Timmins and McGowan2008, BirdLife International 2015).
Currently 25% of galliform species appear to be at risk of extinction (Hilton-Taylor et al. Reference Hilton-Taylor, Pollock, Chanson, Butchart, Oldfield, Katariya, Vié, Hilton-Taylor and Stuart2009). South-East Asia is part of the Indo-Burma global hotspot (Myers et al. Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000), harbouring a number of species in the order Galliformes, higher than those in any other part of the world (BirdLife International 2015). In this region, there are 54 species of Phasianidae (excluding introduced species), including one listed as ‘Critically Endangered’ on the IUCN Red List (Edwards’s Pheasant Lophura edwardsi), two listed as ‘Endangered’, (Green Peafowl Pavo muticus and Bornean Peacock-pheasant Polyplectron schleiermacheri) and 10 others listed as ‘Vulnerable’ (BirdLife International 2015). In addition, three main genera of Phasianidae, Lophura, Arborophila, and Polyplectron, are restricted to South-East Asia, but their ecology and conservation status are poorly known (Grainger et al. in review).
In this study, we focus on Germain’s Peacock Pheasant Polyplectron germaini, hereafter GPP, and Orange-necked Partridge Arborophila davidi, hereafter ONP, dwelling in lowland forest in southern Vietnam and a part of eastern Cambodia. Both are ‘Near Threatened’ (NT) species as there is little to suggest that the overall population of the GPP is in decline and the ONP is probably tolerant to some degree of forest degradation, though it is supposed to have decreased due to clearance within its very small range (BirdLife International 2015). However, other galliforms in south-central Vietnam have severely declined over the past decades even when inhabiting national parks (Sukumal et al. Reference Sukumal, McGowan and Savini2015). The ONP is resident in forest and foothills at an elevation range of 120–600 m, and the GPP resides in forest from sea level up to 1,500 m (BirdLife International 2015). The ONP and GPP are relatively common in some protected areas, especially in Cat Tien National Park. This makes them excellent focal species for quantitatively accessing the effect of habitat degradation on galliform species. In addition, the species’ narrow geographical ranges fall within lowland forest, which has significantly contracted over the last several decades both in southern Vietnam (Wege et al. Reference Wege, Long, Vinh, Dung and Eames1999, Sterling et al. Reference Sterling, Hurley and Minh2006) and eastern Cambodia (Poole and Duckworth Reference Poole and Duckworth2005) putting the species at a higher risk of extinction.
The objectives of this research were: (1) to estimate the density of the GPP and ONP in suitable remaining habitats, (2) to assess the species current and historical distribution, (3) to evaluate the reduction of distribution ranges of the species as a surrogate for estimating population declines and (4) to reassess Red List categories and criteria of ONP and GPP.
Methods
Study areas
The data were gathered in protected areas: (1) Bu Gia Map and (2) Cat Tien National Parks (NP), and (3) Vinh Cuu and (4) Tan Phu Nature Reserves (NR) in southern Vietnam (Figure 1a). Bu Gia Map National Park (1205’–12020’N, 10703’–107012’E) is located east of the Cambodian border. Total area of the park is 259 km2. The main forest types in the park are evergreen (40%), mosaic (50%), and plantation (2%). The elevation ranges from 200 to 950 m above sea level. Cat Tien National Park (11020’–11050’N, 107009’–107035’E) has a total area of 720 km2 divided in two separate sectors, north and south. The topography differs between these two sectors. In the south, the elevation ranges from 110 to 300 m, including isolated hills (200–300 m) located along the Dong Nai River in the east of this sector. The north sector lies at elevations of 300–670 m. The vegetation of the park comprises various types including evergreen forest (52%), mixed deciduous forest (11%), bamboo (1%), and mosaic forest consisting of patches of the three previous forest types (26%). Total area of the remaining land cover types of the park including wetlands, grasslands, and agricultural land is 10%. The first four vegetation types were said to contain populations of the GPP and ONP (Vy et al. Reference Vy, Savini and Carroll2014, BirdLife International 2015). Vinh Cuu Nature Reserve (11005’–11030’N, 106010’–107012’E) has a total area of 1,003 km2, including evergreen (45%), mosaic forest (15%), plantation (10%), and wetland (30%). The elevation ranges from 60 to 300 m. Tan Phu Special Use Forest (11002’–11010’N, 107022’–107027’E) has a total area of 141 km2 and an elevation range from 80 to 250 m. The dominant forest types in Tan Phu are evergreen forest (85%), mosaic forest (5%), and plantation (10%). All these sites are in the north Mekong region lying between elevations of a few meters above sea level to 710 m (Sterling et al. Reference Sterling, Hurley and Minh2006).
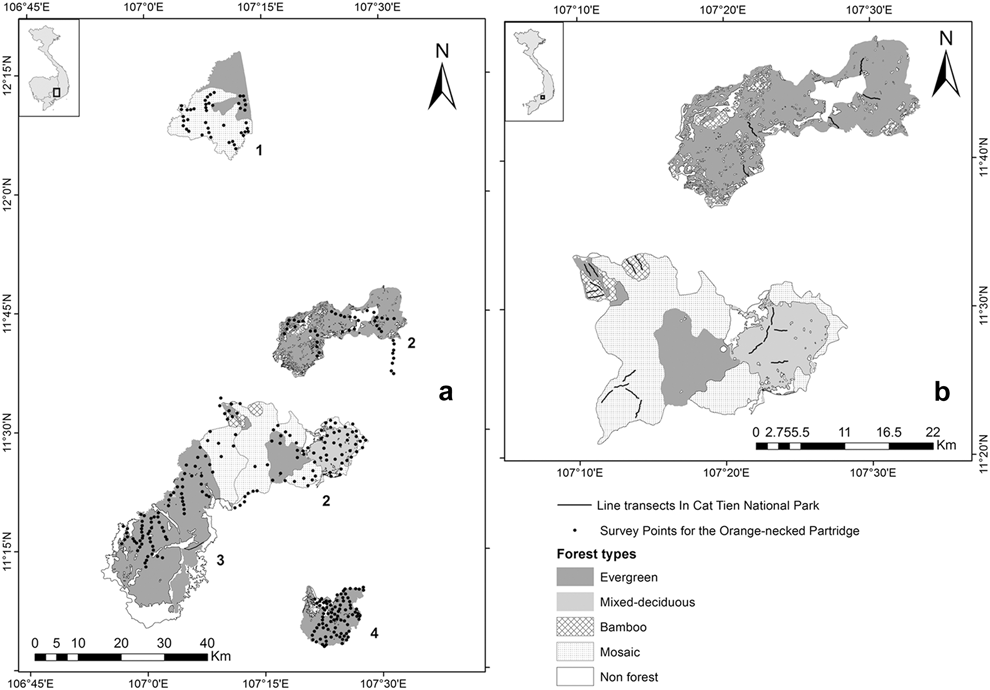
Figure 1. Study sites for Orange-necked Partridge and Germain’s Peacock Pheasant: (a) (1) Bu Gia Map National Park (NP), (2) Cat Tien NP, (3) Vinh Cuu Nature Reserve (NR), and (4) Tan Phu NR; (b) Map of Cat Tien NP with five transects each in Bamboo, mixed-deciduous, and mosaic forests, and seven transects in evergreen forest.
Density estimation
Surveys were carried out in Cat Tien NP during the breeding season (February–May 2014) when the number of calls were expected to be highest (Johnsgard Reference Johnsgard1999). The surveys were conducted using line transects, based on the existing ranger patrolling trails. In each forest type we set four 2-km transects and one 1.5-km transect, except in evergreen forest which had six 2-km transects and one 1.5-km transect (Figure 1b). The surveys were implemented twice daily, in the morning (06h00–08h00) and in the afternoon (16h00–18h00) by one surveyor per transect for three consecutive days at a speed of 1 km per hour. Birds were detected both aurally and visually. To minimise differences between surveyors, prior to data collection, all surveyors undertook several surveys together in order to standardise distance estimations (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). Detected bird locations were based on bearings, distances and calling time, then subsequently mapped using ArcGIS 10.3 (ESRI, Redlands, USA). This also estimated the number of detected birds during each survey and eliminated potential double counts prior to the density estimation analysis.
Density estimation was based on distance sampling (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001). As most detections were from calling males, we excluded sighting detections from the density analysis because there were too few and the detection probability was different. Program DISTANCE 6.0 (Thomas et al. Reference Thomas, Buckland, Rexstad, Laake, Strindberg, Hedley and Burnham2010) was used to estimate the detection probability and density of GPP and ONP. Key functions uniform, half-normal, and hazard with cosine adjustments were used to run the analysis and the best models were selected based on the lowest Akaike’s information criterion (AIC) (Akaike Reference Akaike, Petrovand and Csàki1973). Because the number of detections per habitat were small for all but one habitat, we pooled all detections of a given species to generate a single per species global detection function following Buckland et al. (Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001) and then estimated density per habitat using the different detections per habitat. Finally, we selected the best models based on the coefficient of variance (CV) of each model (Buckland et al. Reference Buckland, Anderson, Burnham, Laake, Borchers and Thomas2001).
Habitat use by GPP and ONP
We examined the impacts of four environmental variables on habitat use by each species: elevation, slope, distance to water sources, and forest types. We derived these variables using ArcGIS10.3 (ESRI, Redlands, USA) from the following sources: (1) elevation and (2) slope were extracted from the ASTER GDEM at a scale of 30 x 30 m (Global Digital Elevation Model) downloaded from the Earth Remote Sensing Data Analysis Center (http://www.jspacesystems.or.jp/ersdac/GDEM/E/4.html), (3) river and stream system maps were sourced from the Ministry of Science and Technology, (4) forest types were classified from LANDSAT 8 (2015) using supervised classification (ESRI 2015), based on the extensive experiences of the author (VNT) who has worked in the areas for over 10 years, in ArcGIS 10.3 (ESRI, Redlands, USA). All data was re-projected to the WGS84 datum before analysis.
For German’s Peacock Pheasant habitat use, we analysed data collected during the 2014 survey in Cat Tien NP. Poisson regression was used to define the association between ecological variables and relative abundance (counts) of GPP (Table 1). Explanatory variables were elevation, slope, forest types, and distance to water sources. Habitat selection models were developed using 132 surveys from 22 transects and the number of calling birds from each survey was treated as the response variable. The models were performed as described below (see Regression models). A set of 14 Poisson regression models including the null model were developed to determine the association between the selected ecological variables and the relative abundance of the GPP.
Table 1. Summary data of Germain’s Peacock Pheasant (GPP) and Orange-necked Partridge (ONP). Data includes number of transects surveyed in 2014, number of point locations in 2000–2008, and environmental variables (means ± standard errors) including elevation (m), slope (degrees), distance to water (m) and forest type.
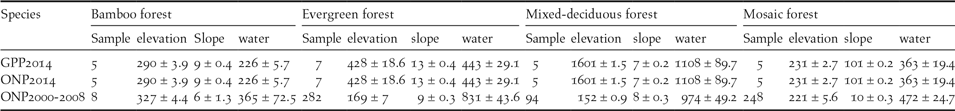
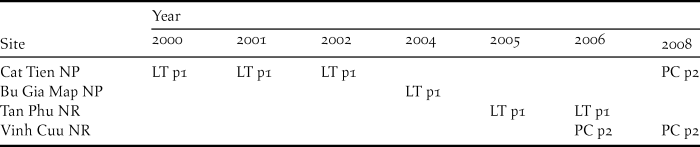
For Orange-necked Partridge habitat use, we analysed data from multiple datasets in order to increase the sample size. Data were collected during 2000–2008 (no surveys were conducted in 2003 and 2007) in four protected areas: Cat Tien and Bu Gia Map NPs, Vinh Cuu and Tan Phu NRs (Figure 1a). The surveys were conducted mainly during the dry season from January to May, which included the breeding season of the ONP (Fuller et al. Reference Fuller, Carroll and McGowan2000), from 06h00 to 10h00 and 15h00 to 18h00 daily. Data on the location of birds were collected using both point count and line transects (see Table 2 for details on when and where each method was used) where both visual and aural detections were recorded. To increase detection probability, we used playback in all our surveys. Line transects were walked along existing patrolling trails at a speed of 1 km per hour. Playback was opened continuously until the species was detected (Playback 1). As the ONP’s call can be heard from a distance of up to 120 m, after detecting the species, playback was stopped until we had moved approximately 300 m away from this last detection in order to avoid double counts. Point counts were conducted at fixed points spaced at 600 m along patrolling trails. At each point, we spent five minutes and used playback for the first two minutes (Playback 2). There was no playback between points. At each detection location, we generated a 300-m radius buffer for habitat measurement. We also defined 188 non-detection points, randomly generated along trails using ArcGIS, for comparison. Habitat selection models were developed using 641 locations (Playback 1: n = 302 locations and Playback 2: n = 339 locations). Each location was visited from one to three times; thus the total survey effort was 802 surveys for all surveyed sites from 2000 to 2008. We used multi-level logistic regression (Gelman and Hill Reference Gelman and Hill2007) to determine the variables influencing the presence of the ONP. The random (intercept) effect was the detection method (Playback 1 and Playback 2) and fixed effects included elevation, slope, distance to water source, and forest type. As we did not detect the ONP in mixed-deciduous forest (238 locations), this forest type was excluded from the habitat selection analysis.
Table 2. Details of different survey methods used in different protected areas during different years for Orange-necked Partridge (ONP). (LT = line transect and PC = point count; p1 = playback 1 and p2 = playback 2).
Regression models
Prior to running the models, the continuous variables including elevation, slope, and distance to water sources were checked and outliers were removed; these variables were then standardised by dividing the values by twice the standard deviation (Gelman Reference Gelman2008). We did not include highly correlated (r > 0.5) variables in the same regression model. The survey effort (number of visits to each point) was treated as a fixed coefficient and set to 1 by using an “offset” (Gelman and Hill Reference Gelman and Hill2007). Model selection was based on comparing Akaike information criterion (AICc) values adjusted for small samples. Akaike model weights (wi) were calculated as the weight of evidence in favor of model i among the models being compared. We assessed model classification accuracy of multilevel logistic regression by using the area under the receiver operating characteristic (ROC) curve (AUC) (Hosmer and Lemeshow Reference Hosmer and Lemeshow2000, Franklin Reference Franklin2010), and calculated AUC using the “PresenceAbsence” package (Freeman and Moisen Reference Freeman and Moisen2008). We chose an optimal threshold cut-off value for classification using the minimised difference between the proportion of presences correctly predicted (sensitivity) and the proportion of absences correctly predicted (specificity) (Fielding and Bell Reference Fielding and Bell1997, Franklin Reference Franklin2010). All analysis was performed using program R version 3.2.1 (R Development Core Team 2015).
Historical and current distribution
Current distribution maps for GPP and ONP in the study sites and across their ranges were predicted using results from the best fitted model containing those ecological variables that best explained the abundance of GPP and presence of ONP. Historical distribution maps of the species were created using data from published literature (BirdLife International 2001, Goes Reference Goes2013, Gray et al. Reference Gray, Pollard, Evans, Goes, Grindley, Omaliss and Sophoan2014, Vy et al. Reference Vy, Savini and Carroll2014) and unpublished reports (Dang and Osborn Reference Dang and Osborn2004, Tordoff et al. Reference Tordoff, Bao, Tu and Hung2004). Based on historical detections of the ONP, the species was not detected at elevations higher than 600 m (Vy et al. Reference Vy, Savini and Carroll2014); therefore, the upper elevation limit for the historical distribution range analysis for the ONP was 600 m. Due to a lack of detailed mapping regarding specific forest types in the past, it is difficult to be precise about rates of loss for particular forest types in the species’ ranges. Therefore, the declines of the species’ distribution ranges over the last 70 years were assessed by comparing total natural forest cover within the potential distribution range in 1943 (Wege et al. Reference Wege, Long, Vinh, Dung and Eames1999) with the total current forest cover in 2015. All these analyses were carried out using ArcGIS 10.3 software (ESRI, Redlands, USA).
Results
Density estimation of GPP and ONP
For the GPP, there were 150 detections collected in four forest types combined. The half-normal key function was the most supported model with detection probability P = 0.61 with an effective strip width of 61 m. The densities were highest in mosaic forest (8.80 calling males/km2), with likely lower densities (∼50% less) in evergreen forest and mixed-deciduous forest although the differences were not statistically significant (Table 3). The lowest density was in bamboo forest with only five detections, which was significantly lower than the mosaic forest, but not the other forest types. The overall density of GPP for all study sites combined was 4.33 calling males/km2 (Table 3).
Table 3. Density estimates for Germain’s Peacock Pheasant (GPP) and Orange-necked Partridge (ONP) in Cat Tien National Park, 2014.
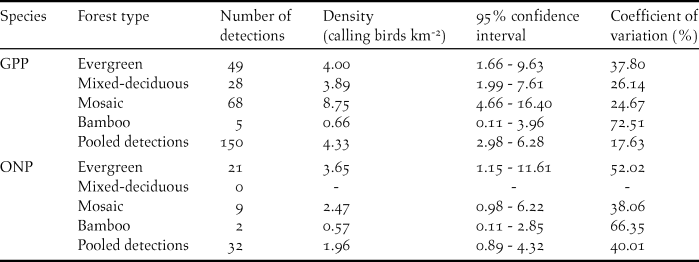
For the ONP, there were 32 detections collected in three forest types (evergreen, mosaic, and bamboo forests), and there were no detections in mixed-deciduous forest. As above, we pooled detections from all forest types to derive a detection function, and then estimated density separately for each forest type. The densities of the ONP were 2.47 individuals/km2 for mosaic forest, 3.65 individuals/km2 for evergreen forest, and 0.57 individuals/km2 for bamboo. The differences were not significant, presumably due to the small number of detections; the density of species for all study sites was 1.96 individuals/km2 (Table 3).
Habitat variables associated with the relative abundance of the GPP
The best model, based on ΔAICc and AICc weights, consisted of three variables: slope, forest type, and distance to water source (Table 4), suggesting that the abundance of the GPP was positively associated with evergreen, mixed-deciduous, and mosaic forest types, but not bamboo. In addition, slope and distance to water source had a significantly negative association with the relative abundance of GPP in the study site (Table 5).
Table 4. The top models derived from a set of regression models examining habitat selection of Germain’s Peacock Pheasant (GPP) and Orange-necked Partridge (ONP). K is the number of parameters in the model, ΔAICc is the difference in AICc (model score) value: models with ΔAICc value 0 have the most support, values between 0 and 2 have substantial support and values greater than 2 have less support (following Akaike Reference Akaike, Petrovand and Csàki1973), wi= Akaike model weights. Forest types include bamboo, evergreen, mixed-deciduous, and mosaic forest.

Table 5. Estimates of coefficients derived from the top models with standard errors and 95% confidence intervals.

* Coefficient of random effect (playback): Playback1 = 0.75 and Playback2 = - 0.74.
Habitat associations with the presence of ONP
A candidate set of seven multilevel logistic regression models were fitted to determine habitat associations of the ONP. The best model consisted of elevation, forest types (evergreen and mosaic), and the interaction between elevation with forest type (Table 4). We omitted bamboo and mixed-deciduous from the habitat association analysis as there were only two and no detections in these habitat types, respectively. The results of the regression analysis showed that mosaic forest at higher elevations positively influenced the presence of ONP. In addition, the best model also indicated that the probability of detecting the species using continuous playback (Playback 1) (intercept = 0.75) was higher than that of using only two minutes of playback (Playback 2) (intercept = -0.74). This best fitted model provided reasonable discrimination between the ONP presence and absence (AUC = 0.80). The AUC suggested the threshold cut-off value for classification at a probability of occurrence of 0.4 minimized the difference between sensitivity and specificity with the highest percent correct classification at 76%.
Historical and current distribution range of the GPP
Within the potential historical range of the GPP, total forest cover in 1943 was approximately 98,600 km2 but the total current forest cover from our LANDSAT classification was approximately 32,700 km2. Thus, the total potential suitable habitat loss over the last 70 years was about 65,900 km2 (67%). A current potential distribution map of GPP in southern Vietnam was created using results of habitat association analysis indicated in Figure 2b. Based on current detections and density of the species in Cat Tien NP, the relative abundance in the species’ maps in Cat Tien NP and in Southern Vietnam were divided into three density categories: (1) low (< 1 individuals/km2), (2) moderate (2–8 individuals/km2), and high (> 8 individuals/km2). The current potential distribution of the GPP in Southern Vietnam covers approximately 27,600 km2, of which only 5,800 km2 are in protected areas and 21,800 km2 appear to be within a matrix of human dominated landscape, agricultural or disturbed areas.
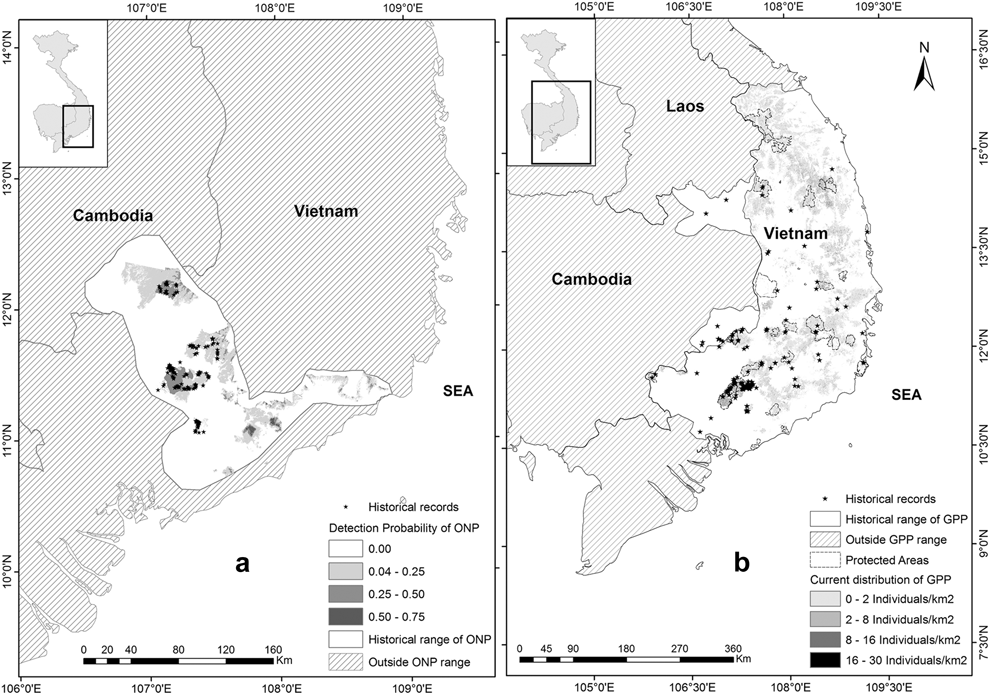
Figure 2. (a) Current potential distribution of the Orange-necked Partridge and its historical range; (b) Current potential distribution map and historical range of the Germain’s Peacock Pheasant.
According to historical detections, records occurred from 10030’N to 15000’N. Based on these records, the distribution range of the GPP may extend to 16000’N as there are some low and flat lands along the coast from 15000’N to 16000’N. Beyond 16000’N in the north, the terrain is mainly steep in hilly areas of the Annamites, particularly Hai Van pass, running west to east (Sterling et al. Reference Sterling, Hurley and Minh2006), which are probably not suitable habitat for the species (Figure 2b). In Cambodia, their range is confined in two areas adjacent to Vietnam: (1) Vinachey and surrounding areas in the north of Ratanakiri; (2) Seima Protected Forest and Snuol Wildlife Sanctuary in southern Mondulkiri Province (Figure 2b). These areas are covered mainly by evergreen and mixed-deciduous forest.
Current and historical distribution range of the ONP
Recent records show the ONP are distributed in the lowlands in the north of the Mekong region in Southern Vietnam and areas in Cambodia, adjacent to Vietnam, ranging from 100 m to 600 m in evergreen, mosaic, patches of bamboo with a canopy of broadleaved trees, but not in extensive bamboo stands (Goes Reference Goes2013, Gray et al. Reference Gray, Pollard, Evans, Goes, Grindley, Omaliss and Sophoan2014, Vy et al. Reference Vy, Savini and Carroll2014, BirdLife International 2015). A potential current distribution map of the species was predicted using the best fitted model from our habitat association analysis (Figure 2a). Based on current records of the ONP, the probability of species’ detection in its range was divided into categories: (1) very low (0–0.25), (2) low (0.25–0.50), and (3) moderate (0.50–0.75). Total area of the current potential range of the ONP is roughly 3,600 km2 in which 3,000 km2 occurs in southern Vietnam (including 1,000 km2 in protected areas and the remaining 2,000 km2 probably within disturbed areas), and 600 km2 in Cambodia.
Historical detections of the species in Vietnam and Cambodia, and its specific habitat use suggest that the historical range of the species covered the lowlands from southern Vietnam (north of the Mekong region) to south-eastern Mondulkiri Province which is characterised by hilly terrain. Within the potential historical range of the ONP, total forest cover in 1943 was about 14,200 km2 (Wege et al. Reference Wege, Long, Vinh, Dung and Eames1999) but total current forest cover from our classification was approximately 4,400 km2. Thus, the total suitable habitat loss over the last 70 years was about 9,800 km2 (69%).
Discussion
The estimated density of GPP found in Cat Tien National Park, was twice as high in the mosaic as in evergreen and mixed deciduous forest, although the differences were not significant, while they appeared to be largely absent from bamboo forest. Density estimates of ONP were higher in evergreen than that in mosaic forest, and lower in bamboo forest although the differences were not significant due to the small number of detections. No detections of this species were recorded along the 9.5 km of transects in the mixed-deciduous forest. Habitat use models suggest that the GPP mainly used flat areas near water, dominated by mosaic, evergreen and mixed-deciduous forests. Habitat use by ONP showed that elevation was positively associated with their presence, which was also significantly associated with evergreen and mosaic forests. On a larger scale, our results show that the remaining habitat for these species is limited to the remaining lowland forest of south-central Vietnam and in a small portion of eastern Cambodia. Based on the forest loss within the ranges of both species, it is estimated that the suitable habitat within the range of both species has shrunk by at least 60–70% over the last 70 years.
This research has provided essential, initial population status quantification of the GPP and the ONP for long-term conservation of the species as Cat Tien is a well-known example of a remaining sizable lowland tropical forest ecosystem in southern Vietnam, and one of the largest national parks in Vietnam, harbouring a variety of rare and endangered species including at least 48 species on the IUCN Red List (Polet Reference Polet, Persoon, Van Est and Sajise2003, Polet and Ling Reference Polet and Ling2004). Cat Tien is most famous for tourism not only because of its high biodiversity, but also stable population of some key species, including Green Peafowl Pavo muticus (Sukumal et al. Reference Sukumal, McGowan and Savini2015). Although Cat Tien is relatively well protected compared to other protected areas, some parts of the park still face various threats as access improves. In the Cat Loc sector, the northern part of the park, a large new road was built to reach Bu Sa Commune within the region, making accessibility to the forest much easier. This might increase impacts on the park, including poaching which could be the main reason for the extinction of the lesser one-horned rhinoceros Rhinoceros sondaicus annamiticus in Cat Loc in 2010 (Brook et al. Reference Brook, Van Coeverden de Groot, Mahood and Long2011). Consequently, we may assume that the overall population status of the GPP and ONP in Cat Tien NP is stable but perhaps somewhat lower than they should be.
Our results suggested that flat mosaic forest (evergreen and mixed-deciduous) near water sources is mainly selected by GPP, as it might provide higher food availability and perhaps reduce predation risk. Both evergreen and mixed-deciduous forests have structural complexity with multiple layers, which produces high coverage (Park Reference Park2003, Corlett Reference Corlett2009), especially dense understorey in areas along streams (Doyle Reference Doyle1990), whereas areas dominated by bamboo forest have a more open understorey (Soderstrom and Calderon Reference Soderstrom and Calderon1979, Corlett Reference Corlett2009). A number of galliform species are likely to use habitats with high coverage of understorey vegetation, which provides suitable shelter to help avoid predators and raise their young effectively (Lima Reference Lima1993, Martin Reference Martin1993). White-eared Pheasant Crossoptilon crossoptilon in China (Wang et al. Reference Wang, Jia and Zheng2006), Hume’s Pheasant Syrmaticus humiea (Iamsiri and Gale Reference Iamsiri and Gale2008) and Siamese Fireback Lophura diardi in Thailand (Suwanrat et al. Reference Suwanrat, Ngoprasert, Sukumal, Suwanwaree and Savini2014) showed similar habitat preferences.
Although the diet of GPP has never been studied in the wild we can assume that they are omnivorous like the closely related Grey Peacock Pheasant Polyplectron bicalcaratum which mainly consumes seeds, fruits, invertebrates and a relatively high proportion of insects, mostly while rearing chicks (Johnsgard Reference Johnsgard1999, Madge and McGowan Reference Madge and McGowan2010). Food availability could explain the high density of GPP observed in flat areas close to streams with high vegetation cover and available surface water (Malanson Reference Malanson1993, Naiman and Décamps Reference Naiman and Décamps1997), which creates a local microhabitat rich in flowering and fruit-bearing plants (Lovett and Price Reference Lovett and Price2007) and a greater abundance of insects and other invertebrates compared to adjacent upslope habitats (Catterall et al. Reference Catterall, Piper, Bunn and Arthur2001, Chan et al. Reference Chan, Yu, Zhang and Dudgeon2008). Moreover, riparian areas also show more loose and friable soil (Roberts et al. Reference Roberts, Howe, Major and Sands1977, Lovett and Price Reference Lovett and Price2007) which probably facilitates foraging in species like galliforms which often scrape the ground for food.
The densities of ONP were higher in evergreen and mosaic forests, and lower in bamboo forest while it was absent from mixed-deciduous forest. Our results also suggested that for this species the understorey structure is important perhaps because it lowers the risk of predation. This micro-habitat preference pattern was also observed in other Arborophila species: Common Hill Partridge A. torqueola (Liao et al. Reference Liao, Hu and Li2007a) and Sichuan Hill Partridge A. rufipectus (Liao et al. Reference Liao, Li, Hu and Lu2007b, Bo et al. Reference Bo, Dowell, Garson and Fen-Qi2009) in China. We did not detect ONP in mixed-deciduous forest in Cat Tien NP where the terrain is quite flat between 120 and 160 m and mostly covered by Lagerstroemia-dominated mixed-deciduous forest, although this elevation range is within the distribution range of the species (Vy et al. Reference Vy, Savini and Carroll2014, BirdLife International 2015). Their absence from this mixed-deciduous forest was also reported by other observers (Robson et al. Reference Robson, Eames, Nguyen and Truong1993, Atkins and Tentij Reference Atkins and Tentij1998). We predict that ONP density is perhaps affected by the presence of other Arborophila species, such as Scaly-breasted Partridge A. chloropus, which also occurs in the area.
Historical distribution of GPP and ONP
Southern Vietnam consists of two regions: Central and Mekong regions. The Central region is comprised of the Annamite hills (500–2,000m) ranging from the Lao border in the west and the central coastline in the east, ending south of the DaLat Plateau and a narrow coastal plain between the Annamites and the sea. The Mekong region in the south covers the flat Mekong Delta with an average elevation between 20 and 750 m. The forests in the Central region contain different forest types dominated by evergreen, semi-evergreen and dry dipterocarp forests. The forests in the north part of the Mekong region are dominated by lowland evergreen, semi-evergreen, secondary grassland, and bamboo habitats (Sterling et al. Reference Sterling, Hurley and Minh2006). The central region is high in biodiversity with many endemic species at country or at regional scale (Tordoff Reference Tordoff2003, Sterling et al. Reference Sterling, Hurley and Minh2006) but, together with the Mekong region, it also suffers from high conversion rates to agriculture and fragmentation (Müller and Zeller Reference Müller and Zeller2002, Brickle et al. Reference Brickle, Duckworth, Tordoff, Poole, Timmins and McGowan2008) as well as high hunting pressure, mainly through snaring (Brickle et al. Reference Brickle, Duckworth, Tordoff, Poole, Timmins and McGowan2008).
GPP was formerly considered very common with its range extending from north of the Mekong region (100N) to north of the Central region (160N) in Vietnam and a small pocket in the eastern part of Cambodia (BirdLife International 2001). With the species occupying both highland and lowland, a historical range of about 122,000 km2 might be suggested. However, the vast dry dipterocarp forest covering much of the central highland of Vietnam to east of the Mekong river in Cambodia, except some parts north of Ratanakiri and the south of Mondulkiri (Sterling et al. Reference Sterling, Hurley and Minh2006), could be a natural barrier to the species in the central highlands of Vietnam and in Cambodia. Furthermore, mangrove forest and wetlands in the south of the Mekong Delta region could have limited its range in the south and the coastal areas in the east.
ONP was mainly found in the northern Mekong region at a range of elevation from 100 to 600 m (Vy et al. Reference Vy, Savini and Carroll2014, BirdLife International 2015), in areas covered by evergreen, semi-evergreen (mixed-deciduous), and bamboo forests. The species historical range was most likely constrained to the south-east in Cambodia (Seima) and in the northern part of Mekong Delta region in Vietnam which were covered predominantly by evergreen and semi-evergreen forests on rolling hills up to 600 m. The historical range in Vietnam was restricted by steep hilly terrain on elevations higher than 600 m to the south of the Annamites in the north, and flat lowlands in the southern Mekong region.
Shrinking distribution of GPP and ONP
Results of the habitat loss analysis showed that forest cover within the range of GPP and ONP has declined by 60–70%, mainly in lowland areas in southern Vietnam, especially in the southern Annamites, and north of the Mekong region (Wege et al. Reference Wege, Long, Vinh, Dung and Eames1999, Sterling et al. Reference Sterling, Hurley and Minh2006). The total remaining suitable forest in the GPP’s range from our land cover assessment was estimated at 32,700 km2 of which only 7,500 km2 (23%) fall inside protected areas, the rest is unprotected and highly fragmented. In addition, most Vietnamese national parks and nature reserves face various threats such as habitat loss and illegal hunting (Polet and Ling Reference Polet and Ling2004, Brook et al. Reference Brook, Van Coeverden de Groot, Mahood and Long2011). GPP habitat preferences suggest that Cat Tien NP, Bu Gia Map NP and Vinh Cuu NR could be considered the most important patches supporting the largest remaining populations. Strengthening protection around flat areas close to water sources should be a priority as the species tends to gather here, especially during the dry season. Regarding potential remaining suitable areas outside the protected area network, it is necessary to define populations of the GPP in sizeable remaining suitable habitats to maintain these populations due to limitations of protection in such areas. Five provinces, including Dong Nai, Lam Dong, Dac Nong, Kon Tum and Quang Nam, which contain relatively large patches of remaining suitable habitats for GPP, should be priority areas for surveys.
The geographical range of ONP, about 19,500 km2 (16,400 km2 in Vietnam and 3,100 km2 in Cambodia) has contracted by at least 60–70% over the last 70 years based on forest cover in 1943 (Wege et al. Reference Wege, Long, Vinh, Dung and Eames1999) and forest cover in 2015, making the current remaining potential area for the ONP about 3,600 km2 (about 3,000 km2 in Vietnam and 600 km2 in Cambodia) but in Vietnam only about 1,000 km2 are protected. In addition, current land cover maps suggest that forests of some unprotected areas where the ONP was detected such as Nghia Trung State Forest Enterprise, Da Teh Special-used Forest, Bu Dop, Dak Ơ, and Bu Dang Special-use Forest (Vy et al. Reference Vy, Savini and Carroll2014) have lost 60–80% of their area between 2008 and 2016. Currently the most important remaining ONP populations are in Cat Tien and Bu Gia Map NP as well as the northern part of Vinh Cuu Nature Reserve. La Nga State Forest Enterprise, adjacent to the southern edge of Cat Tien, could potentially be suitable habitat as it is mainly characterised by gentle hills with mosaic and evergreen forests. Implementing surveys in La Nga to determine the current population status both for ONP and other Galliform species should also be a conservation priority.
Reassessment of Red List criteria and categories of ONP and GPP
This study indicated that natural forests within the range of ONP and GPP declined by almost 70% during the last seven decades. Moreover, less than 25% of the remaining potential suitable habitats for these species are in protected areas such as national parks or nature reserves. Research suggests that compared to areas outside protected areas, rates of loss of natural habitat are reduced inside protected areas (Bruner et al. Reference Bruner, Gullison, Rice and Da Fonseca2001) and local biodiversity is also higher inside protected areas (Gray et al. Reference Gray, Hill, Newbold, Hudson, Börger, Contu and Scharlemann2016). Unfortunately, with almost 75% of their available habitats occurring outside of protected areas, we suggest that in the next few decades, the habitats and populations of these two species would likely continue to decline. Globally, ONP has a very restricted range and with its current occurrence we suggest revision of its global status to ‘Endangered’ (A1cd; B1,2; C1). For the GPP, we suggest revision of its global status to ‘Vulnerable’ (A1cd; B1, C).
Acknowledgements
We would like to express our sincere gratitude to L.C. Kiet, V. Quy, G. A. Gale and P. McGowan for their assistance throughout the project. Support to field surveys was provided by the Institute of Tropical Biology and Cat Tien National Park. Additional financial support was provided by the WWF Vietnam Country Programme, the Nagao Natural Environment Foundation, the Oriental Bird Club, the Birdfair/RSPB Research Fund for Endangered Birds, Rufford Small Grants Foundation, Russell E. Train Education for Nature Program, WWF-US, and apart of field equipment from IDEA WILD. We also thank N. T. Hung for his help with fieldwork. Lastly, we thank L. A. Hung, W. Chutipong, N. Tantipisanuh, P. K. Diem and K. Xaisavanh for their help with data analysis.









