INTRODUCTION
Pneumonia is an important cause of mortality and morbidity in children aged <5 years [Reference Wardlaw1, Reference Jokinen2]. According to the Mortality Registry data in 2005, 4% of infants in Taiwan died from infectious diseases, and about one quarter of these deaths were caused by pneumonia [3]. Therefore, pneumonia represented the most important defined cause of infection-related death in young children. Several risk factors are associated with childhood pneumonia. These include: male gender, congenital cardiopulmonary disease, congenital immunodeficiency, lack of vaccination, environmental tobacco smoking (ETS), low socioeconomic status, living with school-aged children, and environmental crowding [Reference Jokinen2, Reference Greenberg4, Reference Breton5]. Among children, infants are especially susceptible to this disease, probably because their immunity and respiratory anatomies are still under-developed. However, studies on the risk factors for infantile pneumonia have been less conclusive than those of other periods of childhood.
For the identification of risk factors for infantile pneumonia, factors from prenatal and early postnatal periods need to be considered. Prenatal exposure as a cause of later health problems has attracted much attention in recent years. However, studies on the relationship between prenatal exposure and infantile pneumonia are still limited. After birth, infants spend most of their time indoors. Therefore, the postnatal indoor environment may play an important role in their health conditions, including respiratory health [Reference Mendell6]. Studies looking into effects of the indoor environment on infectious respiratory outcomes in infants are also limited. We conducted a cross-sectional study on a representative birth cohort to determine prenatal and early postnatal factors to pneumonia in infants aged <6 months.
MATERIALS AND METHODS
Population and study design
This study was approved by the Institutional Review Board of the Bureau of Health Promotion, Department of Health, Taiwan. The study population was comprised of all infants enrolled in the Taiwan Birth Cohort Study (TBCS) in 2005. In the TBCS study, representative samples were obtained by a multistage stratified systematic sampling design according to Taiwan national birth registry data. Based on the administrative division (four strata: county, town, city, area) and the total fertility rate (three strata: low, medium, high), 369 townships in Taiwan were split into 12 strata. A total of 88 townships were randomly selected from the 12 strata by using the principle of proportion probability to size. Beginning in January 2005, newborn candidates and their main caregivers were randomly sampled from study areas every month. Of the 206 714 live births in 2005, 24 200 (12%) were enrolled into the TBCS, as well as their main caregivers. These infants were followed up by home interviews at the age of 6 months. A home interview was conducted after the mother's informed consent by 89 well-trained interviewers who had at least 2 years' experience in questionnaire interviews conducted by the Bureau of Health Promotion, Taiwan. The children's mothers were the primary subjects for interview. If the mother was unavailable due to death, communication problems, family breakup, etc., the main caregiver with best knowledge of the baby was interviewed instead.
Assessment of outcome and risk factors
The assessments were made using a structured questionnaire. The primary outcome was the occurrence of pneumonia and was assessed by the questions ‘Has this baby ever been diagnosed as having pneumonia by a doctor?’ and ‘Was this baby admitted to a hospital due to this condition?’ The infants whose mothers/caregivers answered ‘yes’ to both questions were considered as having pneumonia.
Potential determinants of childhood pneumonia mentioned in published literature were inquired about in the interviews, including the sex of the baby, gestational age, birth weight, vaccination of Haemophilus influenzae type B, parental education level, family income level, number of siblings at home, and congenital cardiopulmonary disease [Reference Dennehy7]. In addition, the following prenatal conditions of the mother were included as potential risk factors: medications used during pregnancy, body mass index (BMI) before pregnancy, weight gain during pregnancy, morbidities of gestation, tobacco smoking and betel nut chewing habits, shift working, and exposure to ETS. Mothers with a BMI ⩾24·0 were considered ‘overweight’, according to criteria for Asian people [Reference Deurenberg, Yap and van Staveren8].
For postnatal risk factors, we focused on indoor environmental determinants, including the presence in the home of: cockroaches; water damage; visible mould on walls, carpet flooring; incense burning; and pet ownership.
Statistical analysis
The demographic characteristics, as well as the potential determinants and confounders of the outcomes, were compared between infants with and without pneumonia before age 6 months using the χ2 test for categorical variables and the Student's t test for continuous variables.
Univariate logistic regression was performed to determine the relationship between prenatal and postnatal environmental factors as well as the risk of having infantile pneumonia. Odds ratios (ORs) with 95% confidence intervals (CIs) were estimated using individual models. Because of a relative lack of information on risk factors in Asian populations, we decided to include all possible variables in our analysis of risk factors. After an initial univariate analysis, only significant factors (at P<0·05) were included for the backward stepwise logistic regression to construct a best-fitted multivariate model. Adjusted ORs (aORs) with 95% CIs were calculated after mutual adjustment. In order to elaborate the effects of tobacco smoke, we created dummy variants to stratify prenatal tobacco exposure into ‘maternal smoking’, ‘prenatal ETS exposure every day without maternal smoking’, ‘prenatal ETS exposure <7 days per week without maternal smoking’, and ‘unexposed to tobacco smoke’.
Population attributable risks (PARs) were calculated to estimate the contribution of various risk factors to infantile pneumonia. The PAR for any risk factor represents the preventable proportion of pneumonia cases if infants were not exposed to the specified factor. PAR was calculated using the formula
where P is the prevalence of the exposure and R is the relative risk due to the exposure [Reference Coughlin, Benichou and Weed9]. For a better estimate of attributable risk, we further calculated the adjusted attributable risk using the formula
where n is the total number of cases, n j is the number of cases within stratum j of the concerned exposure, and RRj is the relative risk for stratum j compared to the baseline stratum [Reference Bruzzi10]. Owing to the overall low prevalence of infantile pneumonia, an OR of multiple logistic regression is substituted for relative risk according to the rare case assumption [Reference Zhang and Yu11]. Statistical significance was set at P<0·05 based on two-sided calculations. Analyses were conducted using the JMP 5.0 statistical package (SAS Institute Inc., USA).
RESULTS
A total of 21 248 interviews were completed. This corresponded to an overall response rate of 87·8%. The urban and rural response rates were 86·6% and 89·6%, respectively. Of the non-responders, 1739 refused to participate, 358 could not be reached after three attempts, 373 were travelling abroad, and 67 infants died before age 6 months.
A total of 21 248 infants were included for the final analysis, 132 (0·62%) of which were hospitalized pneumonia cases. Table 1 outlines the demographic characteristics of the babies and mothers of the target population. Overall, boys had a higher prevalence of infantile pneumonia than girls before age 6 months. Vaccination of H. influenzae type B and lower birth weight were not significantly associated with pneumonia. Preterm babies were at higher risk of pneumonia in the first 6 months of life. For socioeconomic status, both low parental education and low family income were associated with pneumonia risk. Infants with more than two siblings at home also carried a higher risk of contracting pneumonia.
Table 1. Prevalence of pneumonia in study infants and association with potential risk factors

OR, Odds ratio; CI, confidence intervals; ETS, environmental tobacco smoke; BMI, body mass index.
* Numbers of subjects do not add up to total due to missing data. ORs are crude odds ratios for each risk factor.
Diagnoses of congenital cardiopulmonary diseases in infants were reported by 185 mothers, including 85 with a ventricular septal defect, 33 with an atrial septal defect, 41 with patent ductus arteriosus, six with Tetralogy of Fallot, two with endocardial cushion defects, five with great vessel transposition, 20 with pulmonary artery stenosis, nine with a complex congenital cardiac anomaly, three with congenital tracheal stenosis, and ten with pulmonary hypoplasia. The infants with congenital cardiopulmonary diseases had a much higher risk than those without any congenital cardiopulmonary diseases.
Among the prenatal factors (Table 1), preterm birth, maternal overweight before pregnancy, gestational antibiotic use, prenatal ETS exposure every day, and maternal smoking during pregnancy were found to be associated with infantile pneumonia. Among the postnatal factors (Table 1), visible mould on the walls at home was found to be related to infantile pneumonia. Furthermore, there was a dose–response trend for indoor mould, with the highest risk in infants whose mothers reported mould found on ⩾3 walls (test for trend P=0·03).
After mutual adjustment, the factors that were found to be significantly associated with a higher risk of infantile pneumonia were: male gender, preterm birth, congenital cardiopulmonary diseases, use of antibiotics during pregnancy, maternal overweight before pregnancy, prenatal ETS exposure daily, maternal tobacco smoking, and visible mould on the wall at home (Table 2). About 59·8% of infantile pneumonia could be attributed to these potential modifiable factors.
Table 2. Adjusted odds ratios and adjusted attributable risks for prenatal and postnatal factors associated with pneumonia in 6-month-old infants
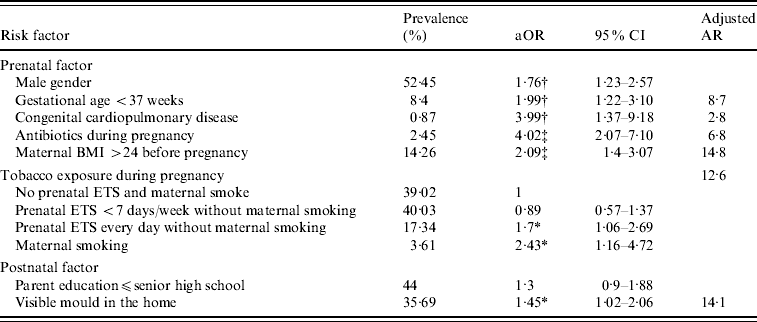
aOR, Adjusted odds ratio; adjusted AR, adjusted attributable risk; BMI, body mass index; ETS, environmental tobacco smoke.
* P<0·05, † P<0·01, ‡ P<0·001.
DISCUSSION
This is the first study to examine prenatal and early postnatal risk factors for infantile pneumonia and their respective contributions to the disease, based on a representative sample drawn from a nationwide birth registry. Several novel prenatal conditions were identified as risk factors for infantile pneumonia before age 6 months, including the use of antibiotics during pregnancy, maternal overweight, prenatal ETS exposure, and maternal smoking during pregnancy. Of the postnatal factors studied, visible mould was the most important risk factor and appears to contribute significantly to infantile pneumonia.
In our infant population, the ‘⩽6 months old’ prevalence rate of pneumonia was 0·62%, which was lower than reported in other studies, e.g. 3·2% for ‘⩽8 months old’ toddlers by Quigley et al. [Reference Quigley, Kelly and Sacker12]. This was probably due to the tight criterion used in our study, i.e. only hospitalized pneumonia.
Antibiotic use during pregnancy was found to be associated with infantile pneumonia in our study. However, the mechanisms of this association were unclear, and the exact antibiotics associated with the occurrence of pneumonia were unknown due to the limitations of our study design. Furthermore, our finding should not hamper adequate antibiotic use during pregnancy when clinically indicated. In general, the most common antibiotics used during pregnancy were penicillin and cephalosporins, due to their low teratogenicity. These antibiotics cross the placenta readily, thus causing fetal exposure [Reference Philipson13]. Several antibiotics are known to suppress bone marrow cells, causing leukopenia and neutropenia in adults [Reference Anagnou14, Reference Timmis15]. The developing immune system of the fetus is potentially more susceptible to xenobiotics than that of an adult [Reference Dietert and Zelikoff16]. Whether prenatal antibiotic exposure impairs or modifies the development of immunity in early life, and which gestational period is the most vulnerable time-window are an areas for further investigation.
The use of Chinese herbal medicines during pregnancy is not uncommon in Taiwan, with a prevalence of up to 10% of pregnant women using pearl powder and huang-liang (Rhizoma coptidis or Chinese goldthread). It is commonly believed that herbs are harmless because they are natural products. Although the use of pearl powder and huang-liang is not associated with infantile pneumonia, other potential adverse effects warrant further monitoring.
Maternal overweight was found to be an independent risk factor of infantile pneumonia in our study. Maternal obesity is known to increase the risks of hypertension, gestational diabetes, delivery complications, and thromboembolism in mothers, and of congenital malformations, macrosomia, and antepartum stillbirth in fetuses [Reference Catalano and Ehrenberg17, Reference Yu, Teoh and Robinson18]. Long-term health effects in the offspring, especially the metabolic effects, have been reported [Reference Whitaker19, Reference Boney20]. Overweight-related insulin resistance might not fully explain our findings, because in our study, gestational diabetes did not increase the risk of pneumonia in infants. Higher BMI was negatively associated with serum surfactant-D protein levels, which have been shown to play an important role in the innate immunity of the airway [Reference Zhao21, Reference Sorensen22]. Further study is needed to determine whether maternal overweight affects surfactant-D levels of the fetus.
Due to the high prevalence of overweight mothers, the estimated PAR of this condition was as high as 14·8%. Since overweight and obesity are becoming worldwide epidemics, their effects on fetal risks of pneumonia and potential changes to the immune system should be a priority research area.
Although visible mould growth and water damage are often found together in homes, our findings showed that only visible mould is associated with infantile pneumonia. This causal relationship was further supported by the observed dose–response relationship. Previous investigations did not find indoor mould to be a significant factor for pneumonia in schoolchildren [Reference Andriessen, Brunekreef and Roemer23, Reference Yang24]. However, infants have less mature systemic and secretory immunities, which could make them even more vulnerable to mould. The exact mechanisms of mould's adverse effects on the early infantile respiratory tract are unclear. However, mycotoxin may play an important role. Mould-related toxins could affect respiratory health through the inhalation of mycotoxin-containing spores or dust [Reference Brinton25–Reference Hodgson29]. Animal and in vitro studies suggest that several indoor moulds produce mycotoxins, which in turn damage the innate immunity of the respiratory tract, the major defence for infants. Fusarium-produced T2 toxin was able to induce apoptotic death of alveolar macrophages, decrease secretory IgA titres, and potentiate reovirus-induced inflammation and tissue injury in mice [Reference Li30]. Stachybotrys chartarum toxins induced apoptosis in pulmonary alveolar macrophages, reduced alveolar type II cell function and anti-oxidative ability, and interfered with surfactant homeostasis [Reference Rand31–Reference Wang and Yadav34]. Additionally, several indoor mould-related mycotoxins were able to inhibit airway ciliary function in chickens [Reference Pieckova and Kunova35].
The PAR of visible mould on walls at home was as high as 14·1%. Again, a high prevalence rate (35·7%) of such exposure caused a high attributable risk. The high temperature and humidity in Taiwan, a subtropical island, provide an ideal environment for mould growth. Countries with similar weather conditions may need to consider mould as a significant factor for pneumonia.
Tobacco smoking compromises an individual's primary pulmonary defence, including mucocilliary clearance and alveolar macrophage activity. Infants with parents who smoke have a higher risk of lower respiratory tract infections [Reference Harlap and Davies36, Reference Strachan and Cook37]. In our study, children exposed to maternal smoking in utero had a higher risk of acquiring pneumonia later on than those only exposed to ETS during pregnancy. However, since prenatal and postnatal exposure to tobacco smoke are highly correlated, a clear distinction between the effects of prenatal tobacco exposure and those of postnatal exposure is difficult to demonstrate.
The strengths of this study include: (1) its large number of participants, which were drawn from a representative nationwide survey; (2) a reasonably high participation rate; (3) reliable diagnosis and medical attention to infants with pneumonia due to the broad coverage (>98% population) of Taiwan's National Health Insurance programme and good accessibility to medical care regardless of socioeconomic status.
The key limitations of this study include: (1) information was obtained from questionnaires; (2) the clinical diagnoses, imaging studies, and bacteriological data could not be obtained; (3) several types of exposure data were provided only by caregivers, and potentially subject to misclassification or random error; (4) the causes of death of the 67 infants were unknown. However, in 2005, pneumonia accounted for ~1% of all-infant mortality in Taiwan. Thus, excluding these was unlikely to change our results; (5) the cross-sectional design of this study allowed for assessment of associations. Causal relationships should be interpreted with caution.
An important limitation is the lack of confirmation of the diagnosis of pneumonia. Due to confidentiality concerns, individual information is not accessed in the TBCS, therefore confirmation by chart review could not be performed. Previous reports have validated the accuracy of the reported diagnoses of pneumonia of early postnatal children with an acceptable specificity of 85–90% for mortality cases and 60–73% for hospitalized cases [Reference Kalter38, Reference Coldham39]. The interval between the event of pneumonia and interview of previous reports was 1–22 months (13 months on average). Our survey was performed when the children were aged 6 months, allowing for a shorter recall interval of 0–6 months. Therefore, recall bias is less than previous reports. Furthermore, hospitals in Taiwan usually recommend that caregivers of hospitalized children should stay in the hospital with the children. The caregivers would be able to observe the signs and symptoms of their children and to discuss with the attending physicians, resulting in the caregivers' having a better understanding of their children's diseases.
The information of visible mould on the wall and water damage was obtained from the questionnaire and not by objective measurements. Reported visible mould and water damage in the home were found to be correlated with the measured total fungal spore counts and the spore counts of Aspergillus and Penicillium. However, smokers tended to underreport mould, and people with allergies tended to overreport [Reference Dales, Miller and McMullen40]. In our study, 98% of those reporting to the TBCS were the children's mothers. The effect of underreporting in smoking mothers might lead to an underestimation of mould-related risk in this study. This effect was probably small because only 3·6% of the mothers in this study smoked and the reported prevalence of visible mould in the home for smoking and non-smoking mothers was similar (37·8% vs. 35·6%). Regarding the overreporting among atopic mothers, its effect is also small because parental atopy is not a known risk factor for the outcome of interest in this study, i.e. infantile pneumonia. Of the mothers in this study, 18% reported having atopic conditions, either asthma, atopic dermatitis, or allergic rhinitis. A similar percentage of mothers with atopic conditions (39%) reported visible mould in the home, as those without atopy (35%). While including maternal atopy into the logistic regression modelling (data not shown), the effect size and significance of visible mould were unchanged. Misclassification of reporting visible mould and water damage would have reduced the strength of the observed relationship of this study, moving the observed results towards the null hypothesis. Since we still observed significant effects of visible mould, it is likely that mould plays an important role in infantile pneumonia.
In conclusion, this investigation observed a rate of 0·62% of hospitalized pneumonia in infants aged ⩽6 months. This condition was associated with maternal overweight, gestational antibiotic use, maternal smoking, prenatal exposure to ETS, and living in a mould-growing environment. Further study is warranted to determine the mechanisms and specific types of antibiotics related to infantile pneumonia when used during pregnancy. Maternal overweight and visible mould in the home should be monitored carefully because they may be important contributors to infantile pneumonia.
ACKNOWLEDGEMENTS
This article used a confidential unit record file from the Taiwan Birth Cohort Study. The authors thank all the public health workers, mothers, and caregivers for their participation in the Taiwan Birth Cohort Study.
DECLARATION OF INTEREST
None.




