Background
Avian influenza (AI) is an important viral disease caused by type A influenza viruses belonging to the viral family Orthomyxoviridae. Avian influenza viruses (AIVs) in general are a major cause of acute respiratory tract diseases in poultry and continue to cause high morbidity and mortality worldwide with sporadic transmission events to a wide range of mammals, including humans [Reference Webster1, 2]. Some AIV subtypes (e.g. H5, H7 and H9) have spread rapidly to and among domestic poultry and cause large-scale outbreaks resulting in severe damage to the poultry industry and have also been reported to cross the species barrier [Reference Alexander3, 4] causing severe clinical signs and even death [Reference Webster1]. Most of the AIV strains are non-pathogenic or cause only mild clinical disease. Pathogenic AIVs are broadly divided into two groups based on their ability to cause systemic disease. They are designated as low pathogenic avian influenza (LPAIV) and highly pathogenic avian influenza viruses (HPAIV) [Reference Alexander5]. The HPAIV H5N1 is currently deeply entrenched in poultry populations in many countries like Bangladesh, China, Indonesia, Vietnam, Egypt and this virus is transmitted incidentally to human [6]. Human infections with LPAIV and HPAIV of subtype H7N9 have recently threatened public health in China [7]. AIV H9N2 is generally associated with milder clinical signs in poultry, but this subtype may be transmitted to humans where it is known to cause milder disease and thus is less well identified [Reference Uyeki8]. During the last two decades, AIV subtypes H5, H9 and H7 have played a major role in influenza outbreaks in poultry in Asia and Europe [Reference Alexander3, Reference Brown9]. Much scientific and public health interest has focused on the HPAI H5N1 viruses in the recent years, but H7 and H9 viruses are also considered to bear pandemic potential. Most of the type A influenza viruses that are found in birds do not infect humans. However, some subtypes, such as H5N1, H7N3, H7N7, H7N9 and H9N2, have been observed to cause infection in people [Reference Nathanson10]. In particular, the goose/Guangdong (gs/GD) lineage of HPAIV H5N1 has evolved into a virus strain that is highly lethal in birds as well as in humans. Significant bird mortality with HP H5N1 is recorded in Bangladesh, Cambodia, China, Egypt, Indonesia and Vietnam [11]. Transmission of AIV between wild bird populations and domestic poultry species occasionally occurred and complicated the epidemiology and control of HPAI [Reference Suarez and Senne12]. Complex interactions between several viral and host factors are needed for successful AIV transmission and adaptation to new host species [Reference Yassine13]. Point mutations in the virus genome as well as reassortment may allow viruses to cross species barriers, adapt to new hosts and potentially increase virulence [Reference Neumann and Kawaoka14].
Both LPAIV H9N2 and the HPAIV H5N1 are co-circulating in the poultry population in Bangladesh since 2007. Continued co-circulation has raised possibilities of further evolution of these viruses, which created additional obstacles to virus eradication. Here, we undertook a systematic literature review in order to evaluate in-depth and in relation to the global situation the genetic variations among AIV circulating in Bangladesh. We also analysed studies on epidemiologic factors of AIV, especially H5N1 and H9N2, infections. In this review, we summarised insights into AIV distribution, routes of transmission, other potential risk factors, genetic diversity and pandemic potential in Bangladesh. We also tried to formulate recommendations for future improved AI surveillance and control strategies.
Methods
Search strategy
The following information and data sources were screened using a combination of terms related to ‘AIV H5N1’, ‘AIV H9N2’, ‘genetic characterization’, ‘genetic evolution’ ‘AIV Bangladesh’ and ‘AIV host ranges’ at ScienceDirect, PubMed, SCOPUS, EMBASE and Research Gate. In addition to the scientific literature search with special focus on recent publications, we also collected information from OIE, WHO, CDC and FAO websites regarding influenza outbreaks and classifications. For the sequence analysis, NCBI nucleotide and protein (GenBank) and GISAID databases were searched for HA gene of Bangladeshi (BD) H5N1 and H9N2 sequences as well as other representing strains of different genetic clades, sub-clades, lineages and sub-lineages. Reference tracking was undertook to verify further information for selection of the preferred literature.
Data extraction and analysis
The nucleotide and protein sequences of HA gene of BD AIV H5N1 and H9N2 were extracted and downloaded from GenBank and GISAID databases. In addition, other relevant sequences and representative strains were identified by NCBI blast search and downloaded. Relevant data were extracted, tabulated and key information extracted and correlated with our own analysis. Any bias was excluded during literature selection and extraction. The extracted sequence data were subjected to Clustal W multiple sequence alignment, and the deduced amino acid sequences were analysed with the BioEdit7.2 software program. Phylogenetic trees were generated using MEGA 6 software.
Results
AIVs in Bangladesh
With nearly 150 million human inhabitants on a landmass of 147 570 km2, Bangladesh is among the most densely populated countries in the world. The economy of Bangladesh heavily depends on its agricultural resources. Livestock, especially poultry, farming is a promising sector for poverty reduction. Approximately 75% of the human population in Bangladesh depends directly on poultry as a source of meat and eggs and household income [15]. Unfortunately, outbreaks of diseases, especially AI, are major constraints in poultry farming in Bangladesh. The high density and interdigitating of both human and poultry populations resulted in rapid disease spread among poultry flocks with a high risk of transmission to humans as a potential event of pandemic emergence.
AI in Bangladesh caused significant mortality resulting in huge economic losses of the country. Almost all the subtypes of AIV have been detected in wild aquatic birds in Bangladesh; however, AIV H5N1 and H9N2 are the main two pathogenic subtypes causing disease outbreaks in commercial poultry. In Bangladesh, since the first reported HPAIV H5N1 infection in 2007 [16], 550 additional outbreaks were notified at the OIE until the end of 2016 [16]. Worldwide, only four countries have reported a higher incidence so far. Although a reduced incidence of H5N1 HPAI outbreaks has been reported to OIE since 2013, H5N1 HPAIVs are still being isolated frequently from live-bird market samples in Bangladesh [Reference Marinova-Petkova17, Reference Turner18]. Following the control measures against HP H5N1, LP H9N2 became the predominant subtype throughout the country [Reference Turner18]. The most frequently identified other subtypes in different studies included H11N3, H4N6 and H1N1, whereas H1N2, H1N3, H1N5, H2N4, H2N5, H3N2, H3N6, H3N8, H4N2, H5N2, H6N1, H6N7, H7N5, H7N9 and H10N7 were less frequently detected in the country [Reference Sarker19–Reference Gerloff21]. LPAIV and HPAIV have frequently been detected in poultry samples from commercial firms and live-bird markets as well as in environmental samples [Reference Negovetich20–Reference Haque24].
In many countries, subtype H5 viruses circulated in low pathogenic form before the emergence of HP H5 was noted [Reference Kawaoka, Naeve and Webster25–Reference Suarez27]. However, no such indications exist for Bangladesh, as there was no systematic surveillance of AI before the HPAI outbreaks. Based on the fact that HP gs/GD lineages were identified during the first and subsequent outbreaks, it is considered highly likely that the highly pathogenic form has been introduced. Following the start of the present panzootic of HPAI H5N1 in 2003 and 2004 in Far-East and South-East Asia, an AI preparedness and response plan was developed for Bangladesh and a national reference laboratory for AI was established at Bangladesh Livestock Research Institute (BLRI) for confirmatory diagnosis of AI in Bangladesh. The first cases of H5N1 HPAI were detected in February 2007 [28], whereas H9N2 LPAIV was first isolated retrospectively in 2007 from a sample of a poultry farm collected in September 2006 [Reference Parvin29]. Since then both HPAI H5N1 and LPAI H9N2 viruses have co-circulated in poultry and village chickens. HPAI in Bangladesh has an apparent seasonal pattern. Each wave of outbreaks usually started in late autumn, peaking in spring and then declining gradually [Reference Ahmed30]. For many years, LPAI H9N2 virus caused mainly mild clinical infections with incidental significant losses in poultry production, but nowadays, observations of infection accumulated describing: visible clinical signs like depression, off feed, congested wattles, respiratory distress, nasal discharge on pressure, periorbital oedema or swollen face. In some cases, yellow-whitish-greenish feces, drop in egg production, morbidity up to 90% and mortality up to 45% (unpublished). Necropsy finding also may be confused with that of HPAI.
Host range
AIVs are detected mainly in waterfowl that inhabit wetland and aquatic environments, for example, in species of the orders Anseriformes and Charadriiformes. AIV infects a wide variety of bird species in nature. Wild aquatic birds are generally considered as the primary natural reservoir for AIVs [Reference Olsen31, Reference Webster32] as the 16 haemagglutinin (HA; H1–H16) and nine neuraminidase (NA; N1–N9) subtypes of influenza A viruses have been found in these birds worldwide [Reference Webster32, Reference Kaleta, Hergarten and Yilmaz33]. Globally, AIVs have been isolated from a number of avian and mammalian species demonstrating a wide host range. Generally, wild birds are believed to be responsible for the introduction of AIV to domesticated birds such as chickens, turkeys, quail and other game birds. These transmission events either occur through direct contact or contact with contaminated surfaces and water. Once the virus has established itself in domesticated birds through adaptation, the potential to spread to mammals is increased [Reference Olsen31–Reference Kaleta, Hergarten and Yilmaz33]. In Bangladesh, AIVs have already been isolated from a wide range of hosts including chickens, ducks, quails, pigeons, crows, migratory birds, humans, environmental samples [Reference Negovetich20, Reference Islam22, 34–Reference Parvin36] and recently from turkeys (unpublished) (Fig. 1). This wide host range facilitates the spread of the virus as well as it increases the chances of genetic recombination. The co-circulation of several subtypes in multiple hosts could contribute to enhanced adaptation of the viruses and their persistence in the country.
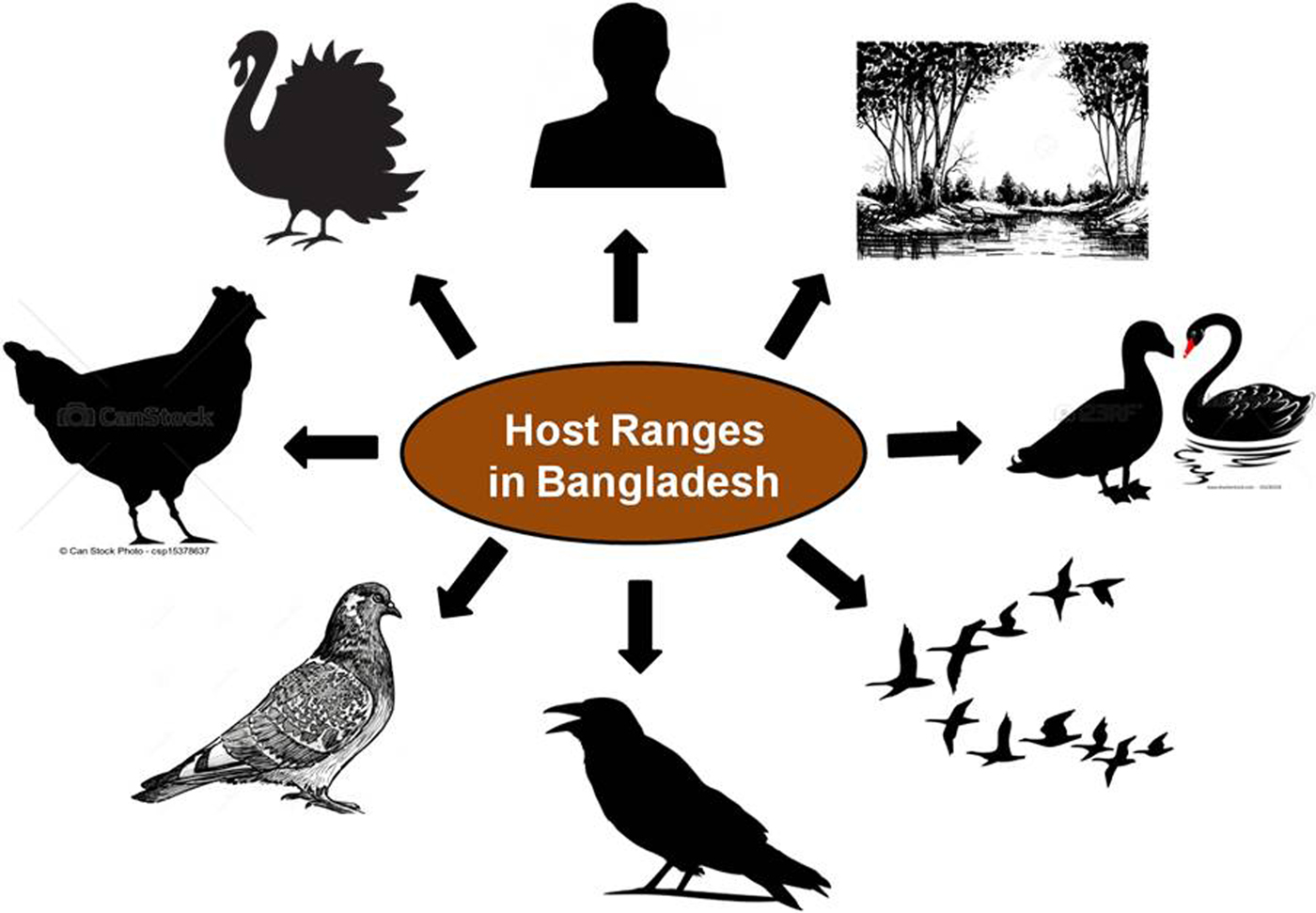
Fig. 1. Host range of avian influenza viruses in Bangladesh.
Genetic diversity of H5N1 in Bangladesh
The HPAIV H5N1 is of major concern of public health. The present devastating H5N1 virus was first observed in goose in the Chinese province of Guangdong in 1996 [Reference Smith37], hence the lineage is referred to as gs/GD. The first human infection with gs/GD viruses was detected in 1997 in Hong Kong [Reference Claas38]. Human cases with significant mortality rates are still being reported. Phylogenetic analyses of the HA genes of the gs/GD viruses resulted in the distinct classification of 12 clades [Reference Smith37]. These clades are further classified into several smaller sub-clades because HPAIVs have evolved through maintenance in the poultry and wild bird populations, in part, and possibly due to vaccination of domestic birds in particular regions [Reference Smith37, Reference Cattoli39]. A simplification to the previously defined criteria, which adds a letter rather than a number to the right-most digit of fifth-order clades, has been proposed to facilitate this and future updates [Reference Smith37]. Out of the 856 documented human HPAIV H5N1 infections worldwide between 2003 and 2016, 452 patients died [40]. There are a few countries, especially in South and South-East Asia, where HPAI H5N1 is no longer considered an epidemic but an endemic disease. These countries suffer from recurring outbreaks of HPAI H5N1 with occasional zoonotic spillover infections to humans [Reference Smith41]. An FAO–OIE–WHO collaboration has been working on measures in order to reduce the risks associated with zoonotic infections of influenza viruses. The collaboration project collects and updates information on HPAIV H5N1.
Genetic analyses previously reported and carried out here (Fig. 2 and Supplementary Fig. 1) revealed that, from February 2007 until the end of 2010, the circulating HPAI H5N1 viruses in Bangladesh clustered with gs/GD clade 2.2.2. However, at the beginning of 2011, new incursions of viruses of clades 2.3.2.1 and 2.3.4.2 were detected in chickens, quails, ducks, crows and migratory birds [Reference Islam22, Reference Haque24, Reference Parvin36]. A phylogenetic analysis of the isolates of 2012 and 2013 has revealed that all the isolates exclusively belonged to clade 2.3.2.1, and no further clades were detected in 2012 [Reference Haque24]. Now only clade 2.3.2.1 viruses are circulating in the country. Recently this clade exhibited genotypic variations as reported from different areas of the world. The BD H5N1 viruses previously grouped as clade 2.3.2.1 are now designated 2.3.2.1a. An extensive surveillance is needed to confirm whether clade 2.2 and clade 2.3.4 viruses have completely disappeared from Bangladesh or any new clade has introduced during the most recent years. HA protein sequence analysis of selected BD HP H5 isolates revealed genetic variations in the receptor binding site (RBS) and in glycosylation sequins. It was reported that four mutations in HA (H119Y, Q238L, G240S and T172A) of the H5N1 virus might be required to render it transmissible between mammals via respiratory droplets [Reference Herfst42]. Among the 78 analysed BD HP H5N1 isolates, 54 showed the T172A mutation, and this mutation led to the loss of a glycosylation site at position 170–172. As many as 52 isolates had a N170D mutation, which was shown to be responsible for improved virus replication and transmission in vivo by altering its receptor-binding preference from α2,3 to α2,6 sialic acid [Reference Imai43]. In summary, BD HP H5N1 belonged to three clades, and had four variations in HA cleavage site, two major mammalian host-specific mutations at the RBS and eight different types of glycosilation sites (Fig. 3), which might have implications with regard to pathogenicity of the viruses. There have been eight human cases of HPAI H5N1 so far in Bangladesh with one lethal outcome [40].
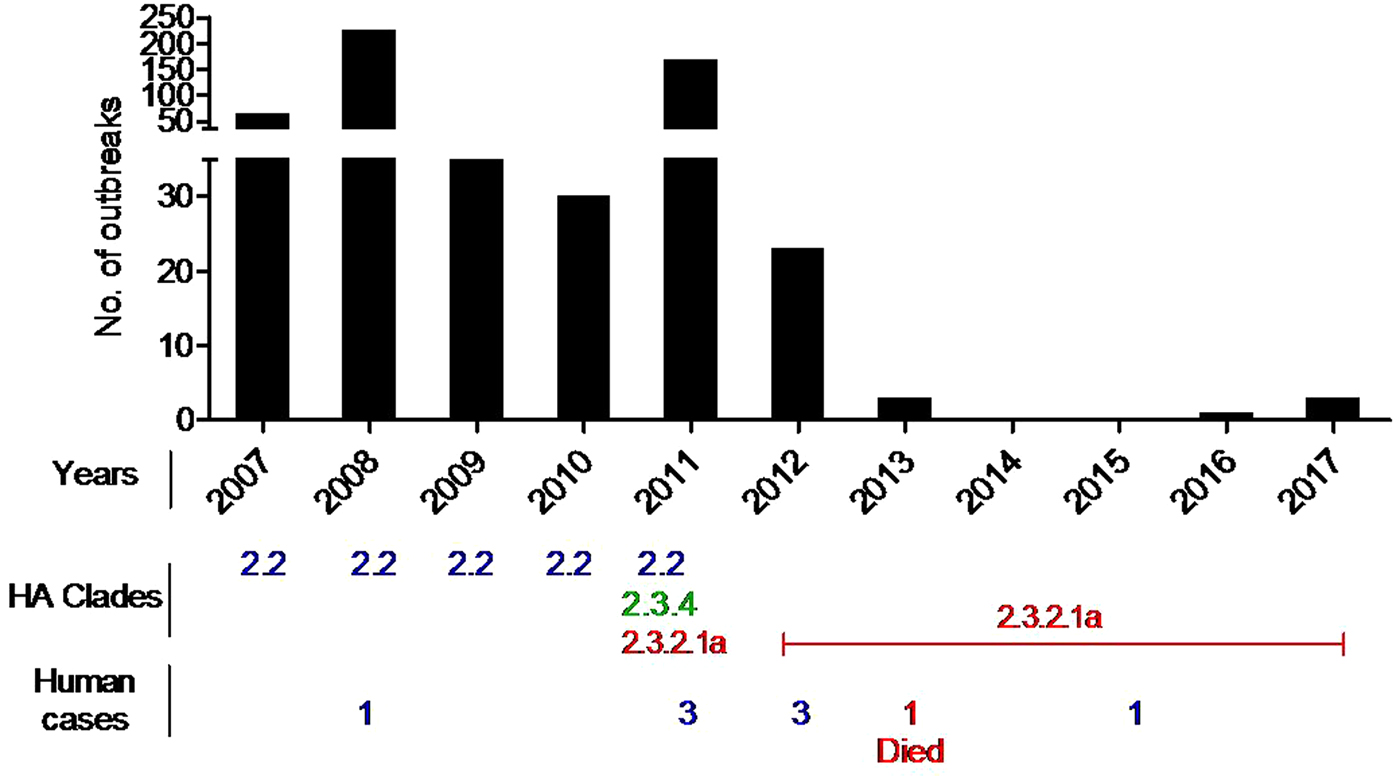
Fig. 2. HP H5N1 outbreaks in birds and humans in Bangladesh during 2007–2017.
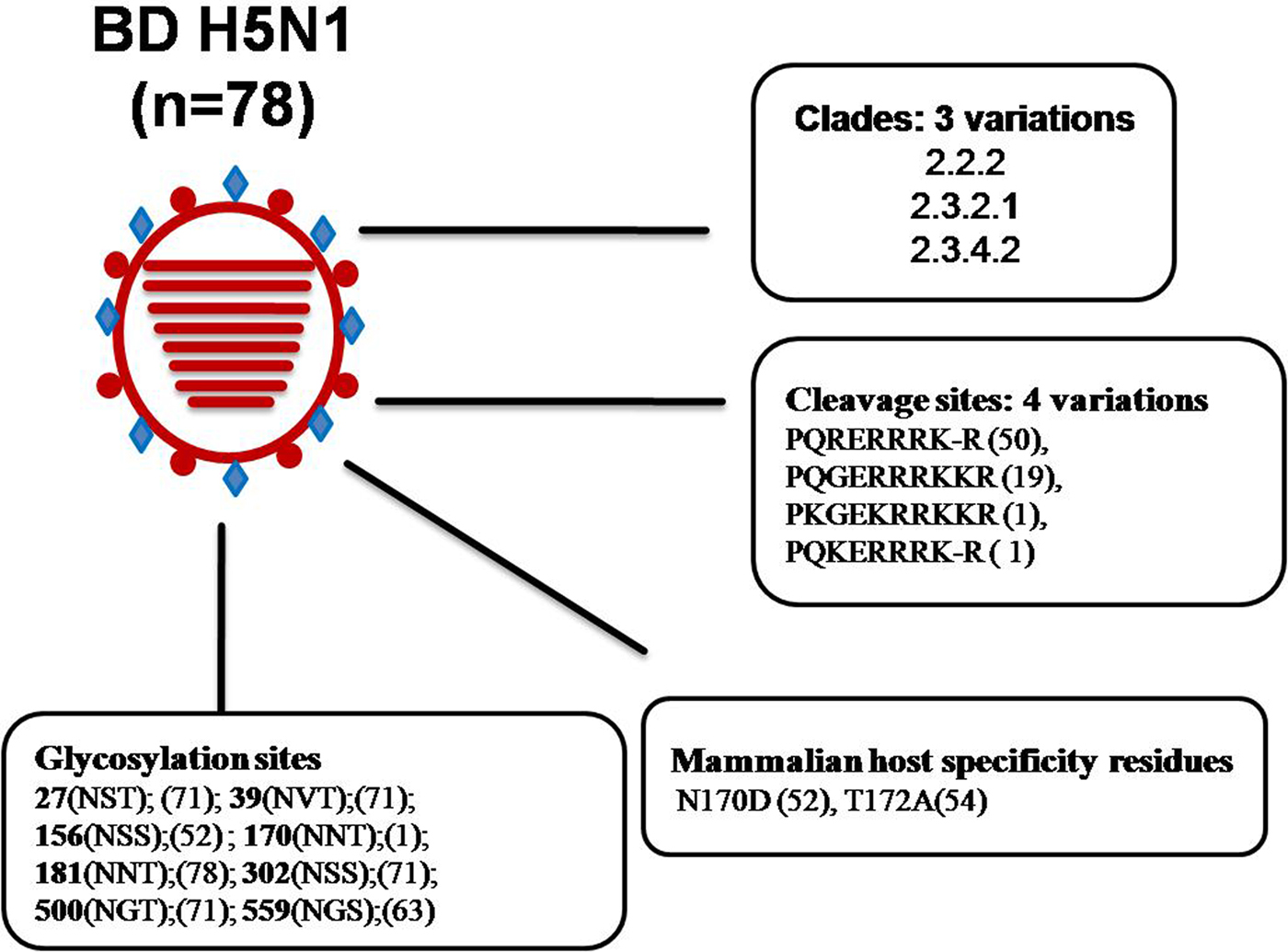
Fig. 3. Analysis of genetic determinants of host range and virulence in HA proteins of Bangladeshi (BD) H5N1 viruses. *Numbers in parentheses indicate number of viruses containing specific amino acid residues/glycosylation sites. *Abbreviations: A, alanine; D, aspartic acid; E, glutamic acid; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; N, asparagine; Q, glutamine; R, arginine; S, serine; T, threonine; V, valine.
Genetic diversity of H9N2 in Bangladesh
LPAIV H9N2 was first detected in the USA in 1966. AIVs of subtype H9N2 are widespread in nature, and are routinely isolated from wild birds, poultry and occasionally from pigs and other mammalian species [Reference Yu44]. They generally caused mild illness and became panzootic in the mid-1980s among chickens, ducks, turkeys, pheasants, quails, ostrich and migratory birds. By 1997, H9N2 viruses have been isolated from multiple avian species throughout Asia, the Middle East, Europe and Africa as well as for the first time from humans in Hong Kong and China in 1999. H9N2 viruses might emerge as human pathogens through mutations or reassortments in an intermediate host such as pigs [Reference Peiris45] or in avian species, or through direct adaptation to the human host [Reference Guan46]. Two distinct major lineages of H9N2 influenza viruses have been defined, the North American and the Eurasian lineages. The latter consists of at least three sub-lineages represented by their prototype strains: A/quail/Hong Kong/G1/97 (G1), A/duck/Hong Kong/Y280/9 (Y280), A/Chicken/Beijing/1/94 (BJ94) or A/chicken/Korea/38349-P96323/96 (Kr-P96323) [Reference Guan47]. The G1-H9N2 is thought to be the donor of six internal genes to the gs/GD HP H5N1 viruses isolated in 1997 [Reference Guan46]. During the last two decades, antigenic and genetic analyses of H9N2 isolates have shown gradual and complex diversification [Reference Guo48, Reference Dong49]. Several distinct sub-lineages of the Eurasian lineage became established in poultry [Reference Guan47, Reference Guo48]. Moreover, reassortments have occurred between H9N2 and H5N1, H7N3 and H7N9 viruses [Reference Parvin29, Reference Guan47, Reference Iqbal50, Reference Liu51]. In addition, some LPAIV H9N2 isolates have acquired increased affinity to human-like α2, 6 SA receptors. Therefore, the potential risk for these viruses to cross species barriers and affect humans is of major public health concern.
In Bangladesh, poultry is experiencing frequent H9N2 infection, where mitigation is often complicated by wrong diagnosis due to the lack of typical clinical symptoms in infected flocks. At present, LPAIV G1-H9N2 is the predominant AIV in domestic land-based poultry in Bangladesh, followed by HPAI H5N1 clade 2.3.2.1a. In addition to the sporadic outbreaks of HP H5N1, H9N2 viruses have been isolated frequently from commercial poultry flocks, live-bird markets as well as from the environment in Bangladesh [Reference Negovetich20, Reference Parvin29, Reference Shanmuganatham35]. Phylogenetically, it appears that all BD H9N2 viruses belong to the G1 lineage. The single exception is isolate A/environment/Bangladesh/1041/2009, which belongs to the Korean (Kr) lineage. Within the G1 lineage, BD H9N2 can be divided broadly into two groups with the formation of many sub-branches. A distant relationship with H9N2 isolates from India, Pakistan and the Middle East was maintained over years indicating towards autochthonous viral circulation in Bangladesh (Supplementary Fig. 2). Previously it has been shown that BD H9N2 shared internal genes with HPAI H7N3 virus from Pakistan [Reference Parvin29], but this reassortment might have taken place before these viruses introduced to Bangladesh. This finding is consistent with the available evidence to date, which suggested that co-circulation/reassortment of H7N3 and H9N2 viruses has only been observed in Pakistan [Reference Iqbal50]. Therefore, it is tempting to speculate that the H9N2 viruses were introduced into backyard poultry in Bangladesh from Pakistan via other neighbouring country through cross-border trading during the early 2000s [Reference Shanmuganatham23]. Later, it has been reported that H9N2 viruses of different genetic constellation have circulated in Bangladesh in the last decade [Reference Shanmuganatham23]. Moreover, LPAIV H9N2 also shared internal genes with contemporary circulating HP H5N1 [Reference Parvin29, Reference Shanmuganatham35]. The HA protein analysis of BD H9N2 isolates revealed that the isolates from 2006 to 2010 had a monobasic cleavage site (KSSR*GLF), whereas from 2010 to 2013 isolates showed a dibasic HA cleavage site (KSKR*GLF). Only one additional mutation at the cleavage site could lead to the emergence of H9N2 AIVs with a polybasic (four basic amino acid residues at a stretch) cleavage site like highly pathogenic AIVs. Most of the BD H9N2 viruses exhibited the Q234L substitution, which is critical for replication and direct transmission of viruses in mammals [Reference Wan and Perez52]. Furthermore, BD H9N2 isolates also contain amino acid residues N166D, H191, E399K located within the RBS of the HA protein, which favours transmission of H9N2 in mammals via respiratory droplets [Reference Guan46]. All the analysed H9N2 subtypes from Bangladesh have seven potential glycosylation sites (position 29, 105, 141, 298, 305, 492 and 551). On the other hand, two potential glycosylation sites are lost at positions 206 and 218, which were originally present in A/quail/Hong Kong/G1/97 (G1 lineage) and A/duck/Hong Kong/Y280/9 (Y280 lineage) ancestral viruses. It has been mentioned that acquisition and loss of glycosylation sites within the HA can affect host specificity, virulence and infectivity of an influenza virus directly by changing the biological properties of HA [Reference Schulze53]. In summary, LPAIV H9N2 of two different lineages (G1 and Kr) have been introduced in Bangladesh, having five variations in the HA cleavage site, four mammalian host-specific mutations at the RBS and 10 different patterns of glycosilation sites (Fig. 4), indicating continuing evolution of H9N2 viruses in Bangladesh.
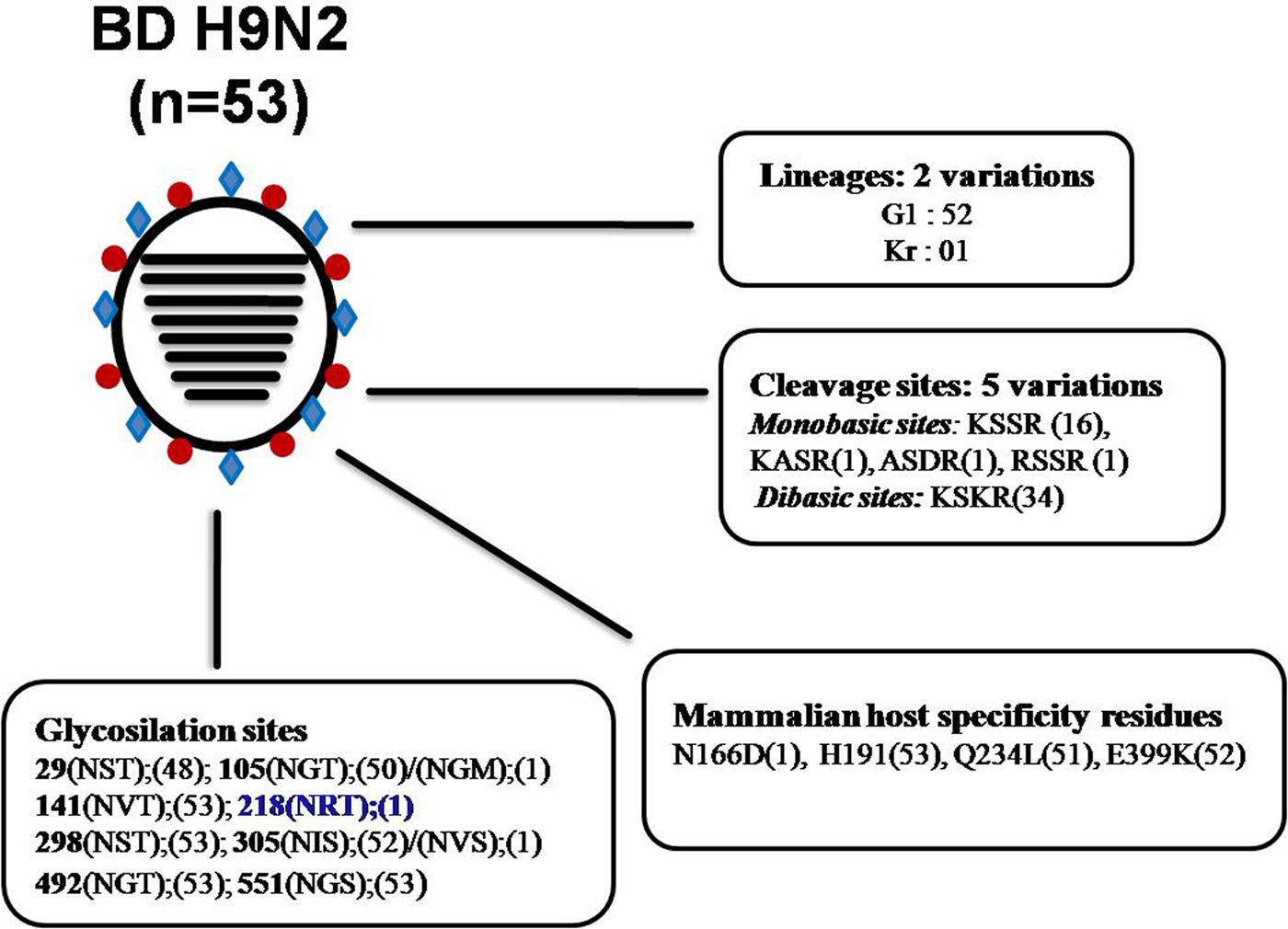
Fig. 4. Analysis of genetic determinants of host range and virulence in HA proteins of Bangladeshi (BD) H9N2 viruses. *Numbers in parentheses indicate number of viruses containing specific amino acid residues/glycosylation sites. *Blue bold indicates an additional glycosylation site only present in A/environment/Bangladesh/1041/2009 H9N2 isolate. *Abbreviations: A, see figure 3.
The continuing evolution of H9N2 poses a constant threat with regard to the future AI situation in Bangladesh and beyond. Three human infections with LPAIV H9N2 have been identified in Bangladesh so far [40]. The G1-H9N2 viruses are reportedly donors of internal genome segments to subtype H5N1, H7N3, H7N9 and H10N8 viruses all of which have already been reported to infect humans in China. The BD H9N2 viruses share genes with two highly pathogenic viruses of subtype H7N3 and H5N1 (Fig. 5). The continued evolution of H9N2 viruses characterised by a steady accumulation of mutations has led to the increasing differentiation of BD H9N2 viruses from other H9N2 lineages circulating in neighbouring countries.
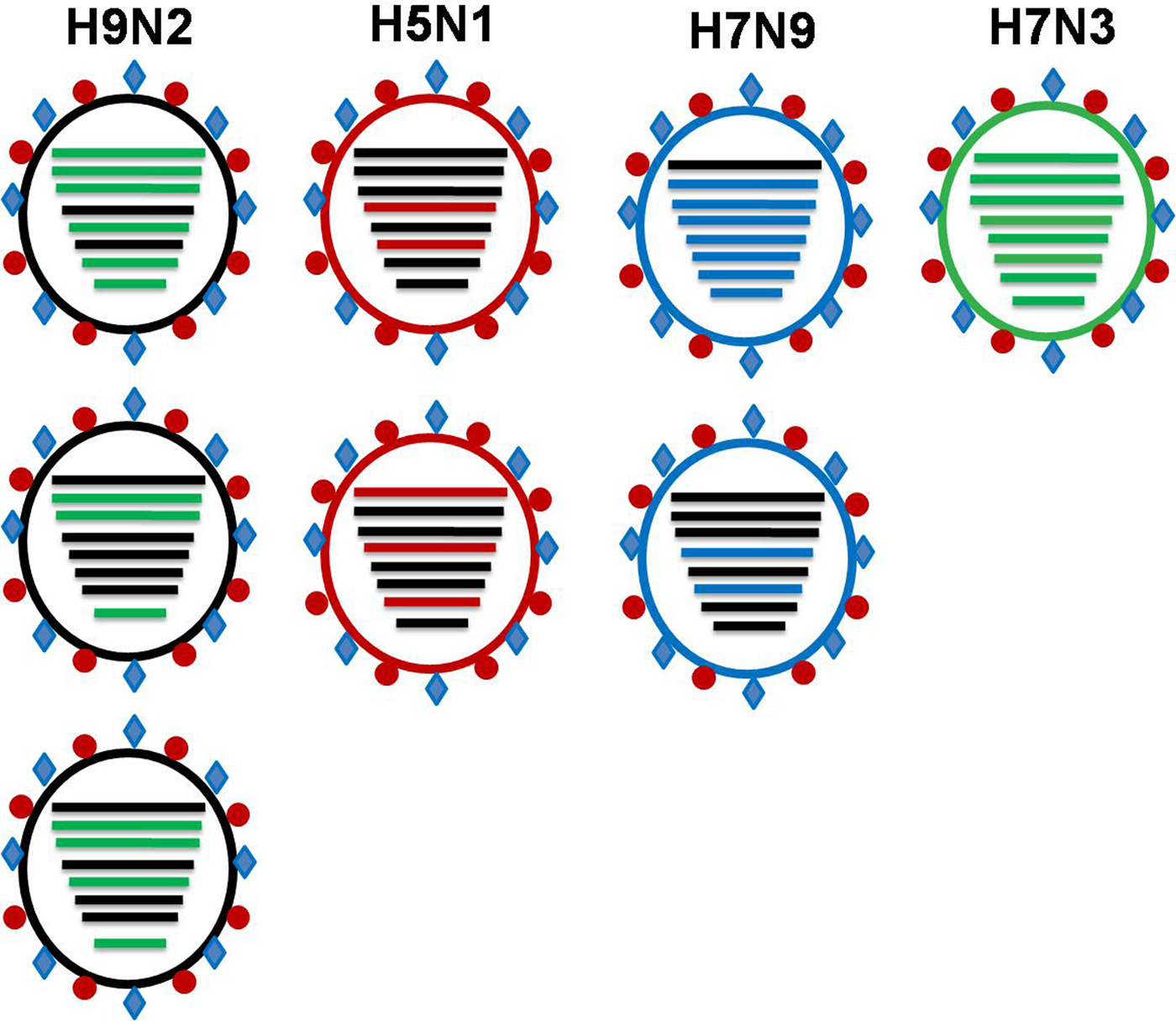
Fig. 5. Commonly observed sharing of internal genes among pathogenic subtypes of avian influenza viruses (Adopted from references [Reference Shanmuganatham23, Reference Parvin29, Reference Guan46, Reference Iqbal50, Reference Wu54]).
Risk factors and control strategies of AI in Bangladesh
The territory of Bangladesh consists of a broad deltaic plain subject to frequent flooding of two major rivers, the Jamuna (Brahmaputra) and Padma (Ganges), and their many tributaries. The abundance of shallow coastal waters provides a large reservoir for wildlife, especially wild waterfowl, which migrate from various parts of Northern and Central Asia to overwinter in the delta [55]. In addition, it is estimated that about 90% of rural households in Bangladesh rear poultry at their backyards, which is an important source of animal protein and income for the household [Reference Sultana56]. Backyard poultry comprises chickens and ducks that are reared together and commonly left to scavenge for food during the day, thereby having frequent contact with wild birds. AIVs spread either in their natural hosts (aquatic wild birds) or through poultry trade within the country. The panzootic spread of HPAIV H5N1 has been attributed to the movement of infected poultry and poultry products and, in some cases, transmission by wild migratory birds [Reference Chen57, Reference Kwon58]. Migratory birds were considered one of the potential sources of introductions of new clades of HP H5N1 in Bangladesh in 2011 [Reference Parvin36]. Infection with and subsequent spread of HPAI H5N1 virus strains in various avian species poses a constant and acute threat to human health worldwide [Reference Claas38, Reference Peiris59]. In Bangladesh, both HPAI H5N1 and LPAIV H9N2 caused human infections usually after direct contact exposure with infected poultry flocks [Reference Parvin36]. Isolation of H9N2 viruses of the same genotype from poultry but not wild birds in different geographic regions of the country at different time points indicate that the virus has been dispersed via human-driven poultry movements, e.g. in live-bird markets. Moreover, lack of awareness and lack of knowledge and practices of biosecurity in small-scale poultry farms also contributed to continuing HPAI and LPAI infections in Bangladesh. Stamping-out of poultry holdings with notifiable AI infections has been the national policy in Bangladesh in combating H5N1 HPAI, which was implemented after the incursion of H5N1 HPAI infection. However, high population densities of both human and poultry, the heterogeneous structure of the poultry industry and inadequate capacity of veterinary services hindered the success of stamping out. Towards the end of 2013, vaccination against HPAIV H5N1 has been introduced. However, the vaccination campaign is no longer strictly regulated by the veterinary authority. Farmers themselves decide to vaccinate their birds or not. This leads to poor vaccination coverage making the control of the disease more difficult. In contrast to H5N1, H9N2 infection is not notifiable in Bangladesh and vaccination against H9N2 is not permitted. While the risk of reintroduction of AIV through migratory birds will always remain, awareness of the farmers, strict biosecurity at farms and gradual phasing out of live-bird markets are the key measures for controlling the spread of AIVs, where stamping out is not a practicable option. Vaccination also could be an additional tool but that need to be time bound, well planned and well regulated with comprehensive coverage. Finally, AIV control programmes must be complemented by systematic and continuing surveillance of AIVs at farms, live-bird markets and the environment.
Conclusion
Many subtypes of AIVs have been detected in aquatic birds, live-bird markets and environment in Bangladesh. So far, H5N1 HPAI and H9N2 LPAI have been responsible for economically important clinical AI outbreaks in poultry. Until to date, three different clades of HP H5N1 viruses and two different lineages of H9N2 viruses are circulating. The co-circulation of these viruses may foster further genetic modifications, e.g. through gene reassortments. Possible emergence of a pandemic influenza virus from zoonotic AIV remains a valid threat for public health. AIV control measures in Bangladesh, where the viruses are well entrenched in the poultry population, need to be revisited with greater emphasis on biosecurity. Continuing AIV surveillance and genetic characterisation of the viruses are essential for monitoring the effectiveness of control measures and analysing the future risks.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818001292.
Acknowledgements
The review analysis helped by the previous research work that has been supported by a research grant from the World Academy of Science (TWAS) and German Academic Exchange Service (DAAD). The authors also are thankful to Professor Dr Timm Harder, Head of the O.I.E. and NR Laboratory for Avian Influenza, Federal Research Institute for Animal Health (FLI), Insel Riems, Greifswald, Germany, for his critical review and suggestions on this manuscript.
Conflict of interest
None.








