Published online by Cambridge University Press: 23 June 2020
X-ray reference powder patterns and structures have been determined for a series of cobalt- and tungsten-containing cubic alkaline-earth perovskites, (BaxSr1–x)2CoWO6 (x = 0.1, 0.2, 0.3, 0.5, 0.7, and 0.9). The structure of the end members of the series, Sr2CoWO6 and Ba2CoWO6, were tetragonal and cubic, respectively, agreeing with the literature data. From Rietveld refinements, it was found that when x = 0.1 and 0.2, the structure was tetragonal I4/m (a = 5.60481(6) and 5.62305(11) Å and c = 7.97989(12) and 7.9847(2) Å, respectively; Z = 2). When x > 0.2, the structure was cubic (Fm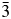 $\bar{3}$m, No. 225; Z = 4) (from x = 0.3 to 0.9, a increases from 7.98399(13) to 8.08871(10) Å). This tetragonal series of compounds exhibit the characteristics of a distorted double-perovskite structure. The bond valence sum values for the alkaline-earth (Ba, Sr) sites in all (BaxSr1−x)2CoWO6 members are greater than the ideal value of 2.0, indicating over-bonding situation, whereas for the W sites, as x increases, a change from under-bonding to slightly over-bonding situation was observed. Density functional theory calculations revealed that while Sr2CoWO6 is a semiconductor, Ba2CoWO6 and SrBaCoWO6 are half-metals. Powder X-ray diffraction patterns of this series of compounds (BaxSr1−x)2CoWO6, with x = 0.1, 0.2, 0.3, 0.5, 0.7, and 0.9, have been submitted to be included in the Powder Diffraction File.
$\bar{3}$m, No. 225; Z = 4) (from x = 0.3 to 0.9, a increases from 7.98399(13) to 8.08871(10) Å). This tetragonal series of compounds exhibit the characteristics of a distorted double-perovskite structure. The bond valence sum values for the alkaline-earth (Ba, Sr) sites in all (BaxSr1−x)2CoWO6 members are greater than the ideal value of 2.0, indicating over-bonding situation, whereas for the W sites, as x increases, a change from under-bonding to slightly over-bonding situation was observed. Density functional theory calculations revealed that while Sr2CoWO6 is a semiconductor, Ba2CoWO6 and SrBaCoWO6 are half-metals. Powder X-ray diffraction patterns of this series of compounds (BaxSr1−x)2CoWO6, with x = 0.1, 0.2, 0.3, 0.5, 0.7, and 0.9, have been submitted to be included in the Powder Diffraction File.
Please note a has been issued for this article.