Regular physical activity has been suggested to have many beneficial effects on health (Physical Activity Guidelines Advisory Committee, 2008), but it is difficult to carry out very long-term randomized and controlled exercise trials. Moreover, selection issues, including genetic pleiotropy, are problematic when trying to evaluate causality on the basis of observational follow-up studies, since it is easier to participate in physical activity or increase the level of activity for genetically more physically fit, lean, and metabolically healthy individuals than for those who are less fit (Kujala, Reference Kujala2011). In addition, compared to the benefits from high aerobic capacity and related good oxidative lipid metabolism among physically active individuals, unfit subjects may have undiagnosed pre-disease states that cause inactivity and increase the risk of chronic diseases or death.
The Finnish Twin Cohort has been used as a natural experimental approach to investigate whether genes or other familial factors can cause selection bias in epidemiological studies of the associations between physical activity and future morbidity or mortality. Findings suggest that genetic pleiotropy may explain some of the association between high physical activity and low mortality, because in pairwise analyses among physical activity-discordant twin pairs, the association is more apparent among DZ than among MZ pairs (Kujala et al., Reference Kujala, Kaprio, Sarna and Koskenvuo1998; Reference Kujala, Kaprio and Koskenvuo2002). However, larger collaborative studies are needed to confirm these findings.
The aim of the TWINACTIVE study was to describe associations of long-term physical activity versus inactivity with health-related outcomes, after controlling for sequence-level genetic differences. Twin pairs in which the co-twins of a pair had different leisure-time physical activity habits throughout their adult life were comprehensively identified from the older Finnish Twin Cohort (Leskinen et al., Reference Leskinen, Waller, Mutikainen, Aaltonen, Ronkainen, Alen and Kujala2009b). In this synopsis paper, we summarize our previously published findings from the TWINACTIVE study and discuss the findings compared to some other twin studies and studies on individuals published by other researchers. Our focus is in the TWINACTIVE study findings, but we want to acknowledge that many other twin studies have also evaluated the associations between physical activity and health (e.g., Andel et al., Reference Andel, Crowe, Pedersen, Fratiglioni, Johansson and Gatz2008; Videman et al., Reference Videman, Batti, Gibbons, Vanninen, Kaprio and Koskenvuo2002; Waller et al., Reference Waller, Kaprio, Lehtovirta, Silventoinen, Koskenvuo and Kujala2010; Williams et al., Reference Williams, Blanche and Krauss2005).
Materials and Methods
The comprehensive selection of physical activity-discordant twin pairs from the older Finnish Twin Cohort (Kaprio & Koskenvuo, Reference Kaprio and Koskenvuo2002), which included 5,663 healthy twin pairs in year 1981, was initially based on questionnaire data regarding self-reported leisure-time physical activity volume in 1975 and 1981. The questionnaire included questions of leisure-time physical activity (mean duration, monthly frequency, and mean intensity of sessions) and activity during work journeys, expressed as leisure-time physical activity volume, that is, MET-index as MET hours/day (calculated from intensity • duration • frequency of exercise, where intensity is described in terms of metabolic equivalent task (MET); Kujala et al., Reference Kujala, Kaprio, Sarna and Koskenvuo1998). Later, in 2005, a retrospective telephone interview was conducted for only those pairs who were discordant for their leisure-time physical-activity volumes in 1975 and 1981 (n = 165 pairs, of which 132 pairs were interviewed). The telephone interview included the same leisure-time physical activity questions as in the 1975 and 1981 questionnaires and enabled us to calculate the volume of past years’ leisure-time physical activity (expressed as MET-index) at intervals of five years, from 1980 to 2005. In 54 pairs, the discordance in leisure-time physical activity MET-indexes existed at the majority –– if not all –– of the follow-up time points (see Figure 1). Of these pairs, seven MZ (five male and two female pairs) and nine DZ pairs (six male and three female pairs) (mean age 60 years, range 50–74 years) were capable of and willing to participate in the TWINACTIVE study measurements. The selection of discordant twin pairs is presented in detail in the original article by Leskinen et al. (Reference Leskinen, Waller, Mutikainen, Aaltonen, Ronkainen, Alen and Kujala2009b). The structured physical-activity questionnaire that was used to measure leisure-time physical activity volume (as the MET index) was shown previously to have a high correlation with detailed interview-based physical-activity assessments covering 12 months (Waller et al., Reference Waller, Kaprio and Kujala2008).

FIGURE 1 Flow chart of the selection of physical activity-discordant twin pairs.
The TWINACTIVE study included multiple health-related measurements, which are fully described in the original articles. Briefly summarized, we included: (1) standardized questionnaires and interviews on leisure-time physical activity (Leskinen et al., Reference Leskinen, Waller, Mutikainen, Aaltonen, Ronkainen, Alen and Kujala2009b), smoking habits, use of alcohol, diet (Rintala et al., Reference Rintala, Lyytikäinen, Leskinen, Alen, Pietiläinen, Kaprio and Kujala2011), and psychological exercise motivation (Aaltonen et al., Reference Aaltonen, Leskinen, Morris, Alen, Kaprio, Liukkonen and Kujala2012); (2) echocardiographic measurements (Mutikainen et al., Reference Mutikainen, Perhonen, Alen, Leskinen, Karjalainen, Rantanen and Kujala2009); (3) symptom-limited clinical exercise test with cycle ergometer to calculate peak exercise capacity from the time-weighted peak load (Leskinen et al., Reference Leskinen, Waller, Mutikainen, Aaltonen, Ronkainen, Alen and Kujala2009b); (4) maximal isometric strength tests (Leskinen et al., Reference Leskinen, Sipilä, Kaprio, Kainulainen, Alen and Kujala2013); (5) peripheral quantitative computed tomography measurements (Ma et al., Reference Ma, Leskinen, Alen, Cheng, Sipilä, Heinonen and Kujala2009); (6) anthropometric and body composition measurements (Leskinen et al., Reference Leskinen, Sipilä, Alen, Cheng, Pietiläinen, Usenius and Kujala2009a); (7) collection of fasting blood and DNA samples (Leskinen et al., Reference Leskinen, Sipilä, Kaprio, Kainulainen, Alen and Kujala2013), oral glucose tolerance tests (Leskinen et al., Reference Leskinen, Sipilä, Kaprio, Kainulainen, Alen and Kujala2013), plasma lipidomic analyses (Leskinen et al., Reference Leskinen, Rinnankoski-Tuikka, Rintala, Seppänen-Laakso, Pöllänen, Alen and Kujala2010), and metabolomics analyses (Kujala et al., Reference Kujala, Mäkinen, Heinonen, Soininen, Kangas, Leskinen and Ala-Korpela2013); and (8) magnetic resonance imaging of the thigh and abdominal region (Leskinen et al., Reference Leskinen, Sipilä, Alen, Cheng, Pietiläinen, Usenius and Kujala2009a) and contrast-enhanced magnetic resonance angiography (Leskinen et al., Reference Leskinen, Usenius, Alen, Kainulainen, Kaprio and Kujala2011). In addition, from healthy volunteers (three MZ and seven DZ pairs from the TWINACTIVE participants), we took muscle and subcutaneous adipose tissue samples a few weeks after the first laboratory visit for gene expression arrays (Leskinen et al., Reference Leskinen, Rinnankoski-Tuikka, Rintala, Seppänen-Laakso, Pöllänen, Alen and Kujala2010). The authors declare that all procedures that contributed to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Statistical Analysis
Pairwise analyses were used to study the significance of intrapair differences between the inactive versus active co-twins of twin pairs. The results were analyzed for all twin pairs and for the MZ and DZ pairs separately to find out whether the trends were similar or different. As MZ pairs share all their segregating genes, it can be hypothesized that any intrapair difference between the co-twins of a MZ pair is due to environmental factors (in this study, mostly physical activity). A standardized mean difference was calculated by dividing the difference of means between active and inactive MZ co-twins by the standard deviation of the same outcome among them. When studying individual-based associations, the within-pair dependency of twin individuals was taken into account by using the cluster option in Stata software (svy:regress).
Results and Discussion
Leisure-Time Physical Activity and Fitness
Members of the twin pairs did not differ with respect to other environmental factors, such as smoking, alcohol, or work-related physical activity habits, either at baseline (1975) or after the follow-up (2007; Leskinen et al., Reference Leskinen, Waller, Mutikainen, Aaltonen, Ronkainen, Alen and Kujala2009b). The mean intrapair difference in leisure-time physical activity MET indexes between the inactive and active twin during the follow-up period was 8.8 MET hours/day, which corresponds to a volume of, for example, a light two-hour daily walk (2.2 ± 2.3 vs. 11.0 ± 4.1 MET hours/day; p < .001, n = 16 pairs). Similar significant intrapair differences in mean follow-up MET values were found between inactive and active MZ (3.4 ± 3.0 vs. 12.2 ± 5.4; p = .018, n = 7 pairs) and DZ (1.3 ± 0.9 vs. 10.1 ± 2.8; p = .008, n = 9 pairs) co-twins (Figure 2). According to a detailed physical activity questionnaire administered during the laboratory visit, the favorite types of activities for the active twins during the previous 12 months before taking part in the TWINACTIVE study measurements were walking (25% of the total 12-month physical activity), jogging (11%), and cross-country skiing (9%). The most common types of activities reported by the inactive twins were repair work (27%), walking (22%), and woodworking (such as woodcutting; 10%). Thus, the active twins engaged mostly in aerobic types of activities during their leisure time. As expected, the active twins had at least 20% higher peak exercise capacity (9.3 METs vs. 7.5 METs; p < .001, n = 16 pairs), accompanied by one-fifth higher lower-limb strength levels, compared to the inactive co-twins (Leskinen et al., Reference Leskinen, Sipilä, Kaprio, Kainulainen, Alen and Kujala2013). Similar intrapair differences in fitness outcomes existed when only MZ pairs were included in the analysis (Figure 3).
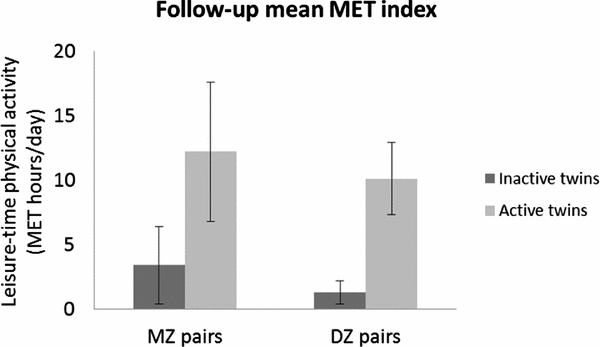
FIGURE 2 Follow-up mean MET indexes in co-twins of physical activity-discordant monozygotic (n = 7) and dizygotic (n = 9) pairs.

FIGURE 3 Standardized mean differences (SMD; pairwise difference/standard deviation of the variable among the members of the pairs) in selected fitness, body composition, and bone and arterial structural outcomes among consistently active versus inactive monozygotic (MZ) co-twins (for details, see original articles by Leskinen et al., Reference Leskinen, Sipilä, Alen, Cheng, Pietiläinen, Usenius and Kujala2009a; Reference Leskinen, Usenius, Alen, Kainulainen, Kaprio and Kujala2011; Reference Leskinen, Sipilä, Kaprio, Kainulainen, Alen and Kujala2013; Ma et al., Reference Ma, Leskinen, Alen, Cheng, Sipilä, Heinonen and Kujala2009).
Exercise Motivation
Pairs of middle-aged and older co-twins who have had totally different leisure-time physical-activity levels during their adult life, yet shared childhood environment, provide a unique opportunity to study reasons for this uncommon divergent behavior. From the questionnaire data regarding motives for and barriers to exercise training, we found that, in particular, intrinsic motivational factors (e.g., exercise enjoyment, sports for health, and willingness to advance in sports) differed between the co-twins of the pairs, in favor of the physically active members of the pairs (Aaltonen et al., Reference Aaltonen, Leskinen, Morris, Alen, Kaprio, Liukkonen and Kujala2012). Interestingly, however, the barriers to exercise training (including lack of time) did not differ between the co-twins of the pairs. On the basis of our younger discordant pair study findings, family- and work-related commitments, accompanied by differences in physical activity habits in adulthood, may contribute to the progression of motivational differences (Rottensteiner et al., Reference Rottensteiner, Leskinen, Niskanen, Aaltonen, Mutikainen, Wikgren and Kujala2015).
Artery Structure
Contrast-enhanced magnetic resonance angiography analysis revealed at least 19% greater lumen diameters in distal aorta, iliac, and femoral arteries at the resting state among active compared to inactive MZ twins (p < .05 for all comparisons; Figure 3). Theoretically, this could indicate that the cross-sectional areas of the vessel lumens were at least 1.5 times greater, allowing for increased blood flow to regularly working lower limb muscles. This finding seems to be site specific, as similar exercise-induced enlargement of carotid arteries could not be demonstrated with this measurement technique (see Leskinen et al., Reference Leskinen, Usenius, Alen, Kainulainen, Kaprio and Kujala2011). These findings correspond with the data from ultrasound-based studies (e.g., Dinenno et al., Reference Dinenno, Tanaka, Monahan, Clevenger, Eskurza, DeSouza and Seals2001; Huonker et al., Reference Huonker, Schmid, Schmidt-Trucksass, Grathwohl and Keul2003; Spence et al., Reference Spence, Carter, Naylor and Green2013).
Lower-Limb Bone Structure
The amount and type of physical activity among the active twins induced detectable positive changes in the tibia bone strength in a site- and direction-specific manner (Ma et al., Reference Ma, Leskinen, Alen, Cheng, Sipilä, Heinonen and Kujala2009). In the tibia shaft, cortical bone was thickened in the anteroposterior direction (Figure 3), and in the distal tibia, trabecular bone density was increased among the active compared to the inactive co-twins. These changes suggest a decreased risk of osteoporosis and related fractures among persistently active individuals, independent of the genetic background. The thickening of the cortical bone among the physically active members of the twin pairs was mostly inward and not due to increases in the external dimensions of the bone, as the enlargement of bones due to loading seems to be related more to loading during growth (Kannus et al., Reference Kannus, Haapasalo, Sankelo, Sievänen, Pasanen, Heinonen and Vuori1995). In addition, compared to the more systematic changes induced by hormone replacement therapy in early post-menopause, the exercise-associated changes were clearly more site specific (Mikkola et al., Reference Mikkola, Heinonen, Kovanen, Cheng, Kujala, Suominen and Sipilä2011).
Body Fat Distribution
The most important finding concerning body weight and composition among the physical activity-discordant twin pairs was that at the end of the follow-up, body weight, body mass index (BMI), and fat free mass did not differ between the members of the pairs as strongly as body fat mass and its distribution (Leskinen et al., Reference Leskinen, Sipilä, Alen, Cheng, Pietiläinen, Usenius and Kujala2009a). This phenomenon was also seen in the genetically standardized analysis of MZ pairs (Figure 3) and can be explained by the fact that both physical activity and genetic background have an influence on body weight and fat free mass but, at least from the physiological perspective, long-term engagement with higher levels of physical activity improves fatty acid usage ability (Achten & Jeukendrup, Reference Achten and Jeukendrup2004, Holloway et al., Reference Holloway, Luiken, Glatz, Spriet and Bonen2008) and can ultimately prevent unhealthy fat distribution (Rottensteiner et al., Reference Rottensteiner, Pietiläinen, Kaprio and Kujala2014). Our visceral and liver fat findings (Leskinen et al., Reference Leskinen, Sipilä, Alen, Cheng, Pietiläinen, Usenius and Kujala2009a), which favored the active and lean co-twins independent of genetic background, agree with the results from other co-twin control studies in younger Finnish MZ twin pairs discordant for obesity (Pietiläinen et al., Reference Pietiläinen, Rissanen, Kaprio, Mäkimattila, Häkkinen, Westerbacka and Yki-Järvinen2005) and those discordant for physical activity and fitness (Hannukainen et al., Reference Hannukainen, Borra, Linderborg, Kallio, Kiss, Lepomäki and Nuutila2011). Our intramuscular fat findings are in line with randomized controlled studies showing that participation in physical activity can prevent further muscle fat infiltration among older participants compared to controls (Goodpaster et al., Reference Goodpaster, Chomentowski, Ward, Rossi, Glynn, Delmonico and Newman2008) and exercise-induced decreased levels of intermuscular fat in middle-aged women and men (Durheim et al., Reference Durheim, Slentz, Bateman, Mabe and Karaus2008).
Risk Factors/Metabolites Measured in Venous Blood Samples
Our comprehensive metabolite findings are more extensively presented in our original article (Kujala et al., Reference Kujala, Mäkinen, Heinonen, Soininen, Kangas, Leskinen and Ala-Korpela2013). Briefly, we found significant intrapair differences in plasma glucose levels and in the ratio of apolipoprotein B to A1; these findings indicate lower cardiovascular risk in physically active versus inactive twins. Sensitive indicators of physical activity associated changes in cardiometabolic risk were increased high-density lipoprotein (HDL) particle diameter and less-saturated serum fatty acid composition, as well as decreased isoleucine and α1-acid glycoprotein levels, among the physically active co-twins. The findings persisted after adjustment for BMI and thus show better metabolic health in physically active individuals (see Kujala et al., Reference Kujala, Mäkinen, Heinonen, Soininen, Kangas, Leskinen and Ala-Korpela2013).
Gene Expression in Muscle and Fat Tissue
In skeletal muscle tissue, gene expression of the central pathways of energy metabolism and supportive metabolic pathways, especially those genes related to the processes of oxidative phosphorylation (OXPHOS), were upregulated among the physically active compared to inactive co-twins (see Leskinen et al., Reference Leskinen, Rinnankoski-Tuikka, Rintala, Seppänen-Laakso, Pöllänen, Alen and Kujala2010). Interestingly, the adaptations in muscle gene expression were similar to those in rats with a higher intrinsic/inherited aerobic capacity compared to low-capacity runner rats (Kivelä et al., Reference Kivelä, Silvennoinen, Lehti, Rinnankoski-Tuikka, Purhonen, Ketola and Kainulainen2010), suggesting that there are common underlying mechanisms for long-term physical activity and inherited high aerobic capacity. Moreover, suppressed expression of oxidative pathway genes in adipose tissue has previously been found among obese MZ twins compared to their non-obese co-twins (Mustelin et al., Reference Mustelin, Pietiläinen, Rissanen, Sovijärvi, Piirilä, Naukkarinen and Yki-Järvinen2008).
In subcutaneous fat tissue samples, the upregulated pathways in active compared to inactive twins included branched-chain amino acid (BCAA) degradation and polyunsaturated fatty acid synthesis. In line with our data, intrapair differences in BCAA catabolism and numbers of mitochondria were observed previously in the adipose tissue of MZ pairs discordant for obesity (Pietiläinen et al., Reference Pietiläinen, Naukkarinen, Rissanen, Saharinen, Ellonen, Keränen and Peltonen2008). Dysregulated BCAA metabolism leads to increased serum BCAA levels and is associated with the development of insulin resistance and glucose intolerance, ultimately leading to type 2 diabetes. Together with previously published data, the findings of our twin study suggest that physical activity maintains normal BCAA catabolism among consistently active individuals (Kainulainen et al., Reference Kainulainen, Hulmi and Kujala2013). This is because, via glyceroneogenesis, BCAA catabolism mediates increased constitutive use of fatty acids for β-oxidation in subjects with increased physical activity (see Kainulainen et al., Reference Kainulainen, Hulmi and Kujala2013). Therefore, high BCAA levels can be regarded as indicators of disorders in normal oxidative lipid metabolism.
Our gene expression data also support the concept that the observed changes in gene expression levels may underlie the muscular and adipose tissue adjustments associated with regular physical activity. We found at least moderate associations between the centroids of the most upregulated gene sets in muscle tissue and estimated peak oxygen uptake and serum HDL concentration levels (Leskinen et al., Reference Leskinen, Rinnankoski-Tuikka, Rintala, Seppänen-Laakso, Pöllänen, Alen and Kujala2010). In addition, the centroids of the most upregulated gene sets in adipose tissue among the active versus inactive twins often had high inverse correlations with visceral fat area, intramuscular fat area, serum triglyceride concentration level, fasting plasma glucose level, and HOMA index (see Leskinen et al., Reference Leskinen, Rinnankoski-Tuikka, Rintala, Seppänen-Laakso, Pöllänen, Alen and Kujala2010).
Strengths and Limitations
As a result of the complete or close match for genes, age, gender, and intrauterine and childhood environment, co-twin control studies are a unique study design with which to investigate the effects of long-term physical activity on health, when both genetic and familial factors are standardized. The fact that it was difficult to identify substantial numbers of twin pairs significantly discordant for physical activity itself suggests a genetic or familial basis for lifetime activity patterns; thus, we were left with a rather small sample size. The TWINACTIVE study included an extensive, retrospective, questionnaire-based follow-up of leisure-time physical activity habits over three decades (from 1975 to 2007), but it is probable that the discordance in physical activity emerged even earlier. Self-reported physical activity can be regarded as a limitation, but continuous objective monitoring of physical activity volume over the course of decades would have been impossible to carry out, and the methods were not available in 1975.
Conclusions
Our co-twin control study aimed to address the problems that generally occur in individual-based studies that attempt to illustrate the long-term effects/associations of physical activity on disease progression and risk factor levels. As summarized here, the TWINACTIVE study findings suggest that the association between physical activity and good cardiometabolic health can be explained mechanistically by the complex network of metabolic and regulatory pathways that underlie the exercise-induced adaptations in metabolic profile, fitness, and body fat distribution. In addition, the TWINACTIVE study findings highlight the specific benefits of very long-term voluntary physical activity with respect to bone and conduit artery structures in a site-specific manner. As many of the associations between physical activity and measured health outcomes were seen in the pairwise analyses among MZ twin pairs, who share all of their genes at the sequence level, our twin study lends support to the hypothesis of a causal relationship between habitual physical activity and the observed findings.
Acknowledgments
We thank the participants of the TWINACTIVE study and the collaborating authors of different specific research reports from the TWINACTIVE study. We thank the Finnish Ministry of Education and Culture (grant to UMK), Academy of Finland (grant to UMK), Finnish Cultural Foundation (grant to TL), and Juho Vainio Foundation (grants to TL and UMK).





