Introduction
Blastocystis sp. is a common intestinal protist found in humans and animals (Udonsom et al., Reference Udonsom, Prasertbun, Mahittikorn, Mori, Changbunjong, Komalamisra, Pintong, Sukthana and Popruk2018), and is distributed worldwide, encompassing both urban and rural settings across developed and developing countries (Scanlan et al., Reference Scanlan, Stensvold, Rajilić-Stojanović, Heilig, De Vos, O'Toole and Cotter2014; Udonsom et al., Reference Udonsom, Prasertbun, Mahittikorn, Mori, Changbunjong, Komalamisra, Pintong, Sukthana and Popruk2018). In Poland, infection rates range from 0.14 to 23.6% across various study groups from 1955 to 2022 (Rudzińska and Sikorska, Reference Rudzińska and Sikorska2023). This figure, meanwhile, often exceeds 50% in developing countries and even 100% among children in Senegal River Basin (Alfellani et al., Reference Alfellani, Stensvold, Vidal-Lapiedra, Onuoha, Fagbenro-Beyioku and Clark2013; El Safadi et al., Reference El Safadi, Gaayeb, Meloni, Cian, Poirier, Wawrzyniak, Delbac, Dabboussi, Delhaes, Seck, Hamze, Riveau and Viscogliosi2014; Popruk et al., Reference Popruk, Adao and Rivera2021). Blastocystis sp. is transmitted through the fecal–oral route, and people or animals are infected by ingesting contaminated food or water (Maloney et al., Reference Maloney, Molokin and Santin2019). Although some cases are asymptomatic, Blastocystis sp. infection is closely linked to conditions such as irritable bowel syndrome, terminal ileitis and ulcerative colitis, manifesting mainly as abdominal pain, diarrhoea, flatulence and constipation (Tunalı et al., Reference Tunalı, Öztürk, Ünver and Turgay2018; Basuony et al., Reference Basuony, M A Basyoni, Negm, Mostafa, El-Wakil, Shemis, Gouda and Saftawy2022).
The morphological diversity of Blastocystis sp. includes vacuolar, granular, amoebic, cyst, avacuolar and multivacuolar forms (Stenzel and Boreham, Reference Stenzel and Boreham1996). Among these, the vacuolar form is the most prevalent and typical form of Blastocystis sp., which is often used as the classical morphological standard of Blastocystis sp. infection. However, the presence of abundant associated bacteria poses challenges for ultrastructural observation of Blastocystis sp. through an electron microscope (Zierdt and Williams, Reference Zierdt and Williams1974).
Isolating Blastocystis sp. from fecal cultures often introduces bacterial contamination, rendering many animal experimental results unacceptable or leading to inconsistencies across studies. Axenic strains, free from contaminating organisms, facilitate more scientific and persuasive in-depth studies on various aspects of Blastocystis sp., such as karyotype modelling, antigen analysis, enzyme activity and surface biochemical components, thereby advancing our understanding of the biological aspects of Blastocystis sp. (Lanuza et al., Reference Lanuza, Carbajal, Villar and Borrás1996b). Since the successful axenic culture of Blastocystis sp. by Zierdt and Williams in Reference Zierdt and Williams1974, numerous scholars have attempted axenic isolation methods.
This paper builds upon previous studies, reflecting a deep understanding of Blastocystis sp. and the significance of its axenic culture. The review encompasses presently available axenic isolation techniques and factors influencing their applicability, emphasizing the importance of axenic isolation for studying Blastocystis pathogenicity, describing its surface biochemical components and elucidating therapeutic effects. The content serves as a valuable reference for clinicians and scientists in selecting appropriate axenic isolation methods.
The axenic cultivation of Blastocystis sp.
Axenic cultivation of Blastocystis sp. involves the culture of Blastocystis sp. free from contamination with other organisms. This necessitates the application of a suitable culture medium and a series of axenic treatment methods, with the selection of the optimal culture medium being crucial (Tan et al., Reference Tan, Ng, Quek, Howe, Ramachandran, Yap and Singh2000). An efficient and economical medium for isolating Blastocystis sp. serves as the foundation for subsequent studies on factors such as its life history, morphological classification and infection immunity (Tan, Reference Tan2008). Axenic methods include antibiotic treatment, monoclonal culture, differential centrifugation, density gradient separation, micromanipulation and the combined application of culture medium. Obtaining axenic Blastocystis sp. often requires the combined application of different methods, presenting a promising avenue for further research.
Commonly used media for the axenic isolation of Blastocystis sp.
The in vitro culture of Blastocystis sp. involves the utilization of monophasic and biphasic media. The biphasic medium comprises 2 media with distinct physical states, while the monophasic medium consists of only 1 physical medium. Based on physical state, the medium is categorized as solid, liquid and solid–liquid biphasic. The solid medium contains agar, and the liquid medium includes Jones medium, Iscove's modified Dulbecco's medium (IMDM) and Dulbecco's modified Eagle medium (DMEM). Typically, a single-phase medium is prepared by sequentially adding necessary raw materials, sterilizing the mixture under high pressure and then packaging it in suitable volumes according to experimental requirements. Biphasic medium includes Locke's egg serum (LES) medium and Boeck–Drbohlav biphasic modified medium (BDMM), composed of solid and liquid components. The preparation involves configuring the solid phase first, followed by the liquid phase and finally covering the solid medium's surface with the liquid medium. Table 1 provides details of specific culture media.
Table 1. Commonly used media for aseptic isolation

Methods for the axenic isolation of Blastocystis sp.
Antibiotic treatment
Antibiotic treatment has been a key approach for the axenic isolation of Blastocystis sp. since the seminal study by Zierdt and Williams (Reference Zierdt and Williams1974). The inclusion of antibiotics in the culture medium (Fig. 1) has become a widely employed method, although it presents challenges. While antibiotics contribute to asepsis, complete elimination of bacteria may not be achieved due to the potential development of antibiotic resistance among the microbial population. It is important to note that low bacterial counts might be mistakenly regarded as axenic (Ng and Tan, Reference Ng and Tan1999). Ampicillin and streptomycin combination stands out as a straight forward and effective addition, proving capable of eliminating a significant portion of bacteria (Teow et al., Reference Teow, Zaman, Ng, Chan, Yap, Howe, Gopalakrishnakone and Singh1991, Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992). Additionally, amphotericin is employed to target yeasts and moulds, common contaminants in the initial culture stages (Zierdt and Williams, Reference Zierdt and Williams1974). Ceftizoxime and vancomycin can eliminate drug-resistant bacteria (Zierdt, Reference Zierdt1991). Despite the benefits of antibiotic treatment in achieving asepsis, it comes with the potential drawback of inhibiting parasite growth, with alterations to the size and vacuole content of Blastocystis sp. (Teow et al., Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992). It is noteworthy that the introduction of antibiotics can impact the longevity of Blastocystis sp., with signs of decline observed after the third passage in a chloramphenicol-containing medium (Zierdt et al., Reference Zierdt, Swan and Hosseini1983). The strategic addition of antibiotics poses a delicate balance, as the direct use of a mixture of multiple antibiotics may result in insufficient growth of Blastocystis sp., while the sequential addition of antibiotics, 1 at a time, has proven beneficial for promoting growth (Lanuza et al., Reference Lanuza, Carbajal, Villar and Borrás1996b).

Figure 1. Antibiotic treatment for the axenic isolation of Blastocystis sp.
Monoclonal culture
Agar cloning technology plays a pivotal role in achieving the physical separation of Blastocystis sp. from bacteria, ensuring the asepsis of Blastocystis sp. (Valido and Rivera, Reference Valido and Rivera2007) (Fig. 2). Monoclonal culture, in the presence of certain bacteria, facilitates the distinct separation of pure Blastocystis sp. colonies from bacterial colonies. The pioneering work of Tan et al. marked the cultivation of axenic Blastocystis sp. colonies in Petri dishes containing soft agar, leading to the successful cultivation of a substantial number of amoebic Blastocystis sp. (Tan et al., Reference Tan, Singh, Thong, Ho, Moe, Chen, Ng and Yap1996a). In the same year, Tan et al. introduced sodium thioglycolate to enhance the colony formation efficiency (%CFE = the number of growing colonies/inoculated cells × 100), resulting in the abundant production of Blastocystis sp. from a single clone (Tan et al., Reference Tan, Singh, Yap, Ho, Moe, Howe and Ng1996b). The monoclonal culture was further applied by Ng and Tan to strains treated with antibiotics, leading to the successful isolation of pure Blastocystis isolates (Ng and Tan, Reference Ng and Tan1999).

Figure 2. Monoclonal culture for the axenic isolation of Blastocystis sp.
The advantage of monoclonal culture lies in the physical separation of Blastocystis sp. colonies and bacterial colonies, with bacterial colonies growing in 0.36% Bacto agar easily distinguishable from the diffuse Blastocystis sp. colonies visible under an inverted microscope. However, this method was associated with the following drawbacks: (1) counting colonies becomes challenging due to the semisolid nature of agar; (2) cloning and growth of Blastocystis sp. in agar necessitate various nutrients, requiring the preparation of fresh culture medium and (3) extracting Blastocystis sp. colonies proves difficult as they are generally embedded in agar. To address these challenges, Tan et al. introduced a simplified and efficient method for clonal growth on solid agar, increasing the agar content from 0.36 to 2%. This modification allowed the successful culture of Blastocystis sp. colonies on the agar surface, facilitating their isolation for further studies (Tan et al., Reference Tan, Ng, Quek, Howe, Ramachandran, Yap and Singh2000). It is crucial to note that abundant bacterial colonies can impede the identification and isolation of Blastocystis sp. colonies. Therefore, antibiotic treatment is deemed necessary in monoclonal culture to reduce the bacterial population and aid in the isolation of Blastocystis sp. colonies (Ng and Tan, Reference Ng and Tan1999; Valido and Rivera, Reference Valido and Rivera2007).
Differential centrifugation technique
Teow et al. employed the differential centrifugation technique in conjunction with antibiotics to eliminate the associated bacteria from 8 Blastocystis sp. isolates (Teow et al., Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992) (Fig. 3). The procedure involved centrifuging Blastocystis sp. in the logarithmic growth phase at 3000 g for 15 min, followed by resuspension in ~10 mL of phosphate-buffered saline (PBS, pH 7.0) containing 4000 μg mL−1 ampicillin and 1000 μg mL−1 streptomycin. Subsequently, the suspension underwent centrifugation at 1000 g for 15 min, the supernatant was discarded and 10 mL of PBS containing antibiotics was added. This entire process was repeated twice. However, limited studies have reported the axenic isolation of Blastocystis sp. using differential centrifugation, possibly attributed to the fragility of Blastocystis sp. cells (Zierdt and Williams, Reference Zierdt and Williams1974; Teow et al., Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992).

Figure 3. Differential centrifugal technique for the axenic isolation of Blastocystis sp. PBS, phosphate-buffered saline; Amp, ampicillin; Strep, streptomycin.
Density gradient separation
To isolate Blastocystis sp. from bacteria, density gradient separation has proven effective (Fig. 4). Upcroft et al. utilized the Ficoll–Paque gradient method to purify Blastocystis sp. and eliminate more than 75% of the bacteria, although complete sterility was not attained (Upcroft et al., Reference Upcroft, Dunn, Dommett, Healey, Upcroft and Boreham1989). Lanuza et al. purified Blastocystis sp. using the Ficoll-metrizoic acid solution and subsequently inoculated them into a fresh medium containing active antibiotics against residual bacteria, thus achieving sterility (Lanuza et al., Reference Lanuza, Carbajal, Villar and Borrás1996b). Defaye et al. reported a method for purifying Blastocystis sp. cysts from feces, employing the Percoll gradient method and an antibiotic mixture to reduce the number of bacteria inoculated per animal to less than 250 colonies (Defaye et al., Reference Defaye, Nourrisson, Baudu, Warwzyniak, Bonnin, Bonnet, Barnich, Ardid, Delbac, Carvalho and Poirier2018). See Table 2 for details.

Figure 4. Density gradient centrifugation for the axenic isolation of Blastocystis sp. P, passage.
Table 2. Introduction of different density gradient methods

Other axenic isolation methods
Hess et al. employed a micropipette, that is, micromanipulation, to isolate Blastocystis sp. from turkey caecal contents for cloning, offering a step towards asepsis (Hess et al., Reference Hess, Kolbe, Grabensteiner and Prosl2006). Shin et al. successfully established an axenic pure culture system for Blastocystis sp. using LES biphasic medium, mLES biphasic medium, liquid IMDM and solid IMDM (Shin et al., Reference Shin, Liao, Liao, Sun, Fu and Liu2017).
Furthermore, several reports have documented the axenic culture of Blastocystis sp. from diverse animal species, including rats, snakes, lizards and crocodiles (Teow et al., Reference Teow, Zaman, Ng, Chan, Yap, Howe, Gopalakrishnakone and Singh1991, Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992; Chen et al., Reference Chen, Singh, Ho, Tan, Ng, Moe and Yap1997; Ng and Tan, Reference Ng and Tan1999). Table 3 provides a summary of axenic isolation methods specific to each animal.
Table 3. Summary of the aseptic isolation methods from humans, rats and some reptiles

Factors affecting the axenic isolation of Blastocystis sp.
Medium composition
Composition of the culture medium
The composition of the culture medium significantly influences the axenic isolation of Blastocystis sp., with components such as growth factors, glucose, mineral salt and sodium thioglycolate playing crucial roles. Zierdt and Williams (Reference Zierdt and Williams1974) axenically isolated Blastocystis sp. using a medium comprising 5 components: (1) boiled traditional Blastocystis sp. culture solution (20%); (2) traditional autoclaved Blastocystis sp. culture solution (20%); (3) supernatant fluid from boiled or autoclaved Blastocystis sp. culture solution (20%); (4) isovitalex (1%) and (5) haemin (5 μg mL−1). Given the strict anaerobic growth requirements of Blastocystis sp., the addition of sodium thioglycolate to the axenic culture medium enhances anaerobic conditions, improving colony growth and yield (Tan et al., Reference Tan, Singh, Thong, Ho, Moe, Chen, Ng and Yap1996a). Furthermore, the combined application of various media was conducive to the axenic culture of Blastocystis sp. Optimization of the media composition, such as enhancing the traditional LES biphasic medium with agar replacement protein, simplifies preparation and reduces contamination risks (Shin et al., Reference Shin, Liao, Liao, Sun, Fu and Liu2017).
Culture temperature
The optimal growth temperature for Blastocystis sp. in axenic culture is 37°C, and attempts to purify Blastocystis sp. in Diamond's TPS-1 medium (The components of TPS-1 medium contained 5 g Trypticase, 10 g Panmede, 2.5 g D-glucose, 0.5 g L-cystein HCl, 0.2 g Ascorbic acid, 2.5 g NaCl, 0.3 g K2HPO4, 0.5 g KH2PO4, 50 mL Bovine serum and 435 mL Distilled water) at 28°C have been unsuccessful (Molet et al., Reference Molet, Werler and Kremer1981). Notably, Blastocystis lapemi sp. nov. has an optimal growth temperature of 24°C, and attempts to adapt from 24 to 37°C resulted in the gradual demise of B. lapemi sp. nov. above 28°C (Teow et al., Reference Teow, Zaman, Ng, Chan, Yap, Howe, Gopalakrishnakone and Singh1991). Blastocystis isolated from land turtles, iguanas, crocodiles and pythons were successfully axenized at 24°C (Teow et al., Reference Teow, Ng, Chan, Chan, Yap, Zaman and Singh1992), and rats were axenized at 37°C (Chen et al., Reference Chen, Singh, Ho, Tan, Ng, Moe and Yap1997). It is highly conceivable that the variation in axenic culture temperature for Blastocystis sp. is linked to the different body temperatures of their respective animal hosts.
Culture medium character
Axenic culture of Blastocystis sp. can be achieved using both monophasic and biphasic media (Tan et al., Reference Tan, Ng, Quek, Howe, Ramachandran, Yap and Singh2000). Compared with the monophasic medium, biphasic medium preparation is complicated and prone to bacterial contamination.
Types and doses of antibiotics used
Due to Blastocystis sp. inhabiting the intestinal tract, the isolation and culture process often involves a mix of unknown species and drug-resistant bacteria. The combination of 1–2 antibiotics at conventional doses may prove ineffective against bacteria due to multiple drug resistance caused by antibiotic abuse (Li et al., Reference Li, Ondon, Ho, Jiang and Li2022). In such instances, considering larger doses of drugs along with physical separation is suggested to achieve asepsis.
Other factors affecting axenic isolation
Axenic culture may be related to the frequency of inoculation, and some bacteria will decrease with each subsequent inoculation until they disappear completely. Centrifugation is employed to separate Blastocystis sp. from agar, creating favourable conditions for its growth. Tan et al. found that emulsifying inoculum in a small amount of pre-reduced IMDM enhances the cloning process, improving colony isolation (Tan et al., Reference Tan, Ng, Quek, Howe, Ramachandran, Yap and Singh2000). The morphology of Blastocystis sp. may also influence axenic culture, with an increase in granular forms of Blastocystis being closely related to a significant increase in bacterial count (Zierdt and Williams, Reference Zierdt and Williams1974).
Applications of the axenic isolation of Blastocystis sp.
Application of the axenic isolation techniques to study the pathogenicity of Blastocystis sp.
The application of axenic isolation techniques is crucial for studying the pathogenicity of Blastocystis sp., which is implicated in various gastrointestinal diseases and may play a role in irritable bowel syndrome (Ismail et al., Reference Ismail, Abbas and Molan2022). Valsecchi et al. showed that the amoebic form of Blastocystis sp. causes urticaria by affecting intestinal immune homoeostasis (Valsecchi et al., Reference Valsecchi, Leghissa and Greco2004). In the study conducted by Moe et al., the injection of axenic Blastocystis sp. isolate B into mice resulted in the discovery of vacuolar and granular parasites in the caecum. Histological examination of the caecum and colon revealed significant inflammatory cell infiltration, lamina propria oedema and mucosal exfoliation (Moe et al., Reference Moe, Singh, Howe, Ho, Tan, Chen, Ng and Yap1997). Intramuscular injection of the same strain led to extensive necrosis and interstitial oedema in the muscle, accompanied by polymorphonuclear leucocyte infiltration (Moe et al., Reference Moe, Singh, Gopalakrishnakone, Ho, Tan, Chen and Yap1998). Puthia et al. observed that the lysate of the pure cultured Blastocystis ST4 WRl isolate induced apoptosis of rat intestinal epithelial cell-6 (IEC-6), altered the distribution of F-actin, reduced epithelial resistance and increased epithelial permeability of the IEC-6 monolayer (Puthia et al., Reference Puthia, Sio, Lu and Tan2006). Furthermore, Ajjampur et al. demonstrated that axenic Blastocystis sp. could degrade mucin in various segments of the large intestine, consequently impacting the mucin barrier (Ajjampur et al., Reference Ajjampur, Png, Chia, Zhang and Tan2016). The observed pathological conditions in the host may be linked to the disruption of in vivo balance by specific isolates or rare subtypes (STs). Yason et al. reported that specific axenic isolates of Blastocystis ST7 could reduce the abundance of Bifidobacterium and Lactobacillus (Yason et al., Reference Yason, Liang, Png, Zhang and Tan2019). Importantly, it is emphasized that studies on the pathogenicity of Blastocystis sp. ideally should be based on axenic isolation for accurate assessments.
Application of axenic cultures in karyotype and ST analysis of Blastocystis sp.
To facilitate accurate karyotype and ST analysis of Blastocystis sp., Upcroft et al. recommended isolating Blastocystis sp. from the associated bacteria, as the presence of bacteria in non-axenic cultures could potentially mask the detection of chromosomes (Upcroft et al., Reference Upcroft, Dunn, Dommett, Healey, Upcroft and Boreham1989). Karyotype analysis of Blastocystis sp. isolated from various hosts, such as sea snakes, rats, tortoises, iguanas and pythons, revealed independent isolates, leading to the designation of distinct species, namely B. lapemi sp. nov., Blastocystis ratti sp. nov., Blastocystis geocheloni sp. nov., Blastocystis cycluri sp. nov. and Blastocystis pythoni sp. nov. respectively (Teow et al., Reference Teow, Zaman, Ng, Chan, Yap, Howe, Gopalakrishnakone and Singh1991; Singh et al., Reference Singh, Ho, Yap, Ng, Tan, Moe and Yap1996; Chen et al., Reference Chen, Singh, Ho, Tan, Ng, Moe and Yap1997). However, Ho et al. found that the karyotypes of different axenic isolates of the same host were not identical, and there were marginal differences in chromosome bands (Ho et al., Reference Ho, Singh, Suresh, Ng and Yap1994).
The classification of the above-mentioned Blastocystis sp. species, isolated from non-human hosts, relied on electrophoretic karyotype as the criterion for defining new species. However, due to the karyotype heterogeneity of some protozoa, this standard may be insufficient to explain the species formation of Blastocystis sp. Host specificity and pathogenicity of different isolates have been correlated with sequence variations in small subunit-ribosomal ribonucleic acid (SSU-rRNA), leading to the division of the genus into 28 STs (Noël et al., Reference Noël, Peyronnet, Gerbod, Edgcomb, Delgado-Viscogliosi, Sogin, Capron, Viscogliosi and Zenner2003; Parija and Jeremiah, Reference Parija and Jeremiah2013; Martiny et al., Reference Martiny, Bart, Vandenberg, Verhaar, Wentink-Bonnema, Moens and van Gool2014). Nineteen axenic isolates of Blastocystis sp. and 9 isolates containing bacteria were analysed using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry (Martiny et al., Reference Martiny, Bart, Vandenberg, Verhaar, Wentink-Bonnema, Moens and van Gool2014). The 19 axenic isolates produced protein maps with good resolution, suggesting that MALDI-TOF mass spectrometry could effectively distinguish the STs of axenic strains (Martiny et al., Reference Martiny, Bart, Vandenberg, Verhaar, Wentink-Bonnema, Moens and van Gool2014). However, the growth hindrance of Blastocystis sp. in the presence of bacteria and fungi resulted in correct identification for only 3 out of the 9 non-axenic isolates (Martiny et al., Reference Martiny, Bart, Vandenberg, Verhaar, Wentink-Bonnema, Moens and van Gool2014).
Application of axenic cultures for immunological studies of Blastocystis sp.
Current immunological studies on Blastocystis sp. infection primarily focus on the detection of antibodies and antigens (Sheela et al., Reference Sheela, Chandramathi and Suresh2020). The potential pathogenic mechanisms of Blastocystis sp. can be preliminarily elucidated through in vitro co-culturing of live cells, lysates, secretions and soluble antigens. Notably, since bacteria can act as antigens and potentially react with cultured cells, the success of these in vitro experiments relies on the use of pure cultures of Blastocystis sp. (Deng and Tan, Reference Deng and Tan2022).
Kukoschke et al. reported at least 2 different peptide patterns and antigenic variants of axenic Blastocystis sp. (Kukoschke and Müller, Reference Kukoschke and Müller1991). Müller (Reference Müller1994) divided 61 axenic isolates of Blastocystis sp. into 4 groups using immunodiffusion, while Lanuza et al. (Reference Lanuza, Carbajal, Villar, Mir and Borrás1999) divided 18 axenic strains into 3 related patterns using sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The findings of the present study corroborated Kukoschke's findings. Serum obtained from mice injected with 20 μg mL−1 soluble antigen of symptomatic and asymptomatic axenic Blastocystis ST3 demonstrated specific cleavage of the corresponding live Blastocystis sp. (Sheela et al., Reference Sheela, Chandramathi and Suresh2020). Serum obtained from mice with soluble antigen of symptomatic Blastocystis ST3 added to asymptomatic Blastocystis ST3, and vice versa, exhibited only 17% cross-reactivity, indicating significant epitope differences between symptomatic and asymptomatic Blastocystis ST3. These results also substantiate Kukoschke's report. However, it is essential to note that there are currently no validated cultures of axenic Blastocystis ST3, and Sheela et al. did not provide relevant evidence of the successful axenization of Blastocystis ST3 isolate.
Blastocystis sp. exerts regulatory effects on cells by modulating cytokine expression. Axenic Blastocystis sp. antigen induces the expression of interleukin (IL)-1β, IL-6 and tumour necrosis factor-alpha (TNF-α) in intestinal explants, colons and macrophages of mice (Lim et al., Reference Lim, Png, Tay, Teo, Jiao, Lehming, Tan and Zhang2014). The axenic soluble antigen of Blastocystis sp. was introduced into peripheral blood mononuclear cells (PBMCs) and human colon cancer cells (HCT116) in a study by Chandramathi et al. (Reference Chandramathi, Suresh and Kuppusamy2010). The study revealed that the gene expressions of interferon-gamma (IFN-γ) and TNF-α were downregulated, while those of IL-6, IL-8 and nuclear factor kappa B (NF-κB) were upregulated in PBMCs. In HCT116, the gene expression of IFN-γ was significantly downregulated, while the expressions of IL-6 and NF-κB were upregulated, suggesting that the Blastocystis sp. antigen (at a specific concentration) has the potential to promote the growth of existing tumours or cancer cells (Chandramathi et al., Reference Chandramathi, Suresh and Kuppusamy2010).
Recently, Deng et al. (Reference Deng, Wojciech, Png, Koh, Aung, Kioh, Chan, Malleret, Zhang, Peng, Gascoigne and Tan2022) conducted a study where axenic Blastocystis ST4 was orally injected into healthy mice and dextran sodium sulphate (DSS)-induced colitis mice. The study revealed that Blastocystis ST4 colonization altered the composition of the intestinal bacterial community in healthy mice (without adverse effects) (Deng et al., Reference Deng, Wojciech, Png, Koh, Aung, Kioh, Chan, Malleret, Zhang, Peng, Gascoigne and Tan2022). Moreover, the colonization of Blastocystis ST4 induced T helper 2 and regulatory T cell immune responses in DSS-induced colitis mice, promoting the recovery of experimentally induced colitis mice (Deng et al., Reference Deng, Wojciech, Png, Koh, Aung, Kioh, Chan, Malleret, Zhang, Peng, Gascoigne and Tan2022). Under similar conditions, orally injecting axenic Blastocystis ST7 into DSS-induced colitis mice exacerbated the severity of colitis by increasing the proportion of pathogenic bacteria and inducing pro-inflammatory IL-17A and TNF-α-producing CD4+ T cells (Deng et al., Reference Deng, Wojciech, Png, Kioh, Gu, Aung, Malleret, Chan, Peng, Zhang, Gascoigne and Tan2023).
In a nutshell, the axenic isolation of Blastocystis sp. plays a crucial role in establishing a Blastocystis sp.–cell co-culture system in vitro and an animal model of Blastocystis sp. infection in vivo. These models serve as the foundation for immunological research.
The application of axenic cultures to study the surface chemical structure and lipid biochemical analysis of Blastocystis sp.
Studies on the surface chemical structure of Blastocystis sp. aid in understanding its pathogenic potential and nutritional impact (Yason et al., Reference Yason, Koh and Tan2018). Axenic cultures offer the advantage of avoiding bacterial interference in experiments. Keenan et al. conducted the pioneering analysis of lipids in 6 axenic strains of Blastocystis sp., revealing that the phospholipid content of all strains constituted ~39% of the total lipids (Keenan et al., Reference Keenan, Huang and Zierdt1992). The primary neutral lipid components were sterol esters, and the predominant polar lipid component was phosphatidylcholine across all strains. However, the study noted the challenge of determining whether the lipids were synthesized by the organisms or derived from the culture media (Keenan et al., Reference Keenan, Huang and Zierdt1992). Subsequent research by Keenan and Zierdt further demonstrated that axenic Blastocystis sp. can synthesize lipids and accumulate cholesterol and intact cholesterol esters directly from the growth medium (Keenan and Zierdt, Reference Keenan and Zierdt1994). Lanuza et al. employed lectin probes to identify specific carbohydrates within the surface coating of axenic Blastocystis sp., revealing that the structure contained α-d-mannose, α-d-glucose, N-acetyl-α-d-glucosamine, α-l-fucose, chitin and sialic acid (Lanuza et al., Reference Lanuza, Carbajal and Borrás1996a). In addition, the axenic Blastocystis ST7 isolate B was not directly destroyed by the antimicrobial peptide LL-37, which may be attributed to the fact that the thicker surface-coated Blastocystis STs are more resistant to osmotic pressure, extreme pH and oxygen exposure (Yason et al., Reference Yason, Ajjampur and Tan2016; Yason and Tan, Reference Yason and Tan2018).
The application of axenic cultures to study the chemotherapy of Blastocystis sp.
Given the individual variations in treating Blastocystis sp. and the absence of a specific therapeutic drug, combination drugs have been employed in clinical treatments to enhance efficacy, reduce toxicity, minimize adverse reactions and delay the onset of drug resistance. The use of sterile blastocysts can effectively evaluate the effect of drugs. Metronidazole is a commonly used drug for Blastocystis sp. infection, induced apoptosis-like features in axenic Blastocystis sp. as observed by Nasirudeen et al. (Reference Nasirudeen, Hian, Singh and Tan2004). These features included nuclear condensation, nuclear DNA fragmentation, reduced cytoplasmic volume, phosphatidylserine externalization and increased cell membrane permeability. Similarly, staurosporine, an apoptosis-inducing agent, prompted similar apoptosis-like features in Blastocystis sp. (Yin et al., Reference Yin, Howe and Tan2010). In vitro testing of 10 antiprotozoal drugs against axenic Blastocystis sp. revealed the order of efficacy as emetine, metronidazole, furazolidone, trimethoprim sulphamethoxazole, 5-chloro-8-hydroxy-7-iodoquinoline (enteric violine) and pentamidine (Zierdt et al., Reference Zierdt, Swan and Hosseini1983). However, certain Blastocystis sp. isolates demonstrated resistance to metronidazole, which has potential mutagenic and carcinogenic effects (Nagel et al., Reference Nagel, Bielefeldt-Ohmann and Traub2014; Eida et al., Reference Eida, El-Shafei, Nomeir and El Safhi2016). Studies on Nigella sativa aqueous extracts revealed their inhibitory effect on axenic Blastocystis sp. Eida et al. found that N. sativa aqueous extracts at concentrations of 100 and 500 μg mL−1 exerted potent lethal effects on axenic Blastocystis sp. isolates in vitro and prevented cytopathic changes in infected mice orally inoculated with Blastocystis sp. in vivo (Eida et al., Reference Eida, El-Shafei, Nomeir and El Safhi2016). Yason et al. used the axenic Blastocystis isolates NUH9, WR1 and B and found good efficacy of diphenyleneiodonium chloride, auranofin and BIX 01294 trihydrochloride hydrate in treating Blastocystis sp. infection, particularly in the axenic Blastocystis ST7 isolate B, which was insensitive to metronidazole (Yason et al., Reference Yason, Koh and Tan2018).
Application of axenic cultures for molecular biology of Blastocystis sp.
The application of axenic cultures in the molecular biology of Blastocystis sp. has played a crucial role in addressing the controversy surrounding the association between Blastocystis STs and its symptomatology (Stensvold et al., Reference Stensvold, Suresh, Tan, Thompson, Traub, Viscogliosi, Yoshikawa and Clark2007). Denoeud et al. took a significant step by reporting the complete genome sequence of axenic Blastocystis ST7 isolates from a patient in Singapore. They proposed candidate genes for the study of physiopathology, opening avenues for comparative genomics with other STs and contributing to the development of typing tools for characterizing pathogenic isolates (Denoeud et al., Reference Denoeud, Roussel, Noel, Wawrzyniak, Da Silva, Diogon, Viscogliosi, Brochier-Armanet, Couloux, Poulain, Segurens, Anthouard, Texier, Blot, Poirier, Ng, Tan, Artiguenave, Jaillon, Aury, Delbac, Wincker, Vivarès and El Alaoui2011).
In the post-genomic era, genetic tools have become instrumental in obtaining genetically modified cells, providing essential insights into the function of Blastocystis genes. Li et al. achieved a milestone by establishing a robust gene delivery protocol using axenic Blastocystis ST7 isolates. Their study involved testing ectopic protein expression with a highly sensitive nano-luciferase reporting system (Li et al., Reference Li, Tsaousis, Purton, Chow, He and Tan2019). The study identified a promoter that includes the upstream region of the 5′ untranslated region (UTR) in legumain gene. A robust transient transfection technique was established in Blastocystis by combining this promoter with the legumain 3′ UTR. This breakthrough technique lays a strong foundation for further investigations into the functions of key molecules in Blastocystis sp.
Conclusion
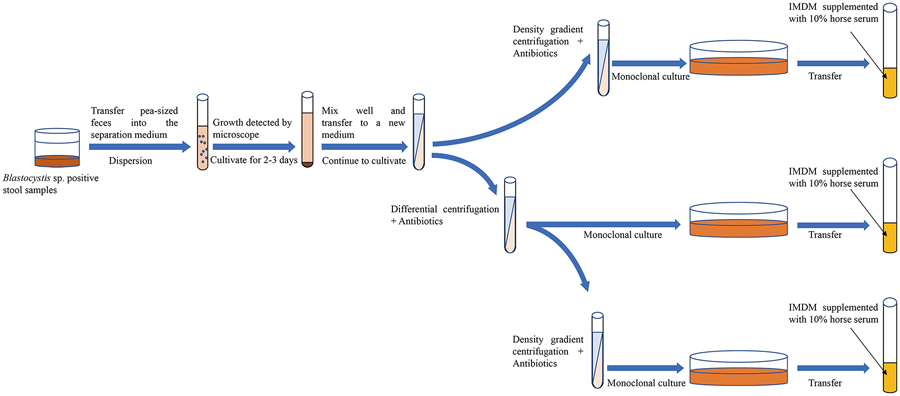
In summary, the axenic isolation of Blastocystis sp. provides a robust foundation for morphological, immunological, drug and molecular biology studies. Currently, the common approach among scholars involves the use of antibiotic treatment in conjunction with another isolation technique to achieve axenic Blastocystis sp. cultures. However, limited reports have explored the combined application of 3 or more axenic isolation techniques. Through an analysis of the pros and cons of various methods, it is posited that employing a combination of several axenic separation techniques proves more advantageous in obtaining pure Blastocystis sp. cultures (Fig. 5). Finally, it is strongly recommended that sufficient data are needed to provide supporting evidence for the successful axenization of Blastocystis sp. in future. This may involve presenting sterile plates to showcase the absence of bacterial growth, utilizing 16S polymerase chain reaction for bacterial detection, and supplying SSU-rRNA sequences for the confirmation of Blastocystis STs.

Figure 5. Combination of multiple methods for the axenic isolation of Blastocystis sp.
Acknowledgements
We wish to thank associate professor Wei Shi from Guangxi Medical University for his technical support.
Author's contribution
X. M., L. W. and C. S. prepared the manuscript and drew figures. Z. Z., X. T., Z. Y. and S. W. critically revised the paper. All authors contributed to the article and approved the final version.
Financial support
This work received support from the Natural Science Foundation of Henan Province (no. 232300421313), the Doctoral Scientific Research Activation Foundation of Xinxiang Medical University (no. XYBSKYZZ202140), the Training Plan for Young Backbone Teachers in Universities of Henan Province (no. 2021GGJS101) and the Research Innovation Project of College Students of Xinxiang Medical University (nos. xyxskyz201907 and xyxskyz202120).
Competing interests
None.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.