Introduction
Schistosomiasis is found in sub-Saharan Africa and it is one of the seven ‘core’ neglected tropical diseases targeted for control by preventive chemotherapy. As outlined by the World Health Organization, preventive chemotherapy involves the co-ordinated access and integrated delivery of safe orally administered anthelminthics offered to selected groups (such as school children) within disease-afflicted communities (Mohammed et al., Reference Mohammed, Haji, Gabrielli, Mubila, Biswas and Chitsulo2008; Stothard et al., Reference Stothard, Sousa-Figueiredo and Navaratnam2013; WHO, 2013). The schistosome, a trematode blood fluke, has a complex lifecycle and a pertinent feature that is often omitted from health education materials is that treatment does not guard against reinfection, a dynamic that needs specific attention with regard to subsequent water contact after treatment (see Fig. 1).
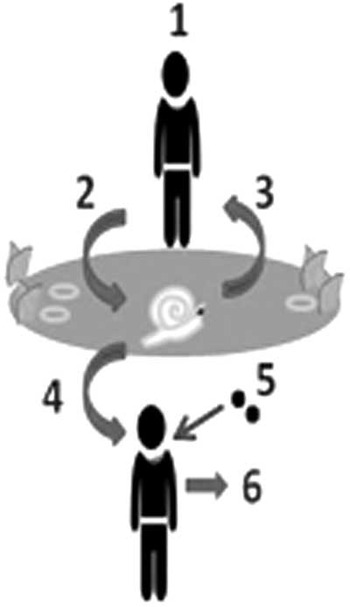
Fig. 1 Epidemiological aspects of the lifecycle of urogenital schistosomiasis; a schematic diagram of the contamination and infection processes as played out in fresh water. Process 1: a previously infected child/adult enters the local area; Process 2: fresh water habitat where susceptible snails exist is contaminated by infected urine containing schistosome eggs; Process 3: hatched miracidia infect and develop in snail(s), which later release schistosome cercariae, often on a daily basis until the snail dies, typically infecting people upon subsequent water contact(s). (Note that the person responsible for original contamination can become hyper-parasitized by further water contact(s)); Process 4: additional people become infected and in turn contribute to Process 2; Process 5: individuals can be treated with praziquantel (40 mg/kg) by school teachers or health centre staff; and Process 6: if treatment is successful and the current infection is cleared the individual cannot contaminate the environment but is still vulnerable to reinfection should water contact continue.
Urogenital schistosomiasis is caused by infection with Schistosoma haematobium and its geographical distribution and zones of transmission closely track those of its fresh water intermediate host snails (Stothard et al., Reference Stothard, Chitsulo, Kristensen and Utzinger2009a). The detrimental effects of schistosomiasis can be contained by preventive chemotherapy campaigns offering treatment with praziquantel. The global demand for this medicine is substantial but is presently not being met because the drug is in limited supply. This is likely to change as following the 2012 London Declaration on Neglected Tropical Diseases, Merck-KGa agreed to scale-up its annual donation to 250 million tablets by 2016. Before this, growing access to praziquantel was fostered in purchasing and subsequently donating the drug for large-scale use by national control programmes with backing from organizations such as the Bill & Melinda Gates Foundation and governmental agencies like USAID (Savioli et al., Reference Savioli, Gabrielli, Montresor, Chitsulo and Engels2009). Although political support is still growing and supportive, concerns over the long-term success of preventive chemotherapy remain, with some academics questioning whether expected levels of treatment coverage will ever be reached and sustained with current strategies (Allen & Parker, Reference Allen and Parker2011; Parker & Allen, Reference Parker and Allen2014). There is a recognized need for successful preventive chemotherapy campaigns to find synergy with complementary control measures, such as appropriate health education, improving water supplies and sanitation and snail control where locally needed (Parker et al., Reference Parker, Allen and Hastings2008; Utzinger et al., Reference Utzinger, Raso, Brooker, De Savigny, Tanner and Ornbjerg2009; Allen & Parker, Reference Allen and Parker2012). This is to ensure that appropriate levels of treatment coverage are attained and progress towards disease-associated targets, as outlined in the WHO 2012–2020 roadmap, is maintained (WHO, 2013).
Control of schistosomiasis in coastal Tanzania
Following support from the Schistosomiasis Control Initiative, a national control programme against schistosomiasis was officially launched in Tanzania in October 2003 (Mazigo et al., Reference Mazigo, Nuwaha, Kinung’hi, Morona, De Moira and Wilson2012). While both intestinal and urogenital schistosomiasis occur in this country, only the latter is a public health concern on the coastal islands of Pemba, Unguja and Mafia. Intestinal schistosomiasis is generally lacking, apart from occasional imported infection, due to an absence of its intermediate snail host. Autochthonous transmission of S. haematobium occurs on both Unguja and Pemba, but not on Mafia (Stothard et al., Reference Stothard, Loxton and Rollinson2002a, Reference Stothard, Sousa-Figueiredo and Navaratnam2013), which track habitats colonized by B. globosus (Stothard & Rollinson, Reference Stothard and Rollinson1997; Stothard et al., Reference Stothard, Loxton, Rollinson, Mgeni, Khamis and Ameri2000; Knopp et al., Reference Knopp, Person, Ame, Mohammed and Ali2013).
In 2003, the President of Zanzibar highlighted the importance of schistosomiasis, which is known locally as kichocho (in Kiswahili), when he announced the Kick out Kichocho (Piga Vita Kichocho) control programme at a large-scale gathering of various health stakeholders and media at Kinyasini School, Unguja. Kick out Kichocho also received support from the Schistosomiasis Control Initiative and the African Development Bank. They provided financial assistance and helped with the procurement and distribution of praziquantel. The programme was implemented by the Helminth Control Laboratory Unguja, Ministry of Health and Social Welfare, while disease monitoring and surveillance was conducted by the Natural History Museum, UK (Stothard et al., Reference Stothard, French, Khamis, Basanez and Rollinson2009b).
The Helminth Control Laboratory Unguja has had a long history of action against schistosomiasis and acts as a referral dispensary for diagnosis and treatment, while also providing a base for the direction and co-ordination of island-wide control activities (Mgeni et al., Reference Mgeni, Kisumku, McCullough, Dixon, Yoon and Mott1990; Stothard et al., Reference Stothard, French, Khamis, Basanez and Rollinson2009b). In brief, Kick out Kichocho aimed to conduct a large-scale preventive chemotherapy campaign for the control of urogenital schistosomiasis in primary schools located in endemic areas and for the control of soil-transmitted helminthiases in all schools by administering albendazole. Since levels of disease awareness in children were known to be low, an associated health education initiative within Kick out Kichocho hoped to improve the knowledge and attitudes towards this disease, diminishing aetiological misconceptions and instigating behavioural change that would promote treatment-seeking behaviour(s) and safe water use. To this end, the health education booklet Juma na Kichocho was utilized as a novel teaching aide.
The booklet’s primary role is to provide health education messages pertinent for kichocho in a child-friendly format (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). The comic-strip layout relates the personal story of Juma, a young boy, who learns about kichocho from his teachers and local health centre staff. The booklet conveys information about primary signs and symptoms of the disease (such as the passing of blood in urine and pain upon micturition), the lifecycle and transmission of the parasite (noting that it is acquired by playing in contaminated fresh water) and appropriate control measures (such as adhering to treatment and reducing water contact). All these messages link well with general health education about schistosomiasis (WHO, 1990).
Context-specific and appropriate delivery of health education is an important aspect of any control programme conducted against schistosomiasis (Parker et al., Reference Parker, Allen and Hastings2008; Aagaard-Hansen et al., Reference Aagaard-Hansen, Mwanga and Bruun2009; Person et al., Reference Person, Knopp, Ali, Mohammed, Khamis and A’kadir2016). However, it is infrequently evaluated (Engels & Mpitabakana, Reference Engels and Mpitabakana1989; Kloos, Reference Kloos1995; Aryeetey et al., Reference Aryeetey, Aholu, Wagatsuma, Bentil, Nkrumah and Kojima1999; Schall & Diniz, Reference Schall and Diniz2001); and measuring the impact of health education can be problematic (Kloos, Reference Kloos1995; Lansdown et al., Reference Lansdown, Ledward, Hall, Issae, Yona and Matulu2002). However, if the impact of health education can be accurately recorded, it has the potential to guide the management of behavioural change, in so doing better justifying funds and resources allocated to it (Teesdale, Reference Teesdale1986; WHO, 1990; Yuan et al., Reference Yuan, Manderson, Tempongko, Wei and Aiguo2000).
Following a pilot study in 2005, a monitoring and evaluation framework for using the Juma na Kichocho booklet was developed, whereby a Kiswahili knowledge and attitudes questionnaire was tested and validated (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). This article reports on the results and experiences of an expanded health education campaign during the 2006–2007 period, assessing both qualitative and quantitative aspects of this initiative.
Methods
Study area and education campaign
As part of the general health education campaign on Unguja, a total of 3850 booklets were purchased at US$5.0 per booklet and donated to the Kick out Kichocho programme. Seventy primary schools participated in the programme, with each school receiving approximately 55 booklets. The locations of the eighteen schools where this formal knowledge and attitudes evaluation took place are listed in Table 1. The prevalence of schistosomiasis at these schools in 2004 (Stothard et al., Reference Stothard, French, Khamis, Basanez and Rollinson2009b) is also shown in Table 1. Before booklets were given out to students in Class V, in December 2005–January 2006, the knowledge and attitudes questionnaire was implemented to record initial knowledge and attitudes responses before the booklet was used in a sub-set of 50 Class V students attending class that day. The booklets were then introduced to the children along with participating classroom teachers and a 30 minute presentation with a portable digital projector, given by a health educator from the Helminth Control Laboratory Unguja. In this session, the booklets were used in the context of discussing broader health issues concerning urogenital schistosomiasis such as the parasite lifecycle, its interplay with fresh water habitats and the need for praziquantel treatment.
Table 1 Locations and names of the eighteen sampled schools with egg-patent prevalences of infection as observed in 2004 surveys and number of pupils completing the knowledge and attitudes questionnaire in 2006 and 2007

a Source: Stothard et al. (Reference Stothard, French, Khamis, Basanez and Rollinson2009b).
Over the following year, all classroom teachers were requested to make structured use of the booklets within their school-health curricula, rotating booklets between classrooms and classes where needed. They were also asked to raise awareness when parasitological surveys were undertaken locally and school children were examined on site. Informal follow-up visits were made by the health educator at each school to encourage the use of booklets and to enquire about the uptake and upkeep of the booklets. At the end of the study period (January 2007), the same knowledge and attitudes questionnaire was again used in Class V as a two-time point cross-sectional comparison, i.e. before and after. In this instance, the health educator deposited the questionnaires at the school and asked the Class V teacher to carry out the questionnaire with the children when convenient later in the week. Once the forms were completed, the Helminth Control Laboratory Unguja was contacted to arrange a time for their collection.
Baseline questionnaire with one-year follow-up
To assess children’s knowledge and attitudes, in May 2005 a standardized written questionnaire in Kiswahili was prepared and pre-tested in five schools: Fujoni, Kitope, Mto Pepo, Mwera and Pwani Mchangani (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). Upon implementation of the questionnaire at baseline in December 2005/January 2006 and follow-up in January 2007, it was requested that participating children in Class V (n=50) completed the forms in ballpoint pen under the supervision of the classroom teacher. This questionnaire used a combination of simple ‘yes’ or ‘no’ answers, as well as multiple choice responses for certain topics where a more detailed understanding was needed (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). A total of eighteen questions were posed, which were assigned to one of three separate sections. Section 1 asked four questions eliciting prevailing knowledge and attitudes of local diseases (e.g. symptoms of febrile illnesses, and assessing whether children understood more memorable aspects of the health surveys such as obtaining finger-prick blood samples as well as collection of stool and urine specimens). Section 2 asked seven questions about malaria (with a focus on knowledge and attitudes about the lifecycle, preventive measures such as the use of bednets and access to treatment); and section 3 asked seven questions about knowledge and attitudes towards urogenital schistosomiasis (kichocho), with a focus on the lifecycle, transmission by fresh water snails, preventive measures such as better sanitation, water hygiene and access to treatment.
The research was approved by the Local Research Ethics Committee (LREC) of Imperial College London (Application 33.18) and by the Zanzibar Medical Research Ethics Committee within the Ministry of Health and Social Welfare.
Data management and analysis
Completed paper copy questionnaire forms were collected from each school and double-entered onto a computer using Microsoft Excel 2007. These data were then exported to Stata v8 (StataCorp LP, TX, USA). The adopted knowledge and attitudes scoring system focused on five questions: 3.0/2.0 What is kichocho/malaria?, 3.1/2.1 What are the main symptoms of kichocho/malaria?, 3.2/2.2 How do you catch kichocho/malaria?, 3.3/2.3 How can you protect yourself from kichocho/malaria? and 3.6/2.6 Can you ever catch kichocho/malaria again after taking treatment? The children were then asked to tick-box their answer from a short list of possible responses. A correct response was awarded a single positive mark while an incorrect response scored no mark (for questions 3.0/2.0, 3.2/2.2 and 3.6/2.6). In multiple choice answers, quarter, half and three-quarters marks were allocated for partially correct answers. Further details of each question can be found in Stothard et al. (Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006).
For each child, a combined knowledge and attitudes score was obtained for schistosomiasis and malaria by tallying correct responses to each of these five questions such that each child’s knowledge and attitudes score could range from 0.0 to 5.0. By progressively pooling children’s knowledge and attitudes scores by sex, and then by schools, a pooled total knowledge and attitudes score could be obtained with an underlying frequency distribution. By assessing pooled changes in this frequency distribution of knowledge and attitudes scores for schistosomiasis and malaria, using the Kolmogorov–Smirnov test (Sokal & Rohlf, Reference Sokal and Rohlf1995) in 2006 and in 2007, it was anticipated that it would be possible to assess booklet-induced changes directly. Using knowledge and attitudes scores for malaria was deemed a neutral or negative control comparator for the expected positive change in knowledge and attitudes for schistosomiasis because there were no equivalent health campaign schemes for malaria at that time.
Results
Upon completion of baseline data entry for 2006, completed questionnaire responses for 751 children (328 boys, 423 girls) attending Class V were available. For follow-up in 2007, completed questionnaire responses from 779 (351 boys, 428 girls) were obtained and numbers by school are shown in Table 1. Collected data from four schools (Pwani mchangani, Kinyasini, Fujoni and Muyuni) were judged to be unreliable, being deemed fictitious, and they were not included in further analyses.
The gender bias in Class V, which favoured girls rather than boys (4:3), was typical. The average age of children participating in the research in 2006 was 13.1 years and 13.3 years in 2007, with ages ranging from a minimum of 10 years to a maximum of 19 years.
Responses and marks to each of the five questions used in the knowledge and attitudes scores are presented by school, year and mean response in Fig. 2. Upon visual perusal of the pie charts little difference in broad trends between years is apparent, although responses at Kizimkazi school for questions 3.0, 3.1 and 3.3 concerning schistosomiasis show considerable improvement, even though this school is not in the endemic area.
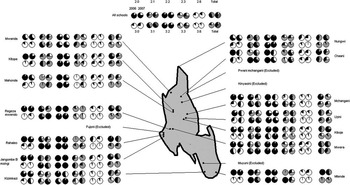
Fig. 2 Outline map of Unguja Island (light grey) with the locations of the eighteen sampled study schools. The associated responses for five knowledge and attitudes (KA) questions each posed specifically for malaria and schistosomiasis are indicated by school. The corresponding pie charts of mean responses are depicted in the central portion of the plot. Owing to poor-quality data, i.e. probably being fictitious, responses from four schools were omitted. Black portion of pie chart corresponds to a correct response while white portion is an incorrect response; sectors in grey are a correct fractional response to a multiple choice question.
Knowledge and attitudes scores across children within the fourteen schools were pooled and mean responses by year are presented as a box-and-whisker plot in Fig. 3. Further pooling of scores across schools to create a final cumulative knowledge and attitudes score for malaria and schistosomiasis in 2006 and 2007 is shown in Fig. 4. Kolmogorov–Smirnov tests used to detect changes in knowledge and attitudes scores, by year, for schistosomiasis (D=0.1, p>0.99) and malaria (D=0.15, p>0.965) were non-significant.
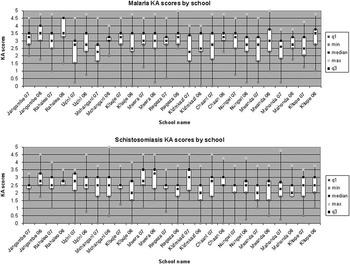
Fig. 3 Changes in average knowledge and attitudes (KA) score in fourteen sampled schools in box-and-whisker plots by year (2006 and 2007). With the overlapping nature of the quintiles, observed changes in scores were not of statistical significance although observed improvements at Kizimkazi, a school in the non-endemic zone, show near significance for knowledge and attitudes for schistosomiasis alone.

Fig. 4 Line plot of cumulative knowledge and attitudes (KA) scores for 2006 and 2007 showing a general decrease in score for malaria with a largely identical distribution of scores for schistosomiasis.
Discussion
The research presented in this article builds on the initial pilot evaluation of the booklets on Unguja (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006), by examining all schools located within the endemic area on Unguja and being assessed over a much longer period, i.e. 1 year rather than 1 month. It should be remembered that at the time of the research, there were just over 110 state-registered primary schools on Unguja, and this survey assessed a very small fraction of the total number of pupils attending primary schools (<2%). At baseline, the levels of awareness for schistosomiasis varied by school (see question 3.0 responses in Fig. 2), but overall levels of knowledge were low (~25%). This is similar to that reported several years earlier (Stothard et al., Reference Stothard, Mgeni, Khamis, Seto, Ramsan and Rollinson2002c) but notably the general levels of awareness for malaria were much better (~75%). This has also been noted before (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006), and is probably due to the greater local priority given to this disease. Moreover, health education for malaria is a permanent feature of the school health curriculum, while schistosomiasis is not. Even though the endemic zone for transmission of S. haematobium is restricted and may suggest an island-wide approach to health education is not needed, primary-school-aged children are known to travel widely within Unguja, often during holiday periods when visiting the homesteads of extended family members within the endemic area (Stothard et al., Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard and Kristensen2002b). The potential importance of this within- or off-island travel in shaping the local epidemiology of transmission should be further investigated, perhaps more quantitatively, by developing a spatial framework estimating local indices of active transmission sites (Vercruysse et al., Reference Vercruysse, Shaw and De Bont2001).
To formalize the role of health education within schistosomiasis control, significant steps were taken by WHO during the late 1980s to promote and reinforce healthy behaviour(s) in the context of developing primary health care (WHO, 1990). This was with a vision of ensuring the full participation of individuals and communities concerned. To this end, a context-specific bill-board poster was produced that encapsulated the diversity of existing interventions and depicted unwanted behaviours that contributed to disease without apportioning blame or shame. Preventive chemotherapy strategies were only beginning to be developed, but they had been piloted much earlier with alternative drugs (Macdonald et al., Reference Macdonald, Forsyth, Rayski and Rashid1968). The approach took off with the widespread availability of praziquantel, following pilot studies in several settings including Unguja at Kinyasini (Mgeni et al., Reference Mgeni, Kisumku, McCullough, Dixon, Yoon and Mott1990) and Pemba (Savioli & Mott, Reference Savioli and Mott1989; Savioli et al., Reference Savioli, Hatz, Dixon, Kisumku and Mott1990). Here, the rapid impact of treatment on the prevalence of disease boosted confidence that long-term success could be achieved simply by promoting better access to chemotherapy. In so doing, the balance of appropriate topics for consideration within health education changed more and more towards supporting and sustaining preventive chemotherapy (Savioli et al., Reference Savioli, Gabrielli, Montresor, Chitsulo and Engels2009). With this increasing focus on chemotherapy, there was little commensurate response in formalized health education, and for children, very little in the way of context-specific materials that explained the need for repeated treatment for this complex disease in a simple, child-friendly manner (Savioli & Mott, Reference Savioli and Mott1989).
With a growing realization that there was a need to develop appropriate health education materials for children, the comic-strip medical booklet Juma na Kichocho was formally designed in collaboration with a local artist and then put into production with the support of WHO (Stothard et al., Reference Stothard, Mook, Mgeni, Khamis, Khamis and Rollinson2006). The original booklet was in a robust and durable format, being suitably robust to the demanding conditions typical of primary schools in Zanzibar. During the 1990s, its use remained very limited. It was not incorporated within the Tanzanian primary school health curriculum and it languished until it was taken up by the Schistosomiasis Control Initiative as an appropriate health education tool for use in primary schools, alongside other health education initiatives to create and sustain a demand for treatment (Beanland et al., Reference Beanland, Lacey, Melkman, Palmer, Stothard, Fleming and Fenwick2006). While there had been no formal qualitative or quantitative evaluation of this booklet, nor of any others developed in this series for other diseases (see http://www.chepe.fr/), it has been further revised and adapted by Merck-KGa into a more modern cartoon format and entitled Bambo has Bilharzia: What Children Should Know about Bilharzia (see http://apps.who.int/iris/bitstream/10665/44636/1/9789241501903_eng.pdf).
Measuring the impact of health education on a disease such as schistosomiasis is not simple, especially as it may also interact and have an impact on other diseases of greater notoriety, e.g. interplay with HIV (Lillerud et al., Reference Lillerud, Stuestoel, Hoel, Rukeba and Kjetland2010). In communities afflicted by schistosomiasis, there are often low levels of awareness (Stothard et al., Reference Stothard, Mgeni, Khamis, Seto, Ramsan, Hubbard and Kristensen2002b, Reference Stothard, Mgeni, Khamis, Seto, Ramsan and Rollinsonc; Mwanga et al., Reference Mwanga, Magnussen, Mugashe, Gabone and Aagaard-Hansen2004) and misconceptions abound across social groups. For example, perceptions of the disease often vary by gender (Parker, Reference Parker1993; Kloos, Reference Kloos1995; Gazzinelli et al., Reference Gazzinelli, Dos Reis, Kloos, Velasquez-Melendez, Dutra and Gazzinelli2006); and this is evident in places such as Sudan (Parker, Reference Parker1995), Kenya (Musuva et al., Reference Musuva, Awiti, Omedo, Ogutu, Secor and Montgomery2014), mainland Tanzania (Mwakitalu et al., Reference Mwakitalu, Malecela, Mosha and Sinionsen2014) and also on Unguja. Recent surveys undertaken by the Zanzibar Elimination of Schistosome Transmission Project, for example, suggest that many still think that kichocho is a boy’s rather than a girl’s disease (Knopp et al., Reference Knopp, Person, Ame, Mohammed and Ali2013a; Person et al., Reference Person, Knopp, Ali, Mohammed, Khamis and A’kadir2016). This illustrates a more general need to combine effective communication with preventive chemotherapy campaigns (Schall & Diniz, Reference Schall and Diniz2001; Fleming et al., Reference Fleming, Fenwick, Tukahebwa, Lubanga, Namwangye, Zaramba and Kabatereine2009).
In line with the lifecycle depicted in Fig. 1, a key feature for both schistosomiasis and malaria was that the majority of children did not realise that reinfection could take place after treatment. This highlights a further problem: there is a tendency to place too little emphasis on personal measures of protection, and too much emphasis on the fail-safe nature of treatment. This can be observed as a group trend, as by pooling questionnaire responses into a single knowledge and attitudes measure by school, it can be seen how this clearly varies by school (see Fig. 2), ranging more widely for malaria than for schistosomiasis. This variation by school might reflect the quality of health education by school or other factors that are school-specific, for example, knowledge and attitudes scores for Rahaleo were much better in 2006 than in 2007. Causal changes in the dynamics of knowledge and attitudes scores of malaria are difficult to explain but can be used in conjunction with knowledge and attitudes scores for schistosomiasis as a useful yardstick or comparator. By pooling scores across schools to obtain school-wide metrics, it is again apparent that scores for schistosomiasis are lower than those for malaria (see Figs 3 and 4). Plotting the changes between years allows greater inference in broad trends and it is clear that knowledge and attitudes for malaria have decreased while those for schistosomiasis have increased. Both changes were statistically insignificant by Kolmogorov–Smirnov tests. It can, therefore, be concluded that the intervention of using the booklets did not achieve a tangible impact in terms of raising awareness and understanding of urogenital schistosomiasis. This is a disappointing finding, given the time and effort allocated to the initiative.
Concurrent parasitological surveys were also undertaken across 28 Ungujan primary schools where the annual dynamics of infections were recorded. This provided an opportunity to administer a simple ‘yes’ and ‘no’ questionnaire on a variety of disease-specific factors. A more general analysis of these data has been reported elsewhere, but a notable decline in the extent of contaminating behaviour, i.e. urinating in fresh water, as a 60.3% decrease (from 14.8% to 5.7%) was observed (Stothard et al., Reference Stothard, French, Khamis, Basanez and Rollinson2009b). No similar decline was observed in levels of reported water contact, playing in fresh water, which probably indicates that although the two activities are connected they are not explicitly interdependent (see Fig. 5). For example, a child may continue to play in fresh water since there is no other choice for recreational play but whilst immersed has the choice to urinate or not to urinate, contingent upon its importance as perceived by the child. Typical of schistosomiasis, there are many socio-cultural drivers behind these dynamics (Aagaard-Hansen et al., Reference Aagaard-Hansen, Mwanga and Bruun2009).

Fig. 5 Bar chart with 95% confidence interval error bars of recalled water contact behaviours of children examined as part of annual surveys within 24 sentinel schools on Unguja Island (Stothard et al., Reference Stothard, French, Khamis, Basanez and Rollinson2009b). Water-contact scores are expressed as percentages of a maximum score of 1 representing positive answers to each of three water-contact questions, i.e. playing, working and washing in water bodies. Water contamination is assessed by the prevalence of positive answers to the question of whether the child urinated in water.
There are two particularly important implications from the above. First, as only a minority of infected children are needed to maintain the transmission cycle at key foci (see Fig. 1), if they continue to urinate schistosome eggs into the environment, reductions in contamination do not correlate in a linear manner with decreases in infected snails and therefore environmental infection risk. To define this aspect more explicitly requires more detailed surveillance of snails and preliminary investigations show that much greater reductions in contaminating behaviours are needed to have any impact on the prevalence of pre-patent infections in snails (Allan et al., Reference Allan, Dunn, Emery, Stothard, Johnston and Kane2013). Second, there is likely to be a sub-set of children in any population who will find it difficult to adhere to safe water-contact behaviour(s) and in so doing facilitate parasite transmission. Clearly, out-of-school children will not only fail to receive appropriate health education but they are also more likely to spend their time engaged in water-contact activities (such as playing, bathing and fishing), thereby contributing to the micro-epidemiology of disease (Rudge et al., Reference Rudge, Stothard, Basanez, Mgeni, Khamis, Khamis and Rollinson2008). Potential gains in children attending school with health messages being relayed to communities by these children, need to be offset against those who are unable or unwilling to attend school and miss or avoid treatment, as seen elsewhere in East Africa (Parker et al., Reference Parker, Allen, Pearson, Peach, Flynn and Rees2012; Allen & Parker, Reference Allen and Parker2016; Hastings, Reference Hastings2016).
Conclusion
The research presented in this article highlights the limitations of health education that attempts to change the knowledge, attitudes and practices of primary school children. Given the pervasiveness of reinfection, there is, too, a pressing need to understand and more seriously engage with local understandings and responses to urogenital schistosomiasis. As things stand, it is clear that an approach that relies on voluntary behavioural change and assumes that knowledge conveyed in a child-friendly booklet translates into ‘rational’ behavioural change is inadequate. Given the intricate association of schistosomiasis with children and their water-contact activities, there is a pressing need to develop approaches that accommodate local social, economic and ecological realities if elimination of urogenital schistosomiasis is to be achieved. One promising way forward is to undertake ethnographic research, grounded in political economy, alongside biological research documenting changes occurring in the micro-epidemiology of Unguja Island following preventive chemotherapy. Such research will enable a more holistic, biosocial approach to be developed and this, in turn, will enable the design of more effective strategies that foster sustained, behavioural change.
Acknowledgments
Funding support from the Health Foundation UK was gratefully received. The authors thank the Schistosomiasis Control Initiative (SCI), Imperial College London, for donation of the Juma na Kichocho booklets and their support of the helminth control programme, together with the African Development Bank (ADB). Special thanks are due to the primary school teachers and children who took part in this exercise and to the field support staff within the HCLU who assisted in this educational campaign. Helpful comments from the reviewers and editors were gratefully received. The authors would like to dedicate this paper to the memory of the late Dr Virginia Schall, a pioneer in health education approaches for schistosomiasis. Copies of the KA questionnaire in English and Kiswahili can be found by following the weblink www.countdownonntds.org/unguja.








