Psychosis encompasses a group of disorders that present with problems in emotion, thought structure, perception, cognition and volition. Such disorders typically follow relapsing–remitting courses and often evolve into highly debilitating chronic disorders. Both schizophrenia and bipolar disorder have been historically conceptualised as ‘functional’ psychiatric disorders, without an identifiable organic basis and with few physical manifestations. However, signs are often present, particularly in schizophrenia, which are suggestive of its ‘organic’ underpinnings, and which contributed to the original Kraepelinian dichotomy (Reference KraepelinKraepelin 1919). Through a wealth of biological research, we now recognise that illnesses across the psychosis spectrum are associated with identifiable genetic risk markers and measurable abnormalities in cerebral structure and function, cognition, and neurological function. These deficits go far beyond the scope of standard psychiatric phenomenology, and suggest that schizophrenia and the affective psychoses lie on a continuum of biological vulnerability.
About 60% of people with schizophrenia have evidence of neurological deficits in both the sensory and motor domains (Reference Buchanan and HeinrichsBuchanan 1989). The relevance of these neurological abnormalities to schizophrenia and psychosis, and their pathophysiological significance in particular, remains elusive. The terminology describing these deficits can be confusing, but in this article we will apply the most broadly accepted terminology, dividing neurological signs into:
-
• hard (or primary) signs
-
• soft or integrative signs, referred to here as neurological abnormalities
-
• extrapyramidal side-effects (EPS).
These terms are clarified in Box 1.
BOX 1 Definitions of neurological deficits in psychosis
-
• Hard or primary signs Broadly meaning neurological signs detected at routine clinical neurological examination and generally indicative of an anatomically localisable central or peripheral nervous system lesion
-
• Soft or integrative neurological signs (neurological abnormalities) Signs that do not localise anatomically or functionally to specific regions of the nervous system
-
• Extrapyramidal side-effects (EPS) Signs that can be attributed to antipsychotic medication
The clinical neurological examination
In everyday clinical practice a thorough neurological examination is an important component in the assessment of each new patient presenting with psychosis. This will often take the form of an unguided screen for hard signs, largely untailored to the psychiatric presentation. The aim of this examination is to exclude the presence of localising neurological signs indicative of gross neuropathology in patients presenting with psychiatric phenomena that could be consistent with a lesion in the central nervous system. In the UK, this examination will often only occur if the individual is admitted to hospital as an in-patient, and it routinely assesses the integrity of the cranial nerves (I to XII) and the peripheries through assessment of tone, power, reflexes, gait and coordination. Some psychiatrists also assess the presence of neurological signs attributable to side-effects of psychotropic medication, such as rigidity and tremor, and even less frequently they assess frontal lobe function through, for example, tests of complex motor-task sequencing. In many clinics and hospitals this will be followed by either a cerebral computed tomography (CT) or magnetic resonance imaging (MRI) scan, and less routinely an electroencephalogram (EEG), irrespective of the earlier clinical findings.
The general validity of routine cerebral imaging of new patients with psychosis is poorly established. Although many ‘routine’ scans detect some evidence of abnormality, usually atrophy or ischaemia, very few are clinically significant or lead to any change in patient care (Reference Borgwardt, Radue and GotzBorgwardt 2006). The fact remains that a normal neurological examination is insufficiently sensitive to absolutely exclude the presence of a significant intracranial lesion (Reference Mueller, Rufer and MoergeliMueller 2006). However, in the context of focal neurological signs, the detection rate of clinically significant pathological lesions using imaging rises, in some cases changing the focus of treatment entirely (Reference Hollister and ShahHollister 1996; Reference Erhart, Young and MarderErhart 2005). Beyond this undoubtedly important consideration, the relevance of the assessment of hard signs to the care of patients with schizophrenia is rather limited. Few studies have suggested any significant link to the disorder, and it is therefore not surprising that when patients are examined by an inexperienced psychiatrist, the results contribute little to further management.
Although some patients with psychosis do exhibit hard neurological signs, it is apparent that many more exhibit more subtle, non-localising soft signs or neurological abnormalities. These do not reflect primary tract or nuclear pathology, but are attributable to impaired integration within and between the sensory and motor systems (integrative signs). The term ‘soft signs’, when applied to these abnormalities, has often raised doubts about their validity and whether they can be defined with any rigour, about their reliability or reproducibility, and about whether they have any neurological significance at all. However, numerous systematic and controlled studies have consistently identified neurological dysfunction in schizophrenia, and lead to the conclusion that doubts over their meaning reflect limitations to our knowledge rather than the unreality of findings (Reference Heinrichs and BuchananHeinrichs 1988).
The greatest weakness in the evaluation of neurological signs is perhaps their subjectivity, with a lack of standardisation affecting both their detection and rating. Another issue to take into account is evidence that many neurological signs, such as the palmomental reflex, have a high prevalence in the general population, making their interpretation difficult and the risk of false positives very high if these signs are taken in isolation. Finally, it should be remembered that normal aging is naturally associated with an increased presence of these signs. In addition to these issues, there is psychiatrists' lack of familiarity with many of the abnormalities themselves, which is a consideration for clinical medicine as a whole, and can be equally applied to the cardiovascular as to the mental state examination.
Assessment scales
To address some of these problems, several structured instruments have been developed specifically to assess neurological abnormalities present in psychosis. These instruments have helped to standardise their assessment as a component of the neurological examination. More importantly, they focus researchers' minds on developing a better understanding of the role of neurological abnormalities in the pathophysiology of psychosis and on trying to understand their relevance to patient care. Current validated schedules include the Neurological Evaluation Scale (Reference Buchanan and HeinrichsBuchanan 1989), the Cambridge Neurological Inventory (Reference Chen, Shapleske and LuqueChen 1995), the Heidelberg Scale (Reference Schroder, Niethammer and GeiderSchroder 1991) and the Condensed Neurological Examination (Reference Rossi, De Cataldo and Di MicheleRossi 1990) (Table 1). Of these, the most widely used and that with the greatest amount of research evidence is the Neurological Evaluation Scale.
TABLE 1 Common validated schedules for assessing neurological abnormalities

| Scale | Areas measured | Rating used |
|---|---|---|
| Neurological Evaluation Scale (Reference Buchanan and HeinrichsBuchanan 1989) | Sensory integration, motor coordination, sequencing of motor acts, ‘others’ (e.g. memory, grasp, gaze, mirror movements) | 3-point scale: 0=absent, 1=mild but definite, 2=marked impairment |
| Cambridge Neurological Inventory (Reference Chen, Shapleske and LuqueChen 1995) | Speech, eye movements, selective examination of cranial nerves, extremity examination, soft signs, ‘others’ (e.g. facial dyskinesia, stereotypy, arm drift/dropping) | 4-point scale: 0=normal response, 0.5=equivocal response, 1=abnormal response, 2=grossly abnormal response |
| Heidelberg Scale (Reference Schroder, Niethammer and GeiderSchroder 1991) | Neurological signs (e.g. station and gait, tandem walking, dysdiadochokinesia, grapaesthesia) | 4-point scale: 0=absent, 1=mild, 2=present, 3=marked |
| Condensed Neurological Examination (Reference Rossi, De Cataldo and Di MicheleRossi 1990) | Neurological hard signs (e.g. palmomental test, suck reflex, blunt–sharp discrimination), neurological soft signs (e.g. gaze impersistence, oral apraxia, imaginary acts) | 2-point scale: 0=absent, 1=present (unless otherwise stated) |
The scales vary in their content, but common themes emerge. Each assesses the presence of multiple neurological abnormalities since no single abnormality is useful in the diagnosis or management of schizophrenia. Common collections of abnormalities aggregate in each scale (Table 2) along specific functional lines. These collections are linked conceptually by our understanding of the functional organisation of the nervous system, and lead to subscales within each scale, the most robust subscales being motor tasks/motor impairment and cognitive/perceptual impairment.
TABLE 2 Functional areas frequently reported as abnormal in schizophrenia and tests that can elicit disturbances in these areas
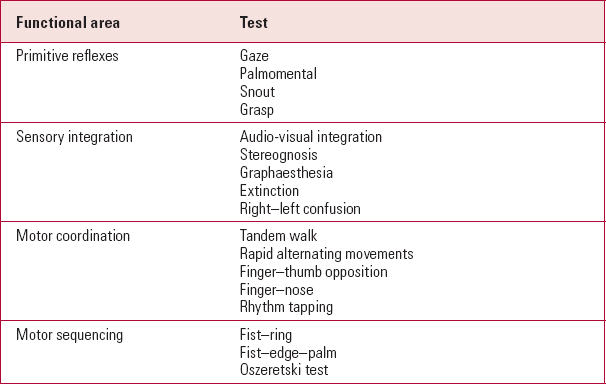
| Functional area | Test |
|---|---|
| Primitive reflexes | Gaze |
| Palmomental | |
| Snout | |
| Grasp | |
| Sensory integration | Audio-visual integration |
| Stereognosis | |
| Graphaesthesia | |
| Extinction | |
| Right–left confusion | |
| Motor coordination | Tandem walk |
| Rapid alternating movements | |
| Finger–thumb opposition | |
| Finger–nose | |
| Rhythm tapping | |
| Motor sequencing | Fist–ring |
| Fist–edge–palm | |
| Oszeretski test |
Even within the existing scales, the categorisation of each neurological sign as hard or soft (primary or integrative) is not straightforward. For example, tests such as the fist–edge–palm test, or the fistring test, require integration between the sensory and motor systems (integrative signs), but are also indicative of frontal lobe damage (primary signs). The same can be said for the tandem walk or finger–nose tests, which can reflect impaired sensorimotor integration, but also focal cerebellar damage. Finally, cortical release signs (primitive reflexes such as the glabellar, grasp and palmomental reflexes) appear as a consequence both of frontal lesions and of more diffuse pathology, and therefore cannot be exclusively classified as hard or soft (Reference Walterfang and VelakoulisWalterfang 2005).
The scales rate each neurological sign numerically, usually from absent to severe, and can produce cumulative scores for the total and sub-scale neurological scores. Although these scores are important, they should not be interpreted as an indication of illness severity. However, as we shall see later, the score can inform clinical management as well as progress.
Neurological abnormalities and defining neurological impairment in psychosis
An excess of neurological abnormalities is an incontrovertible finding in the psychoses. However, the total number of abnormalities is neither a useful measure of ‘neurological impairment’, nor a threshold defining ‘abnormality’. By definition, none of the abnormalities are, either by themselves or cumulatively, associated with significant functional impairment, and so the concept of a threshold is of itself rather arbitrary. The authors of the existing scales recognised this and did not attempt to define their own thresholds for abnormality. Furthermore, the scales that assess the greatest number of abnormalities, those that cast their net widest, detect the greatest number of individuals with ‘impairment’. Consequently, although neurological abnormalities appear to be an integral component of psychoses, it is unrealistic to use their assessment to define patients with psychotic disorders as neurologically impaired, so reflecting the very nature of the abnormalities detected. Rather, as we shall see, the evaluation of neurological abnormality can be used as another source of information to guide the assessment, diagnosis and treatment of patients with psychoses.
Epidemiology of neurological abnormalities
Neurological abnormalities occur in excess in patients with a variety of mental health problems, including obsessive–compulsive disorder and autism. The presence of such abnormalities in disorders with a possible neurodevelopmental origin reflects their hypothetical association with underlying central nervous system pathology. However, outside schizophrenia, very few studies have investigated more than one neurological domain, usually the motor domain. Neurological abnormalities seem particularly prevalent in patients with schizophrenia, with some studies reporting rates as high as 100%, although most report rates between 50 and 65% (Reference Bombin, Arango and BuchananBombin 2005). Greater total numbers of abnormalities have been reported in patients with schizophrenia compared with patients with non-psychotic affective disorders, obsessive–compulsive disorder, alcohol dependence, and healthy controls, although this diagnostic specificity is lost when compared with other psychotic disorders (Reference Whitty, Clarke and McTigueWhitty 2006; Reference Dazzan, Lloyd and MorganDazzan 2008). For example, patients with affective psychoses show signs of frontal and parietal dysfunction and impaired motor performance (Reference Nasrallah, Tippin and McCalley-WhittersNasrallah 1983; Reference Boks, Liddle and BurgerhofBoks 2004; Reference Dazzan, Lloyd and MorganDazzan 2008) in a pattern very similar to that seen in patients with schizophrenic psychoses.
Aetiology of neurological abnormalities
Thus far, very few aetiological risk factors for neurological abnormalities in schizophrenia have been identified. A variety of studies have failed to find any consistent association between such abnormalities and sociodemographic characteristics of patients, and to date the greatest body of evidence relates to the genetic risk for the disorder (Reference Dazzan and MurrayDazzan 2002).
Genetics
Increased rates of neurological abnormalities are seen in the unaffected offspring of mothers with schizophrenia, who have greater abnormality scores than the offspring of mothers with other psychotic disorders (Reference Schubert and McNeilSchubert 2004, Reference Schubert and McNeil2005). Unaffected relatives (parents, siblings and co-twins) of patients with schizophrenia are at also increased risk of neurological abnormalities (Reference Yazici, Demir and YaziciYazici 2002; Reference Gourion, Goldberger and OlieGourion 2004). Interestingly, the number of abnormalities appears to be related to the genetic proximity of the unaffected relative to the proband – the closer this relationship, the greater their number (Reference Egan, Hyde and BonomoEgan 2001; Reference Picchioni, Toulopoulou and LandauPicchioni 2006). This familial aggregation does not extend to involuntary dyskinetic movements, neurological deficits that are conceptually distinct from neurological abnormalities (Reference Tarbox and Pogue-GeileTarbox 2006). These are much more closely linked to the neurological side-effects of antipsychotic medication, supporting a pathophysiological distinction between the two groups of neurological signs.
Obstetric complications
Obstetric complications, in particular perinatal hypoxia, have shown to be a potential environmental risk factor for neurological abnormalities in offspring. However, it is likely that the magnitude of any association between neurological abnormalities and obstetric complications is much smaller than was originally anticipated. Preterm cohort studies have shown that neonatal prematurity is associated with abnormal neural development, and that people born preterm have increased levels of abnormalities and impaired neuropsychological function compared with people born at full-term (Reference Allin, Matsumoto and SanthouseAllin 2001). When individuals at genetic risk of schizophrenia are exposed to obstetric complications, they express even greater levels of neurological abnormalities, suggesting an interactive effect between these genetic and environmental risk factors (Reference Cantor-Graae, McNeil and RicklerCantor-Graae 1994, Reference Cantor-Graae, Ismail and McNeil2000). This effect increases the magnitude of any future neurological deficit, supporting this as an aetiological model of schizophrenia.
Can neurological abnormalities predict proneness to psychosis?
It is clear that neurological abnormalities are an intrinsic part of vulnerability to psychosis and that they are already present in excess in the earliest phases of the disorder (Reference Dazzan and MurrayDazzan 2002). The next question is whether or not this excess pre-dates the onset of psychosis, possibly acting as a vulnerability marker for the illness. Indeed, impairments of motor development and fine motor coordination have been observed in children who later develop schizophrenia (Reference Crow, Done and SackerCrow 1995; Reference Cannon, Walsh and HollisCannon 2001). Their presence in such children suggests that this neurological dysfunction is a marker of latent neurodevelopmental abnormality, itself acting as the foundation for the risk of schizophrenia.
Although neurological abnormalities correlate with psychosis proneness and schizotypal traits in the general population, they are not yet a sensitive enough tool to discriminate between those who will and will not develop schizophrenia from these high-risk populations (Reference Lawrie, Byrne and MillerLawrie 2001; Reference Barkus, Stirling and HopkinsBarkus 2006). Despite a strong argument for a role for neurological abnormalities, and perhaps more specifically for neuromotor dysfunction in this context (Reference McNeil and Cantor-GraaeMcNeil 2000), none of the most widely accepted ‘high-risk mental state’ screening tools include a comprehensive assessment of neurological abnormalities, instead focusing on transient or attenuated psychotic symptoms.
Pathophysiology of neurological abnormalities
Surprisingly few studies have investigated the anatomical substrate of neurological abnormalities in psychosis. At a gross neuroanatomical level, neurological abnormalities are associated with smaller whole brain volume, and enlarged sulci and cerebral ventricles (Reference Weinberger, Wyatt and UsdinWeinberger 1982; Reference Rubin, Vorstrup and HemmingsenRubin 1994). This association is not surprising, given that both are typical features of schizophrenia, and it does not prove causation. More recent studies have investigated patients early in their illness, before exposure to long-term pharmacological treatment. These studies have reported an association between an excess of motor signs and volume reductions in the basal ganglia, cerebellum and motor cortex (Reference Keshavan, Sanders and SweeneyKeshavan 2003; Reference Dazzan, Morgan and OrrDazzan 2004; Reference Ho, Mola and AndreasenHo 2004). In contrast, greater numbers of sensory integration signs in both patients with schizophrenia and healthy individuals (Reference Keshavan, Sanders and SweeneyKeshavan 2003; Reference Dazzan, Morgan and OrrDazzan 2004, Reference Dazzan, Morgan and Orr2005) seem to be associated with volume reduction of the hetero-modal association cortex, including the inferior frontal, medial temporal and inferior parietal cortices.
From a functional perspective we remain very much in the dark since even fewer studies have explored this area. One study reported lower levels of neural activity in the motor cortex in patients with schizophrenia making small hand movements in the scanner (Reference Schroder, Wenz and SchadSchroder 1995), and another demonstrated impaired metabolic activity in the basal ganglia of patients with prominent developmental reflexes (Reference Gangadhar, Jayakumar and VenkatasubramanianGangadhar 2006). More recently, using connectivity analysis Reference Rao, Di and ChanRao and colleagues (2008) found evidence of failed regulation, rather than direct participation, of the prefrontal cortex in the execution of the fist–edge–palm test.
These imaging findings suggest that the underlying neural substrate for neurological abnormality maps, at least in part, to the neural substrate underpinning schizophrenia. The association between abnormalities and smaller basal ganglia volume seen in patients may be particularly indicative of the neural substrate of psychosis. Most importantly, the volume reductions detected in these studies are independent of both current and longitudinal antipsychotic exposure, and support the conclusion that neurological abnormalities and their anatomical correlates genuinely reflect the neural substrate for schizophrenia and not merely a treatment epiphenomenon (Reference Dazzan, Morgan and OrrDazzan 2004).
Clinical associations
Temporal stability of neurological abnormalities
Although neurological abnormalities demonstrate some trait-like characteristics in their relationship to schizophrenia, they are also sensitive to a number of illness severity measures. Findings from first-episode psychosis studies of the temporal stability of neurological abnormalities suggest that, although some signs, such as cortical release signs, may be trait-like (reflecting their neuro-developmental origin), others, such as motor dysfunction, may be more state-like and potentially susceptible to therapeutic manipulation (Reference Emsley, Turner and OosthuizenEmsley 2005). However, any improvement has tended to be reported after only relatively brief treatment and could be ascribed to learning effects. In fact, longer-term follow-up studies in first-episode psychosis have suggested stability or even possible deterioration of neurological abnormalities over time. This deterioration could be due to the long-term effects of antipsychotic medication, or to the action of a progressive degenerative process.
Relationship to positive and negative symptoms
Turning to psychopathology, neurological abnormalities show relatively little relationship to positive psychotic symptoms, although motor dysfunction has been associated with greater positive symptom scores, both improving with treatment (Reference Tosato and DazzanTosato 2005). Disorganisation has been associated with high neurological abnormality scores (Reference Schroder, Niethammer and GeiderSchroder 1991), and specifically high scores for sensory integration and the sequencing of complex motor acts (Reference LiddleLiddle 1987; Reference Arango, Kirkpatrick and BuchananArango 2000), but the majority of recent studies do not report any association between neurological abnormalities and disorganisation.
In contrast, neurological abnormalities seem to be strongly correlated with current and future negative symptoms (Reference Yazici, Demir and YaziciYazici 2002; Reference Prikryl, Ceskova and KasparekPrikryl 2006). In fact, high abnormality scores predict a greater risk of developing features of the chronic ‘defect state’ (Reference Ismail, Cantor-Graae and CardenalIsmail 1998; Reference Galderisi, Maj and MucciGalderisi 2002) and becoming a treatment ‘non-responder’ (Reference Smith, Kadewari and RosenbergerSmith 1999). Neurological abnormalities in this situation might act as clinical markers of the underlying central nervous system pathology, possibly involving the cerebellum and frontal lobes. Abnormalities detected in the first episode of illness may be predictive of the patient's future response to antipsychotic treatment and of their degree of social recovery, with higher levels of neurological abnormalities associated with a poorer functional outcome and more severe cognitive deficits (particularly in executive or frontoparietal function) (Reference Arango, Bartko and GoldArango 1999; Reference Mohr, Hubmann and AlbusMohr 2003; Reference Sanders, Schuepbach and GoldsteinSanders 2004; Reference Bachmann, Bottmer and SchroderBachmann 2005). Finally, rates of abnormalities (particularly in the context of frontal lobe dysfunction) also identify patients who are more sensitive to the side-effects of antipsychotic medication (Reference Convit, Volavka and CzoborConvit 1994).
Clinical significance
By assessing the magnitude of neurological abnormalities in patients with psychosis, we can begin to identify subgroups of patients who are at greatest risk of developing more negative symptoms and cognitive impairment, and who will have a poorer functional outcome. By using this information early on in the course of the illness, we can begin to identify those patients who are most likely to present with complex medical, cognitive and social care needs, and who are likely to require greater levels of support to maximise their functional recovery.
Association with antipsychotic medication
Most, if not all, antipsychotic medication can cause some neurological side-effects, most commonly extrapyramidal motor symptoms. These include acute dystonic reactions, akathisia, Parkinsonism and tardive dyskinesia. Extrapyramidal side-effects are conceptually distinct from neurological abnormalities, and although a wealth of evidence suggests that the excess of abnormalities is not an epiphenomenon of antipsychotic treatment, the relationship between neurological abnormalities and antipsychotic medication remains contentious. Intuitively we may suspect that neurological abnormalities are attributable at least in part to antipsychotic treatment, but if anything the opposite seems to be the case.
Rating scales for neurological side-effects
Just as with neurological abnormalities, several rating scales have been developed to assess neurological side-effects and each scale tends to tap into a specific side-effect domain: dyskinesia, akathisia or Parkinsonism. The most commonly used scales include those listed in Box 2. By and large, these scales are simple, short and easy to administer, reflecting the intention that they should be easy to use in a variety of settings.
BOX 2 Scales for assessing neurological side-effects
-
• Abnormal Involuntary Movements Scale (Reference GuyNational Institute of Mental Health 1976)
-
• Barnes Akathisia Rating Scale (Reference BarnesBarnes 1989)
-
• Simpson–Angus Scale (Reference Simpson and AngusSimpson 1970)
-
• Scale for Targeting Abnormal Kinetic Movements (Reference Wojcik, Gelenberg and LaBrieWojcik 1980)
The hypotheses
The pathophysiological relationship between neurological abnormalities and antipsychotic side-effects is complex, and could take a variety of forms. First, neurological abnormalities might simply represent a direct side-effect of prescribed antipsychotic medication. Second, higher rates of abnormalities might identify a subpopulation of patients with a poorer prognosis and a more aggressive illness, who are consequently prescribed higher doses of antipsychotics, leading to an indirect association between the two. Third, it is possible that individuals with poorer central nervous system development, and therefore more neurological abnormalities, are more sensitive to the neurological side-effects of antipsychotics.
The evidence
Many studies have investigated the relationship between neurological abnormalities and anti-psychotics, with the vast majority reporting little or no evidence of a relationship between abnormalities and either the dose or type of antipsychotic, including clozapine, or between the abnormality and side-effect ratings. In contrast, some studies have reported that neurological abnormalities may improve, particularly in the motor domain, following the initiation of effective antipsychotic treatment (Reference Whitty, Clarke and BrowneWhitty 2003; Reference SchefferScheffer 2004).
Further evidence of the independent nature of neurological abnormalities and extrapyramidal motor symptoms in psychosis has been provided by studies of individuals who are antipsychoticnaive. These studies have found that an excess of abnormalities in patients with schizophrenia can be detected even before antipsychotic treatment has started (Reference Venkatasubramanian, Latha and GangadharVenkatasubramanian 2003; Reference McCreadie, Srinivasan and PadmavatiMcCreadie 2005). By contrast, although EPS can be seen in never-treated patients, there is a wealth of evidence linking EPS score and the dose and type of antipsychotic medication prescribed (Reference McCreadie, Thara and PadmavatiMcCreadie 2002, Reference McCreadie, Srinivasan and Padmavati2005). In comparison with typical antipsychotics, atypicals (or second-generation antipsychotics) are associated with fewer EPS, particularly acute dystonic reactions and Parkinsonism, although this advantage is lost if more than one atypical is prescribed (Reference Boks, Liddle and RussoBoks, 2003; Reference Carnahan, Lund and PerryCarnahan 2006; Reference CaseyCasey 2006). Atypicals also appear to hold an advantage for the longer-term risk of tardive dyskinesia, but this is not yet well established for these relatively new medicines (Reference Miller, McEvoy and DavisMiller 2005; Reference Tarsy and BaldessariniTarsy 2006). In contrast to the findings for neurological abnormalities, there is general agreement about which patient characteristics are associated with the greatest sensitivity to EPS (Box 3).
BOX 3 Factors associated with EPS severity
-
• Increasing age (Reference Miller, McEvoy and DavisMiller 2005)
-
• Young age at onset (Reference Srinivasan, Thara and PadmavathiSrinivasan 2001)
-
• Male gender (Reference Ismail, Cantor-Graae and McNeilIsmail 2001)
-
• Ethnicity (Reference Wonodi, Adami and CassadyWonodi 2004)
-
• Past history of obstetric complications (Reference Ismail, Cantor-Graae and McNeilIsmail 2001)
-
• Longer treatment duration
Among the patients at the greatest risk of long-term EPS are those with a family history of primary movement disorders (Reference Lencer, Eismann and KastenLencer 2004), those with extrapyramidal motor symptoms before treatment begins (Reference Caligiuri and LohrCaligiuri 1997), and those who develop acute dystonic reactions or Parkinsonism very early in the course of treatment (Reference Sachdev, Brodaty and RoseSachdev 1999). Finally, the degree of motor-sequencing impairment present during an acute episode of illness predicts the future degree of dyskinesia at follow-up, suggesting a possible relationship between the two (Reference Emsley, Turner and OosthuizenEmsley 2005).
Clinical implications
Taken as a whole, this evidence emphasises the predictive function of neurological abnormalities and extrapyramidal motor symptoms from early in the course of the illness. Both sets of signs should be assessed early, ideally before antipsychotic treatment is started, and then longitudinally throughout the course of treatment. Detecting and addressing some of these neurological signs early in the course of the illness can pre-date the development of future disabling long-term neurological side-effects that we know can be associated with a greater negative symptom load, a lower sense of well-being and increased levels of illicit drug use (Reference Miller, McEvoy and DavisMiller 2005; Reference Potvin, Pampoulova and Mancini-MariePotvin 2006).
Conclusions
Although the use of comprehensive structured scales assessing neurological abnormalities is still more appropriate to the research setting, a regular physical examination incorporating the neurological system and the evaluation of hard signs and soft signs, as well as signs of extrapyramidal symptoms, should figure in the assessment and treatment of any patient with psychosis and schizophrenia. This examination should be conducted early in the course of the illness, ideally before treatment is initiated, and at regular intervals thereafter. To achieve this, psychiatrists should work to develop and maintain the skills necessary to identify these neurological clinical signs reliably, and view their periodic assessment as much a part of the patient assessment as the mental state examination. Clinicians should develop and monitor their performance against objective standards and with peers in order to maintain their skills.
Given the extensive nature of the standardised schedules and obvious time pressures, clinicians should focus their assessment on a restricted neurological examination, even though the limited research base makes any recommendation only tentative. It has been proposed that cortical release signs (e.g. palmomental, grasp and oral reflexes) should be routinely evaluated in addition to the standard neurological examination, as these signs may be indicators of diffuse cerebral dysfunction requiring further investigation (Reference Walterfang and VelakoulisWalterfang 2005). In addition, audio-visual integration and verbal memory offer both high specificity and predictive value in schizophrenia (Reference Keshavan, Sanders and SweeneyKeshavan 2003), which may help to inform diagnosis at first presentation and inform predictions of future treatment response.
Finally, extrapyramidal symptoms should be objectively rated using a single scale (e.g. Abnormal Involuntary Movements Scale, Barnes Akathisia Rating Scale) owing to the importance of EPS in driving treatment discontinuation (Reference Lieberman, Stroup and McEvoyLieberman 2005). A focused, regular neurological evaluation can then provide important prognostic information regarding the risk of negative symptoms and cognitive deficits and can help to identify individuals with more complex future care needs. It can also identify patients at heightened risk of side-effects from antipsychotics, helping to improve treatment concordance and the therapeutic relationship.
MCQs
-
1 In the evaluation of neurological abnormalities:
-
a soft signs indicate a neurological abnormality which does not localise anatomically or functionally to a specific region of the nervous system
-
b most scales for evaluating neurological abnormalities identify a threshold to define ‘abnormality’
-
c the presence of specific neurological abnormalities is useful in the diagnosis and management of schizophrenia
-
d categorising neurological signs as hard or soft is straightforward
-
e neurological signs cannot be grouped according to functional domain.
-
-
2 As regards neurological abnormalities:
-
a less than 25% of patients with schizophrenia have neurological abnormalities at clinical examination
-
b a patient with schizophrenia with two or more neurological abnormalities should be considered neurologically impaired
-
c neurological abnormalities are more common in schizophrenia than in depressive disorder
-
d minor neurological abnormalities are not found in the general population
-
e neurological abnormalities are only present in individuals with a comorbid substance abuse disorder.
-
-
3 Neurological abnormalities:
-
a are typically associated with positive psychotic symptoms in schizophrenia
-
b are associated with functional outcome in schizophrenia
-
c typically increase after initiating antipsychotic treatment
-
d are more common in males
-
e become more severe after first onset of psychosis.
-
-
4 Regarding the association between neurological abnormalities and use of antipsychotics:
-
a neurological abnormalities are more commonly seen in patients taking typical as opposed to atypical antipsychotics
-
b neurological abnormalities and extrapyramidal side-effects are closely correlated in most patients on antipsychotic medication
-
c extrapyramidal signs can be seen in never-treated patients with schizophrenia
-
d neurological abnormalities are not detectable in individuals who are antipsychoticnaive
-
e neurological abnormalities typically improve following antipsychotic treatment.
-
-
5 As regards neurological abnormalities and brain structure:
-
a neurological abnormalities are associated with larger whole brain volume
-
b motor signs are associated with volume reductions in the basal ganglia
-
c only an excess of motor signs is associated with brain volume reductions
-
d the presence of primitive reflexes always reflects an underlying frontal lesion
-
e an MRI scan is essential to inform the clinical management of patients with psychosis and soft neurological abnormalities.
-
MCQ answers
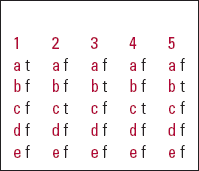
| 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|
| a | t | a | f | a | f | a | f | a | f |
| b | f | b | f | b | t | b | f | b | t |
| c | f | c | t | c | f | c | t | c | f |
| d | f | d | f | d | f | d | f | d | f |
| e | f | e | f | e | f | e | f | e | f |





eLetters
No eLetters have been published for this article.