INTRODUCTION
Yersiniosis is a zoonotic gastrointestinal disease caused by infections with the bacterium Yersinia enterocolitica. Disease is usually characterized by diarrhoea and abdominal pain. Sequelae of infections, such as reactive arthritis or erythema nodosum, occur in some cases. In Germany, yersiniosis caused by Y. enterocolitica is a notifiable disease. In 2010, 3364 cases were reported, corresponding to an incidence of 4/100 000 population [1]. Compared to other countries of the European Union, this incidence is relatively high [2]. Small children, in particular those aged 1 year, are most frequently affected by yersiniosis, with incidence as high as 48/100 000 population in 2010 [1]. Pronounced incidence differences exist between German federal states, which are mainly driven by incidence differences in children aged <5 years [Reference Rosner, Stark and Werber3].
In Germany, infections with Y. enterocolitica typically occur sporadically; disease outbreaks are rare [Reference Rosner, Stark and Werber3]. The main human pathogenic serotype is Y. enterocolitica O:3, but other serotypes such as O:9, O:5,27, and O:8 have also been associated with disease in humans [Reference Bottone4]. The main reservoir of human pathogenic serotypes, in particular serotype O:3, biotype 4, are pigs [Reference Fredriksson-Ahomaa5]. The association of disease with consumption of raw and undercooked pork or pork products is well established [Reference Tauxe6–Reference Huovinen10]. However, other risk factors, for example, consumption of untreated drinking water [Reference Ostroff7], eating in a canteen [Reference Huovinen10], and, for children, use of a pacifier and contact with pet dogs and cats [Reference Boqvist9] have also been described. Studies are scarce in children, the population group most affected in Germany and other European countries [Reference Rosner, Stark and Werber3, 11].
A case-control study was conducted to identify risk factors for sporadic Y. enterocolitica infections in Germany. Additional aims were to find possible explanations for high disease incidence in very young children as well as for marked regional incidence differences.
METHODS
Data collection and storage procedures were approved by the Federal Commissioner for Data Protection and Freedom of Information and by the State Data Protection Commissioners of the federal states participating in the study.
Study design
A population-based case-control study was conducted in five federal states of Germany (Bavaria, Brandenburg, Hesse, Saxony-Anhalt, Thuringia) from 15 April 2009 to 30 June 2010. The mean annual incidence rates (2001–2010) of yersiniosis in the federal states participating in the study ranged from high (Thuringia, 21 notified illnesses/100 000 population; Saxony-Anhalt, 15/100 000) to intermediate (Brandenburg, 9/100 000) and low (Hesse, 5/100 000; Bavaria, 4/100 000).
A case was defined as illness in a person notified to the local health department during the study period with a Y. enterocolitica infection culture-confirmed from stool presenting with at least one of the following symptoms: diarrhoea, abdominal pain, tenesma, vomiting, and fever. Case patients were recruited by local health authorities. Cases were excluded from data analysis if they had travelled abroad in the 7 days prior to onset of symptoms or if disease onset preceded completion of the questionnaire by >60 days.
Controls were frequency-matched to cases by age group and federal state (control:case ratio 3:1). For selection of population-based controls a two-step procedure was applied. First, 10–15 counties within participating federal states were selected randomly with a probability of selection proportional to population size. Second, county authorities provided randomly selected addresses of persons belonging to defined age groups (0–4, 5–14, ⩾15 years) from local population registries. Questionnaires for controls were sent out monthly throughout the study period. Controls were excluded from data analysis if they had travelled abroad in the 7 days before completing the questionnaire.
Data collection
Cases and controls were invited to complete a standard, self-administered questionnaire that had been sent to them by mail. The questionnaire inquired about exposure to potential risk factors such as recent travel abroad, consumption of certain food items, eating habits (including diet, eating out), contact with animals (including pets and farm animals), indicators of person-to-person transmission (e.g. preceding diarrhoeal disease in the same household), use of medication (including gastric acid inhibitors), occupational exposure, as well as basic demographic information (e.g. month and year of birth, postal code, level of professional education, immigration status, number of persons living in the household). Cases were also asked about their illness (e.g. disease onset, duration, symptoms). Certain questions regarding exposure were only posed in the questionnaire for children (e.g. playing in a sandbox, use of a pacifier for children aged <5 years). Questions referred to the 7 days preceding onset of symptoms for cases and to the 7 days preceding completion of the questionnaire for controls. Parents/caregivers were asked to complete the questionnaire for children aged <15 years.
Data analysis
Data was entered into an EpiData database (version 3.1, The EpiData Association, Denmark) and validated by means of double data entry. Where possible, information on serotype of the Y. enterocolitica isolate of cases was obtained from the national surveillance database of notified cases hosted at the Robert Koch Institute. Data was analysed with Stata 11 (Stata Corporation, USA).
Single-risk variable analysis including a total of 78 variables was conducted by computing adjusted odds ratios (aORs) and 95% confidence intervals (CIs) using logistic regression, adjusting for the matching variables age group and federal state. Statistical significance was assessed by Wald tests. Variables with P values of <0·2 in this analysis were considered for multivariable logistic regression modelling, which was employed to investigate the relation of exposure variables with yersiniosis. Pairwise correlation was examined for variables that measured related exposures, for example, consumption of meat products and consumption of pork products. If the correlation coefficient (Cramer's V) was >0·25, only one variable of the pair was selected for model building, based on plausibility. Multivariable analysis commenced by fitting a model with a starting set of variables, selected as described above. Then, terms with the highest P value (Wald test) were eliminated from the model sequentially until P values for the remaining variables were ⩽0·05. Matching variables (age group, federal state) were forced into the model. The variable age was further subgrouped (0–1, 2–4, 5–14, 15–39, 40–59, ⩾60 years) to control any remaining confounding. Finally, the eliminated variables were individually re-introduced into the final model and tested for significance. Statistical interactions between variables in the final model were assessed by likelihood ratio (LR) tests for logistic regression models with and without the multiplicative interaction term.
The proportion of Y. enterocolitica infections attributable to each significant risk factor in the final multivariable model [population attributable fraction (PAF)], assuming causality, was estimated using methods described by Bruzzi et al. [Reference Bruzzi12]. Confidence intervals were calculated in R, version 2.12.0 [13], using the percentile method for samples obtained by an age-group and federal-state stratified bootstrap [Reference Llorca and Delgado-Rodríguez14].
Missing data
Despite detailed instructions on completing the questionnaire, a substantial proportion of study participants answered questions presented as choices from item lists, e.g. on consumption of various meat products, by only marking ‘Yes’ for items they had consumed and leaving the answer options ‘No’ or ‘Don't know’ for other items from the same list blank. For data analysis, missing answers for questions from an item list were converted to ‘No’ if the answer had been left blank and for one or more answers of the same item list only the option ‘Yes’ was chosen. Missing answers were converted to ‘No’ for questions that were skipped because the introductory question had been answered with ‘No’. Missing data regarding exposure variables that did not apply to all age groups (e.g. use of a pacifier in age groups aged ⩾5 years, playing in a sandbox in age group aged ⩾15 years) were converted to the answer ‘No’ for persons in age groups to whom the question was not posed. Variables were not included in multivariable analyses if the proportion of missing values was high (>20%).
RESULTS
Study population
During the study period, 644 questionnaires were returned by case patients, corresponding to about 42% of all cases reported to the Robert Koch Institute by local health authorities participating in the study via state health authorities of the five federal states. Of those, 571 (88·7%) were included in data analysis. Patients were excluded from data analysis because they had travelled abroad in the 7 days prior to disease onset (n=39, 6·1%), were asymptomatic (n=8, 1·2%), or onset of symptoms preceded completion of the questionnaire by >60 days (n=26, 4·0%). The median time interval between disease onset and completing the questionnaire was 19 days (interquartile range 13–26 days). Case patients did not differ substantially from all notified cases in the surveillance database with respect to age, sex, residence in federal state, and serotype distribution. Thirty-six per cent of the questionnaires mailed to controls were returned (n=1892), and 1798 (95·0%) were included in the data analysis. Controls were excluded from data analysis because they had travelled abroad in the 7 days before completing the questionnaire (n=79, 4·2%), or the questionnaire had been answered for more than one person (n=15, 0·8%).
Cases were slightly younger than controls (median age 8 years vs. 9 years, respectively), 38% of cases and 33% of controls were aged <5 years. Fifty-six per cent of cases and 49% of controls were male. Thirty-five per cent of cases resided in the federal state of Bavaria, and 24% in the state of Thuringia (Table 1). The composition of the study population was similar to all cases notified in the five participating states during the study period with respect to age group (all notified cases: 36·5%, age group 0–4 years; 30·4%, 5–14 years; 33·1% ⩾15 years), sex (all notified cases: 56·5% male), and federal state (all notified cases: 33·2% Bavaria, 9·3% Brandenburg, 17·3% Hesse, 13·6% Saxony-Anhalt, 26·6% Thuringia).
Table 1. Descriptive analysis of the study population (N=2389) in the case-control study of risk factors for sporadic yersiniosis conducted in Germany, 2009–2010

Serotypes
Information on the serotype of the Y. enterocolitica isolates was available through the national surveillance database for 563 (98·6%) cases, 514 of which had complete entries specifying the serotype. Of those, 93·6% had been infected with serotype O:3, 5·1% with serotype O:9, 0·4% with serotype O:5,27, and 1·0% with another, unspecified serotype. No case patient included in the data analysis had been infected with serotype O:8. Serotype distribution in the study population was similar to the distribution in all cases notified in the five participating states during the study period: 90·9% serotype O:3, 5·4% serotype O:9, 0·7% serotype O:5,27, 0·7% serotype O:8, 2·3% other, unspecified serotype.
Risk factors associated with Y. enterocolitica infection
In single-risk variable analyses, 22/78 variables were associated with illness (P<0·2). Of those, four variables were excluded because they correlated strongly with another variable (e.g. frequent consumption of pork correlated with recent consumption of heated/cooked pork) or because the proportion of missing values was high (use of medication other than gastric acid inhibitors, 23% missing values), resulting in a starting set of 18 variables for multivariable modelling (Table 2). The proportion of missing values of variables considered for multivariable analysis ranged from 0% to 19% (average 6%) in cases, and from 0% to 9% (average 3%) in controls. The proportion of missing values was higher for variables that described recent consumption of specific food items (e.g. recent consumption of beef) than for variables that described eating habits (e.g. frequency of eating out) or sociodemographic characteristics (e.g. professional education).
Table 2. Single-risk variable analysisFootnote * in the case-control study of risk factors for sporadic yersiniosis conducted in Germany, 2009–2010
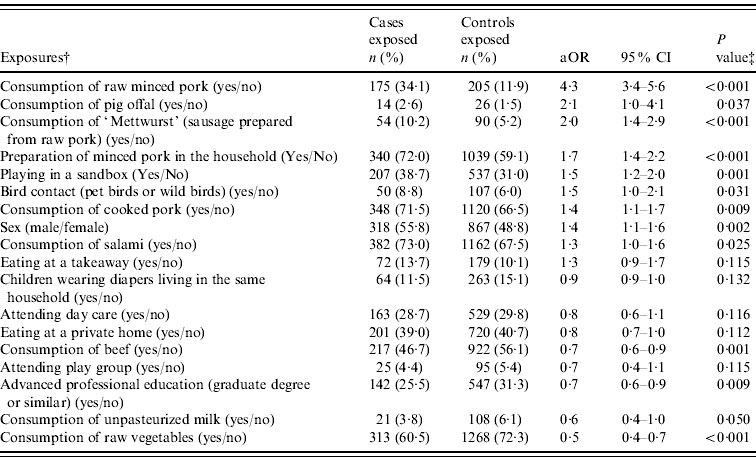
aOR, Adjusted odds ratio; CI, confidence interval.
* Logistic regression, adjusted for age group and federal state.
† Exposure variables listed comprise the starting set of exposure variables used for multivariable logistic regression models.
‡ P value from Wald tests.
The following exposure variables were not significantly associated with illness (P⩾0·2): recent consumption of meat or meat products from sheep, chicken, turkey, wild boar, or deer, sausages containing raw pork (other than those listed in Table 2), heated minced pork, sprouts or lettuce; frequent (once a week or more) consumption of beef or poultry; buying meat and meat products mainly fresh rather than pre-packed or frozen; having recently eaten at a restaurant, fast-food restaurant, or canteen; recent contact with dogs, cats, rodents, reptiles, cattle, pigs, horses, or wild animals; a person with diarrhoea in the same household or in the same environment (e.g. kindergarten, work place); occupational exposure to animals or raw meat; occupational exposure to children aged <6 years; recent visit to private day care (‘day nanny’; question posed in children's questionnaire only); use of a pacifier (question posed only if children were aged <5 years); use of antibiotics or gastric acid inhibitors within the past 4 weeks; chronic medical condition (diabetes, chronic intestinal illness, cancer, or chronic illness that weakens immune system); having an immigration background.
A total of 352/571 cases (62%) and 1495/1798 controls (83%) had complete data on all variables included in the final multivariable logistic regression model. Four exposure variables, referring to the 7 days preceding disease onset, were positively and significantly associated with illness: consumption of raw minced pork, preparation of minced pork in the household, contact with birds, and playing in a sandbox. The association between illness and consumption of raw minced pork was the strongest (aOR 4·7, 95% CI 3·5–6·3). Two exposure variables were negatively associated with illness: consumption of raw vegetables, and consumption of beef (Table 3). The interaction between consumption of raw minced pork and age group was statistically significant, albeit borderline, in the multivariable logistic regression model (LR test, P=0·049). The association between consumption of raw minced pork and illness was strongest for the youngest age group (0–1 years: aOR 17·5, 95% CI 6·0–51·2) and about four times lower for age groups 2–4, 5–14, and 15–39 years. The association was weakest for persons aged ⩾40 years (Table 3). In a separate multivariable model restricted to this age group, only recent eating at a takeaway (aOR 6·6, 95% CI 2·4–18·2) and contact with birds (aOR 4·6, 95% CI 1·7–12·8) were significant risk factors. In a multivariable model restricted to children aged <5 years, consumption of raw pork was the only significant risk factor (aOR 6·3, 95% CI 4·9–10·0) confirming results obtained from the multivariable model for all age groups. The interaction between preparation of minced pork in the household and age group was not statistically significant in the model for all age groups (LR test, P=0·260).
Table 3. Results of a multivariable risk factor analysisFootnote * for sporadic yersiniosis in Germany, 2009–2010

aOR, Adjusted odds ratio (adjusted for age group and federal state of residency); CI, confidence interval; PAF, population attributable fraction.
* Based on 352 cases and 1495 controls.
† P values from Wald tests except P value for interaction term ‘consumption of raw minced pork×age group’ (likelihood ratio test).
The proportion of persons that had consumed raw minced pork was between 28% and 39% in case patients in age groups up to 40 years, and between 4% and 14% in controls in the same age groups. Interestingly, 28% of diseased children aged <2 years and 35% of those aged 2–4 years had consumed raw minced pork in the 7 days preceding onset of illness (Table 4).
Table 4. Consumption of raw minced pork in cases and controls in the 7 days preceding onset of yersiniosis, or completion of the questionnaire, respectively, according to age group and federal state, as determined in a case-control study of risk factors for sporadic yersiniosis, Germany 2009–2010

Population attributable fraction
The PAF, expressed as a percentage, was determined for each risk factor based on the final multivariable model for all age groups without the interaction term. Accordingly, about one third (30%, 95% CI 27–32) of illnesses due to Y. enterocolitica infection could be avoided in the population if consumption of raw minced pork was eliminated. The total attributable fraction for all risk factors (aOR>1) listed in Table 3 was 54% (95% CI 45–62). The PAF was estimated for the main risk factor ‘consumption of raw minced pork’ in each age group and was highest for age groups aged <15 years (Table 3).
DISCUSSION
To our knowledge, this study is the largest case-control study to investigate risk factors for yersiniosis. Consumption of raw pork was the main risk factor for sporadic Y. enterocolitica infections, explaining about 30% of all infections in Germany. Unexpectedly, the association between consumption of raw pork and illness was even more pronounced in young children. This offers one explanation for the more than tenfold higher average annual incidence of yersiniosis in children aged <5 years, particularly 1-year-olds, compared to the German population aged ⩾5 years, and for incidence differences between German federal states, which are primarily attributable to incidence differences in young children [Reference Rosner, Stark and Werber3].
It is biologically plausible that consumption of raw or undercooked pork is the main driver of yersiniosis incidence in Germany. About 90% of notified cases are typically infected with Y. enterocolitica biotype 4, serotype O:3, which is frequently isolated from pigs [2, Reference Fredriksson-Ahomaa5, Reference Fredriksson-Ahomaa15–Reference Fosse, Seegers and Magras17] and pork samples [2, Reference Fredriksson-Ahomaa5], and the disease association has been established in case-control studies conducted in other countries [Reference Tauxe6–Reference Huovinen10]. However, the importance of raw or undercooked pork for yersiniosis in young children in Germany was previously unknown. The association found in the present study varied by age group and was strongest in children aged <2 years. In this youngest age group exposure prevalence in controls was disproportionately lower than in older age groups, whereas exposure prevalence in cases was comparable. Lack of previous exposure and, in consequence, lack of any specific immunity against Y. enterocolitica, in combination with a generally still less mature immune system [Reference Cohen18], may predispose young children to infection and disease. Exposure to raw minced pork generally was unexpectedly frequent, even in young children. About 30% of cases and about 9% of controls aged <5 years had eaten raw minced pork in the 7 days before onset of illness or completion of the questionnaire, respectively. This disturbing finding appears unexceptional for Germany. In a survey of 145 healthy children conducted in Belgium in 1985, 36% of the children had eaten raw pork by the age of 5 years, and the median age of first exposure was 18 months [Reference Tauxe6]. Consumption of raw pork was identified as a risk factor for yersiniosis in Belgium as well [Reference Tauxe6], and, subsequently, measures were taken to prevent contamination of meat during the slaughtering process and to dissuade consumers from eating raw or undercooked pork, which resulted in a significant decrease in Y. enterocolitica infections [Reference Verhaegen19].
Raw minced pork mixed with spices and, optionally, onions (known as ‘Mett’ or ‘Hackepeter’) spread on a bread roll is a commonly consumed dish in Germany, mainly in the northern and eastern regions. This is also reflected in the exposure data presented here. The proportion of cases and controls that reported having eaten raw minced pork was higher in regions where ‘Mett’ or ‘Hackepeter’ are commonly eaten (eastern federal states: Thuringia, Saxony-Anhalt, Brandenburg) than in Bavaria (southern state). Surveillance data showed that incidence of yersiniosis is highest in eastern federal states (Thuringia, Saxony-Anhalt) [Reference Rosner, Stark and Werber3]. Our data indicate that this is due to higher exposure frequency to raw pork in these regions compared to other regions of the country, and only to a lesser extent, if at all, to surveillance artifacts because of regional differences in completeness of reporting, as is sometimes suggested [Reference Held20].
Consumption of raw pork was only weakly associated with yersiniosis in persons aged ⩾40 years. In a multivariable model restricted to this age group, only recent eating at a takeaway and contact with birds were significantly associated with illness. However, the PAFs for these risk factors, based on the final multivariable model, were relatively small (having eaten at a takeaway, 13%; contact with birds, 10%). Further studies, with a larger number of participants, should be conducted to elucidate risk factors of yersiniosis in this age group. It is possible that older persons have been repeatedly exposed to Y. enterocolitica during their life time and developed relative immunity. Thus, clinically symptomatic Y. enterocolitica infections may reflect higher individual susceptibilities or higher infectious doses, rather than differences in exposure frequency compared to controls.
Preparation of minced pork in the household was also a risk factor for sporadic yersiniosis.
Cross-contamination of kitchen utensils or food items may occur during preparation of minced pork if kitchen hygiene is suboptimal, which may explain the association of this variable with illness. We did not find an effect modification of this variable by age group. This indicates that preparation of minced pork in the household is not a risk factor predominantly for adults, who presumably handle and prepare the minced pork, but also for young children in the same household. Transmission to children may occur via cross-contaminated food or through interpersonal spread, suggesting deficiencies in hand hygiene.
Playing in a sandbox has been reported as a risk factor for sporadic infections with Salmonella enterica serotype Typhimurium and Shiga toxin-producing Escherichia coli (STEC) in children [Reference Werber21, Reference Doorduyn22]. Further investigation is needed to discover if sandboxes pose a true risk of yersiniosis, for example, via contamination of the sand with animal faeces, or if a sandbox is a place where the pathogen is transmitted directly or indirectly from person to person [Reference Werber21]. We have no plausible explanation as to why contact with birds would be associated with Y. enterocolitica infections. The proportion of cases attributable to this exposure was small (4%). Our questionnaire did not differentiate between contact with pet birds, e.g. budgerigars or parrots, farm animals, e.g. chickens, or wild-living birds, but direct contact with wild-living birds is likely to be negligible. Yersinia spp. such as Y. pseudotuberculosis or Y. fredericksenii, and, occasionally Y. enterocolitica, have been isolated from a variety of birds; however, human pathogenic Y. enterocolitica serotypes are not typically found in birds [Reference Giles and Carter23–Reference Shayegani28].
There are several possible limitations to our study. Even though our study was relatively large, it still did not have sufficient power to elucidate specific risk factors for Y. enterocolitica serotypes other than O:3 because most (94%) case patients were infected by serotype O:3. Case patients were recruited from notified, laboratory-confirmed cases and may not be representative of all yersiniosis cases in the population. Patients seeking medical care and triggering microbiological examination of stool samples because of a gastrointestinal illness are more likely to be young children or to suffer from a more severe or prolonged course of illness [Reference Scallan29]. Furthermore, detailed clinical information on case patients, for example on complications such as concurrent bacteraemia in young children, is not routinely collected through the notification system [Reference Rosner, Stark and Werber3]. Differential recall between cases and controls is an inherent source of bias in case-control studies. In our study, the recall period for cases was almost 3 weeks further in the past (median of 19 days between disease onset and completion of questionnaire) than for controls (past 7 days). This is reflected by the higher proportion of missing values for questions regarding consumption of various food items in cases compared to controls. Cases may not have remembered consumption of specific food items as well as controls. This may have led to an underestimation of the strength of the association and, in consequence, of the PAF. It is also possible that cases already knew or suspected that raw pork was a risk factor for yersiniosis when completing the questionnaire, which may have resulted in an overestimation of the association between consumption of raw pork and illness. However, yersiniosis and possible risk factors for the disease are not generally known by the public, as opposed to, for example, salmonellosis, and, therefore, it appears unlikely that prior knowledge about this risk factor may have biased our results.
Despite efforts to control foodborne pathogens in animals and food [30], and measures suggested for reducing Y. enterocolitica on pig carcasses in the slaughtering process [Reference Nesbakken31, Reference Nesbakken32], Y. enterocolitica was isolated from about 5% of pork product samples and about 2% of minced pork samples in Germany, according to recent data [2]. Therefore, educating consumers about risks associated with consumption of raw pork products currently appears to be the most effective preventive measure for consumer protection from Y. enterocolitica infections.
CONCLUSIONS
Most yersiniosis cases are foodborne. Consumption of raw minced pork was the main risk factor of sporadic yersiniosis in this study, and is a frequent habit not only in the German adult population, but also in young children. The pork consumption pattern elucidated in this study offers a suitable explanation for the relatively high yersiniosis incidence in young children and the marked regional incidence differences within the country. Prevention efforts should specifically target parents and caregivers of young children and focus on the high infection risk associated with consumption of raw minced pork. This would be likely to reduce incidence in children of other infections caused by pork-associated pathogens such as Salmonella enterica serotype Typhimurium. Furthermore, efforts should be increased to educate the public about proper kitchen hygiene. Finally, pig slaughtering techniques and meat handling processes should be improved to prevent contamination of meat with Y. enterocolitica, particularly meat used for preparation of raw minced pork.
ACKNOWLEDGEMENTS
This study was supported by grant 01KI07127 (Foodborne Zoonotic Infections of Humans; FBI-Zoo) from the German Federal Ministry of Education and Research (BMBF). The authors thank all study participants and the local and state health authorities in Bavaria, Brandenburg, Hesse, Saxony-Anhalt, and Thuringia for their support. We thank Nina Bauer, Diana Buhe, and Kati Steinert for their help with mailing of questionnaires and data entry throughout the study. The authors are grateful to Susanne Behnke and Mario Schummert for assistance with data entry.
DECLARATION OF INTEREST
None.






