Introduction
The Great Basin of western United States contains the most complete and well-exposed sections covering the Cambrian Series 2–Series 3 interval (Montezuman–Marjuman stages of the Laurentian nomenclature; Palmer, Reference Palmer1998). These highly fossiliferous, mixed carbonate-siliciclastic successions have been extensively investigated for their trilobite and brachiopod faunas (e.g., Rowell Reference Rowell1966, Reference Rowell1977, Reference Rowell1980; Rowell and Henderson, Reference Rowell and Henderson1978; Palmer and Halley, Reference Palmer and Halley1979; Sundberg and McCollum, Reference Sundberg and McCollum1997, Reference Sundberg and McCollum2000, Reference Sundberg and McCollum2003a, Reference Sundberg and McCollumb; Hollingsworth Reference Hollingsworth2005, Reference Hollingsworth2011a, Reference Hollingsworthb; Streng and Holmer, Reference Streng and Holmer2006; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a). However, other faunal elements, especially small shelly fossils (SSFs), are generally unconsidered. These SSFs provide important information for biostratigraphic, depositional environment, and paleoecologic reconstructions (e.g., Geyer, Reference Geyer1986; Elicki, Reference Elicki1994, Reference Elicki2005, Reference Elicki2006; Geyer and Shergold, Reference Geyer and Shergold2000; Gubanov, Reference Gubanov2002; Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtmann2007).
Few, non-brachiopod, SSFs have been reported in detail from the Great Basin. Tubes of uncertain affinity and the hyolith Salanytheca sp. occur in the pre-trilobitic Cambrian Deep Spring Formation of western Nevada and eastern California (Signor et al., Reference Signor, Mount and Onken1987). The agglutinated protist Platysolenites antiquissimus Eichwald, Reference Eichwald1860, chancelloriid sclerites, helicoplacoid ossicles, and hyoliths occur in the Montezuman Stage of Indian Springs Canyon (Fig. 1; Streng et al., Reference Streng, Babcock and Hollingsworth2005; English and Babcock, Reference English and Babcock2010). Hyolithellus insolitus Grigorieva in Voronin et al., Reference Voronin, Voronova, Grigor’eva, Drozdova, Zhegaloo, Zhuravlev, Ragozina, Rozanov, Sayutina, Sysoev and Fonin1982, Sphenotallus sp., echinoderm ossicles, and sponge spicules were described from the lower Dyeran Harkless Formation of Gold Point (Fig. 1; Skovsted and Holmer, Reference Skovsted and Holmer2006). Furthermore, the helcionelloid molluscs Anabarella chelata Skovsted, Reference Skovsted2006a and Costipelagiella nevadense Skovsted, Reference Skovsted2006a, the hyolith Parkula esmeraldina Skovsted, Reference Skovsted2006a, and remains of echinoderms, chancelloriids, and sponges occur in the uppermost Dyeran Stage from the basal Emigrant Formation of Split Mountain (Fig. 1; Skovsted, Reference Skovsted2006a). The lower Cambrian hyolith fauna originally described by Walcott (Reference Walcott1886) and Resser (Reference Resser1938) from Nevada were reinvestigated by Malinky (Reference Malinky1988).
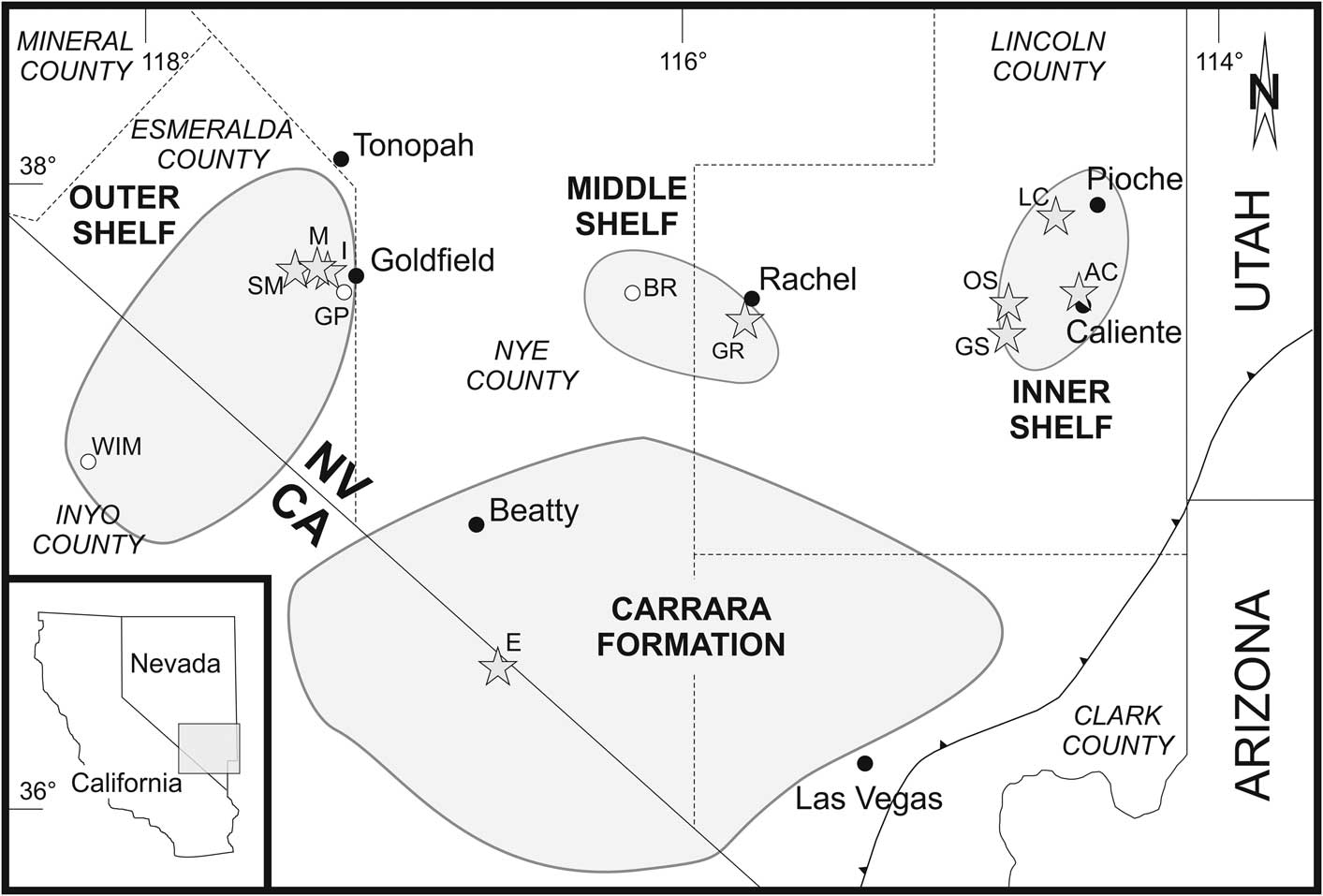
Figure 1 Map of the southern Great Basin, showing the facies realms of the inner, middle, and outer shelf (modified from Palmer and Halley, Reference Palmer and Halley1979; Sundberg and McCollum, Reference Sundberg and McCollum2000, Reference Sundberg and McCollum2003a; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a). Shaded stars mark the sections investigated: AC, Antelope Canyon; E, Echo Canyon; GR, Groom Range; GS, Grassy Spring; I, Indian Springs Canyon; LC, Log Cabin Mine; M, Montezuma Range; OS, Oak Spring Summit; SM, Split Mountain. White circles represent localities mentioned in the text: BR, Belted Range; GP, Gold Point; WIM, White-Inyo Mountains.
Most recent report of SSFs from the Great Basin only mentioned their presence without any illustration, systematic documentation, and/or detailed stratigraphic distribution. Hollingsworth (Reference Hollingsworth2011b) and Hollingsworth and Babcock (Reference Hollingsworth and Babcock2011) reported the hyolith “Ladatheca” cylindrica Grabau, Reference Grabau1900, orthothecid hyoliths, and the bradoriid Dielymella? Ulrich and Bassler, Reference Ulrich and Bassler1931 from the Montezuman Stage and the lower unnamed stage of the Indian Springs Canyon and Montezuma Range sections (Fig. 1). Webster (Reference Webster2011c) mentioned pelagiellids, hyoliths, and chancelloriids from the upper Dyeran from a variety of sections in Nevada. Sundberg and McCollum (Reference Sundberg and McCollum1997, Reference Sundberg and McCollum2003a) and McCollum et al. (Reference McCollum, McCollum and Sundberg2011) mentioned Stenothecoides elongata Walcott (Reference Walcott1886) and Latouchella arguta Resser (Reference Resser1939) and hyoliths, echinoderms, and chancelloriids from the lower Delamaran Stage of Nevada.
The purpose of this report is to document a new small shelly assemblage from a variety of Montezuman–Delamaran mixed carbonate-siliciclastic successions of eastern California and southern Nevada in order to increase the knowledge of the paleogeographic and biostratigraphic potential of these faunal elements.
General geology and stratigraphy
The Montezuman–Delamaran succession of the Great Basin reflects the overall flooding of the western margin of the Laurentian craton (Webster, Reference Webster2011a). During this time the shelf was spatially and temporally heterogeneous, as documented by the multitude of regional lithostratigraphic units (e.g., Palmer and Halley, Reference Palmer and Halley1979; Sundberg and McCollum, Reference Sundberg and McCollum2003b; Webster, Reference Webster2011a, Reference Websterb; Figs. 1, 2). Based on lithofacies and trilobite distribution patterns, the depositional environment is separated into inner, middle, and outer shelf facies realms (e.g., Stewart, Reference Stewart1970; Palmer and Halley, Reference Palmer and Halley1979; Sundberg and McCollum, Reference Sundberg and McCollum2003a; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a; Figs. 1, 2).
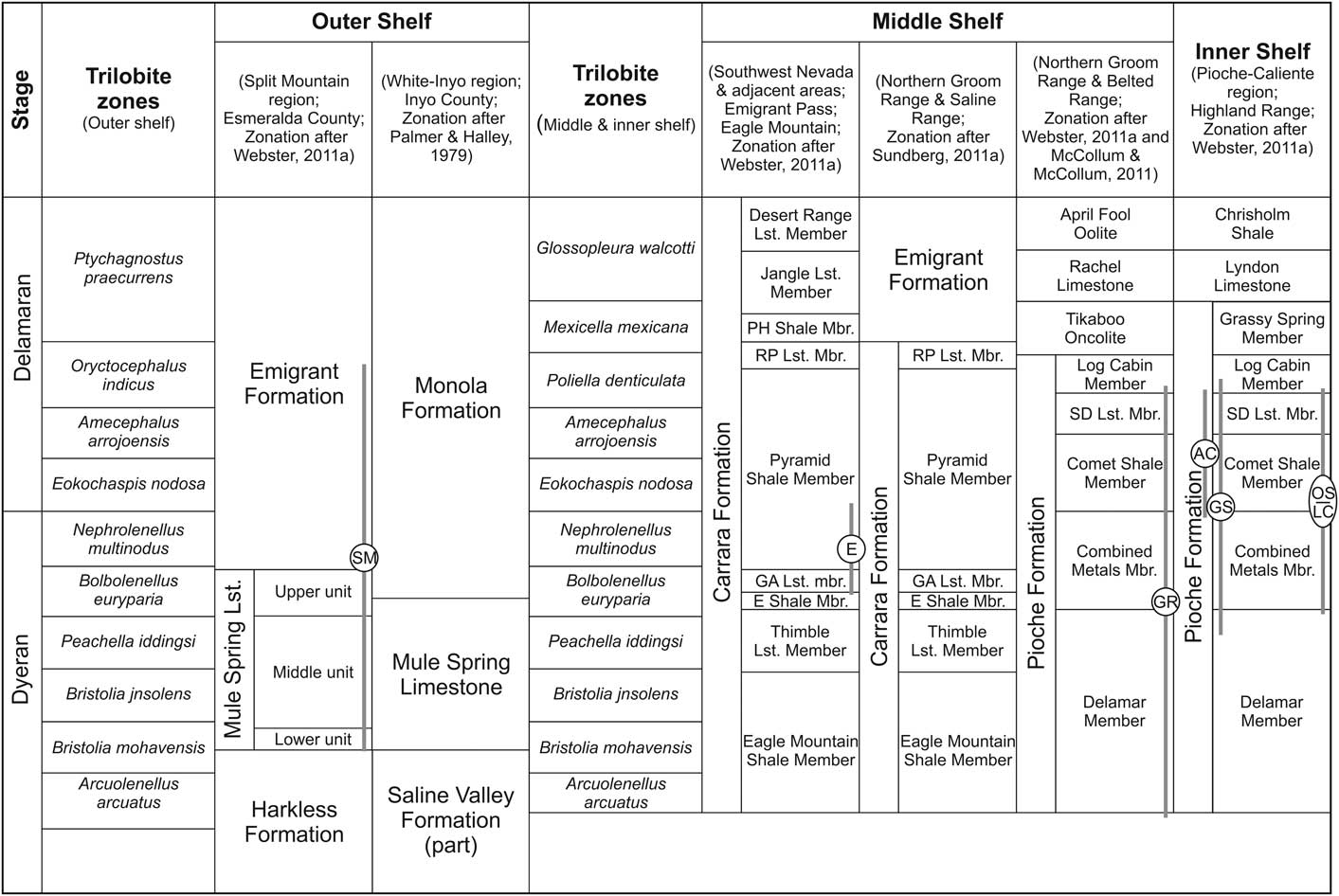
Figure 2 Biostratigraphic zonation of the upper Dyeran–Delamaran interval, and associated lithostratigraphy on the outer, middle, and open shelf of Nevada and SE-California, western Laurentia (modified from Palmer and Halley, Reference Palmer and Halley1979; Sundberg and McCollum, Reference Sundberg and McCollum2000, Reference Sundberg and McCollum2003b; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a). Stratigraphic positions of the analyzed Dyeran–Delamaran sections are marked. Abbreviations: E Shale Mbr., Echo Shale Member; GA Lst. Mbr., Gold Ace Limestone Member; PH Shale Mbr., Pahrump Hills Shale Member; RP Lst. Mbr., Red Pass Limestone Member; SD Lst. Mbr., Susan Duster Limestone Member; Lst., limestone; Mbr., member. See Figure 1 for section abbreviations.
Inner shelf facies
Sections of the inner shelf facies are primarily exposed in the Pioche-Caliente area of eastern Nevada (Fig. 1). The Dyeran-Delamaran boundary interval is represented by the Pioche Formation (Arcuolenellus arcuatus-Mexicella mexicana zones; Sundberg and McCollum, Reference Sundberg and McCollum2000; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a, Reference Websterc; Fig. 2). Its lower part, the Delamar Member, consists of a succession of bioturbated claystone and siltstone interbedded with sandstone and conglomerate layers, with carbonate intercalations at the top (Webster, Reference Webster2011c). The lower cliff-forming portion of the succeeding Combined Metals Member consists of bioclastic oncolitic limestone, nodular limestone, and thin limestone beds. The upper portion of the Combined Metals Member shows a higher siliciclastic content, represented by ribbon limestone, nodular limestone, siltstone, and sandstone intercalations (Webster, Reference Webster2011c). The base of the Delamaran starts with the Comet Shale Member (Eokochaspis nodosa–Amecephalus arrojoensis zones; Fig. 2), predominated by claystone and siltstone with a few thin limestone beds (Sundberg and McCollum, Reference Sundberg and McCollum2000; McCollum and McCollum, Reference McCollum and McCollum2011). It is disconformably overlain by the Susan Duster Limestone Member (Amecephalus arrojoensis–Poliella denticulata zones), which consists of a basal bioclastic limestone, an interval of claystone and nodular limestone, and an upper part of nodular-bedded limestone (Sundberg and McCollum, Reference Sundberg and McCollum2003b; Sundberg, Reference Sundberg2011). The overlying Log Cabin Member (Poliella denticulata Zone) consists of claystone and siltstone with intercalations of sandstone and bioclastic limestone (Sundberg and McCollum, Reference Sundberg and McCollum2003b; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011). The uppermost part of the Pioche Formation is represented by the Grassy Spring Member (Mexicella mexicana Zone) consisting of claystone, siltstone, and sandstone (Eddy and McCollum, Reference Eddy and McCollum1998; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011).
Middle shelf facies
The Dyeran–Delamaran of the middle shelf facies is represented by the Carrara Formation in southern Nevada and southeastern California (Fig. 1). The Carrara Formation is separated into nine siliciclastic and carbonate intervals, ranging from the Arcuolenellus arcuatus Zone to the Glossopleura walcotti Zone (Palmer and Halley, Reference Palmer and Halley1979; Webster, Reference Webster2011a; Fig. 2). The lowermost and uppermost carbonate members (Thimble Limestone and Desert Range Limestone members, respectively) are characterized by thin-bedded argillaceous (dolomitic) limestone (Palmer and Halley, Reference Palmer and Halley1979). In contrast, the other limestone portions are cliff-forming units, composed of oncolitic, oolitic, laminated, and fenestral limestone (Palmer and Halley, Reference Palmer and Halley1979). The lithostratigraphic nomenclature applied by Palmer and Halley (Reference Palmer and Halley1979) does not fit with the sedimentary succession observed in the northern Groom Range and Belted Range (GR and BR in Fig. 1) of central Nevada, which resulted in several synonymous nomenclatures for the region (Fig. 2; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011; Webster, Reference Webster2011a; Webster et al., Reference Webster, McCollum and Sundberg2011).
Outer shelf facies
Sections of the outer shelf facies crop out in western Nevada and eastern California (Fig. 1). The Dyeran Mule Spring Limestone is represented by predominately shallow subtidal–intertidal carbonates and is subdivided into: (1) a lower unit, composed of cliff-forming bioturbated limestone; (2) a middle unit, composed of bioclastic, oncolitic, oolitic, and peloidal limestones with numerous claystone intercalations; and (3) an upper cliff-forming unit composed of oncolitic and fenestral limestones with intercalated intraformational conglomerates (Fig. 2; Nelson, Reference Nelson1962; Stewart, Reference Stewart1970; Albers and Stewart, Reference Albers and Stewart1972; Webster, Reference Webster2011a, Reference Websterb). The Mule Spring Limestone is overlain by the siliciclastic and carbonate, partly highly condensed Emigrant Formation (uppermost Dyeran–Sunwaptan Stage) in western Nevada and by the Monola Formation in Death Valley National Park of eastern California (e.g., Palmer, Reference Palmer1971; Palmer and Halley, Reference Palmer and Halley1979; McCollum and McCollum, Reference McCollum and McCollum2011; Sundberg, Reference Sundberg2011; Sundberg et al., Reference Sundberg, McCollum and McCollum2011). The Monola Formation is subdivided into a lower claystone portion with intercalated limestone and an upper limestone with minor siltstone intercalations (Sundberg and McCollum, Reference Sundberg and McCollum1997). McCollum and McCollum (Reference McCollum and McCollum2011) identified the depositional environment of the Monola Formation as located between the outer shelf position of the Emigrant Formation and the medial to inner shelf positions of the Carrara and Pioche formations.
Materials and methods
The material described in this report derives from nine sections covering the Montezuman–Delamaran interval (Terreneuvian/Cambrian Stage 2–Cambrian Series 3/Cambrian Stage 5) of the different shelf facies realms (Figs. 1–4). All carbonate samples are characterized by a high fossil content observable in thin sections or even macroscopically. However, the major part of small shelly fossils is preserved as carbonate, which hampers extraction from the limestone. Several preparation methods were tested using 95% to pure acetic acid partly in combination with copper(II) sulfate and chloroform (see Nötzold, Reference Nötzold1965; Knitter, Reference Knitter1979; Tarsilli and Warne, Reference Tarsilli and Warne1997). All these methods require a distinct porosity of the limestone that enables the intrusion of chemicals and thus the expansion of the rock due to gassing or crystallization. But, the Laurentian samples are strongly lithified without any porosity, which inhibited extraction of microfossils using these procedures. The best results were realized by dissolving the carbonate samples in buffered 7% acetic acid. The extracted microfossils are often corroded during the chemical preparation, but it seems to be the only way for releasing a significant number of small shelly fossils from the Laurentian samples. However, due to dissolution of a majority of the carbonate fossils, this procedure delivered only few phosphatic internal molds and silicified specimens out of the total fossil content. Acetic residues were sieved, dried, and the faunal elements were hand-picked from the residue under a binocular microscope. Subsequently, they were mounted, sputter-coated with gold, and photographed under a CamScan 44 scanning electron microscope at the Department of Geology of the University of Cologne.

Figure 3 Stratigraphic columns of the studied outer shelf sections with positions of samples investigated. See Figure 4 for legend of lithology. The Fallotaspis Zone corresponds to the illustrated part of the Montezuman Stage.

Figure 4 Stratigraphic columns of the studied middle and inner shelf sections with positions of samples investigated. For abbreviations see Figure 3.
Repositories and institutional abbreviations
The material described and figured is housed in the collection of the Geological Institute of the TU Bergakademie Freiberg under the prefix FG 544/GB/locality/sample/SEM-stub number. For brevity herein, localities and specimens are cited without the prefix FG 544/GB. Localities are listed as AC (Antelope Canyon), E (Echo Canyon), GR (Groom Range), GS (Grassy Spring), I (Indian Springs Canyon), LC (Log Cabin Mine), M (Montezuma Range), OS (Oak Spring Summit), and SM (Split Mountain) (Figs. 1–4). Individual collections are denoted by locality abbreviation and sample number (e.g., SM 14).
Systematic paleontology
The helcionelloid molluscs, Anabarella chelata and Costipelagiella nevadense, were verified from the lowermost Emigrant Formation of the Split Mountain section (SM 14, SM 15; Fig. 3). Stratigraphic position and locality are identical with those published by Skovsted (Reference Skovsted2006a). Thus, these species are figured (Fig. 5.1–5.16), but not discussed herein.

Figure 5 Molluscs from the Delamar and Combined Metals members of Grassy Spring and Log Cabin Mine sections and from the Emigrant Formation of Split Mountain; Dyeran Stage. (1–13) Anabarella chelata Skovsted, Reference Skovsted2006a; scale bar=200 μm; lateral views: (1) FG 544/GB/SM/14/A1-17; (2) FG 544/GB/SM/14/A1-21; (3) FG 544/GB/SM/14/A1-7; (4) FG 544/GB/SM/14/A1-1; (5) FG 544/GB/SM/14/A1-8; (6) FG 544/GB/SM/14/A1-16; (7) FG 544/GB/SM/14/A1-3; (8) FG 544/GB/SM/15/A2-4; (9) FG 544/GB/SM/14/A1-12; (10) FG 544/GB/SM/14/A1-25; (11) FG 544/GB/SM/15/A2-2; (12) FG 544/GB/SM/14/A1-22; (13) FG 544/GB/SM/15/B8-1. (14–16) Costipelagiella nevadense Skovsted, Reference Skovsted2006a; scale bar=200 μm; lateral views: (14) FG 544/GB/SM/15/B8-7; (15) FG 544/GB/SM/15/B8-10; (16) FG 544/GB/SM/14/A1-27. (17–22) Pelagiella aff. P. subangulata (Tate, Reference Tate1892); scale bar=200 μm: (17–19) FG 544/GB/LC/6/A9-11, (17, 18) lateral view, (19) oblique apertural view; (20–22) FG 544/GB/LC/6/A8-26, (20, 21) lateral view, (22) oblique apertural view. (23–32) Microcornus sp.; scale bar=500 μm: (23, 24) FG 544/GB/GS/17/A4-4, (23) dorsal view, (24) oblique view from the aperture; (25, 26) FG 544/GB/GS/17/A4-12, (25) dorsal view, (26) oblique view from the aperture; (27, 28) FG 544/GB/GS/17/A4-10, (27) dorsal view, (28) oblique view from the aperture; (29, 30) FG 544/GB/GS/17/A4-6, (29) dorsal view, (30) oblique view from the aperture; (31, 32) FG 544/GB/GS/17/A5-16, (31) dorsal view, (32) oblique view from the aperture.
Phylum Mollusca Cuvier, Reference Cuvier1797
Class Helcionelloida Peel, Reference Peel1991
Order Helcionellida Geyer, Reference Geyer1994
Family Helcionellidae Wenz, Reference Wenz1938
Genus Pelagiella Matthew, Reference Matthew1895
Type species
Cyrtolithes atlantoides Matthew, Reference Matthew1894; lower Cambrian of southeast New Brunswick, Canada.
Pelagiella aff. P. subangulata (Tate, Reference Tate1892)
Figures 5.17–5.22, 6.1–6.23, 7.1–7.6, 8.35–8.37

Figure 6 Small shelly fossils from the Campito, Delamar, Echo Shale, Combined Metals members of Montezuma Range, Echo Canyon, Grassy Spring, Oak Spring Summit, Log Cabin Mine sections as well as from the basal Emigrant Formation of Split Mountain; Montezuman–Dyeran stages; all scale bars 200 μm: (1–23) Pelagiella aff. P. subangulata (Tate, Reference Tate1892): (1–3) FG 544/GB/LC/6/A9-12, (1, 2) lateral view, (3) oblique apertural view; (4–6) FG 544/GB/LC/6/A9-19, (4, 5) lateral view, (6) oblique apertural view; (7–9) FG 544/GB/LC/6/A9-33, (7, 8) lateral view, (9) oblique apertural view; (10–12) FG 544/GB/SM/14/A1-13, (10, 11) lateral view, colonization of endolithic cyanobacteria on the internal mold, (12) oblique apertural view; (13, 14) FG 544/GB/OS/5/A12-13, lateral view; (15) FG 544/GB/GR/11/A10-11, lateral view; (16) FG 544/GB/GR/11/A10-22, lateral view; (17–19) FG 544/GB/GR/11/A10-6, (17, 18) lateral view, (19) oblique apertural view; (20–22) FG 544/GB/GR/11/A10-31, (20, 21) lateral view, (22) oblique apertural view; (23) FG 544/GB/GS/13/A6-14, oblique lateral view. (24–28) Hyolithellus? sp.: (24) FG 544/GB/M/5/C8-2; (25) FG 544/GB/LC/6/A8-1; (26) FG 544/GB/M/5/C8-1; (27) FG 544/GB/M/5/C8-3; (28) FG 544/GB/GS/17/A5-11.

Figure 7 Small shelly fossils from the Delamar, Echo Shale, and Combined Metals members of Echo Canyon, Grassy Spring, Groom Range, and Oak Spring Summit sections; Dyeran Stage. (1–6) Pelagiella aff. P. subangulata (Tate, Reference Tate1892); scale bar=200 μm: (1–3) FG 544/GB/GS/13/A6-8, (1, 2) lateral view, (3) oblique apertural view; (4–6) FG 544/GB/GR/11/A10-14, (4, 5) lateral view, (6) oblique apertural view. (7–12) Helcionellid gen. and sp. indet. 1; note the fine radial lirae in the apical region: (7, 8) FG 544/GB/OS/3/B2-3; scale bar=500 μm; (7) lateral view, (8) apical view; (9, 10) FG 544/GB/OS/3/B2-5; scale bar=200 μm; (9) lateral view, (10) apical view; (11, 12) FG 544/GB/OS/3/B2-4; scale bar=500 μm; (11) lateral view, (12) apical view. (13–16) Helcionellid gen. and sp. indet. 2; scale bar=500 μm; note the well-developed concentric rugae in the apical region: (13, 14) FG 544/GB/OS/3/B2-8, (13) lateral view, (14) apical view; (15, 16) FG 544/GB/OS/3/B2-9, (15) lateral view, (16) apical view. (17–32) Parkula sp.; scale bars 400 μm except for (25, 26, 31, 32) (200 μm): (17, 18) FG 544/GB/GS/13/A6-2, (17) dorsal view, (18) oblique view from the aperture; (19, 20) FG 544/GB/E/12/B6-17, (19) dorsal view, (20) oblique view from the aperture; (21, 22) FG 544/GB/GS/17/A4-14, (21) dorsal view, (22) oblique view from the aperture; (23, 24) FG 544/GB/GS/17/A5-15, (23) dorsal view, (24) oblique view from the aperture; (25, 26) FG 544/GB/E/12/B6-7, (25) dorsal view, (26) oblique view from the aperture; (27, 28) FG 544/GB/GS/17/A5-16, (27) dorsal view, (28) oblique view from the aperture; (29, 30) FG 544/GB/E/10/A3-5, (29) dorsal view, (30) oblique view from the aperture; (31, 32) FG 544/GB/E/10/A3-2, (31) dorsal view, (32) oblique view from the aperture.

Figure 8 Small shelly fossils from the Echo Shale, Pyramid Shale, Combined Metals, and Comet Shale members of the Montezuma Range, Oak Spring Summit, and Log Cabin Mine sections, as well as from the basal Emigrant Formation of Split Mountain; Montezuman–Delamaran stages. (1–8) indeterminate echinoderm ossicles; scale bar=400 μm: (1) FG 544/GB/E/12/B1-2; (2) FG 544/GB/E/15/B4-9; (3) FG 544/GB/E/15/B4-5; (4) FG 544/GB/E/12/B1-1; (5) FG 544/GB/E/15/B4-10; (6) FG 544/GB/E/16/B4-2; (7) FG 544/GB/SM/15/B8-5; (8) FG 544/GB/E/12/B1-6. (9–11) Allonnia sp.; scale bar=400 μm: (9) FG 544/GB/OS/7/C11-13; (10) FG 544/GB/OS/7/C11-22; (11) FG 544/GB/OS/4/C2-13. (12–15) Chancelloria sp. 1; scale bar=400 μm except (14) (700 μm): (12) FG 544/GB/LC/6/A8-3; (13) FG 544/GB/LC/6/A8-4; (14) FG 544/GB/OS/4/C2-12; (15) FG 544/GB/LC/6/A8-5. (16–22) Chancelloria sp. 2; scale bar=300 μm: (16) FG 544/GB/OS/7/C11-11; (17) FG 544/GB/OS/7/C11-12; (18) FG 544/GB/OS/7/C11-10; (19) FG 544/GB/OS/7/C11-21; (20) FG 544/GB/OS/7/C11-18; (21) FG 544/GB/OS/7/C11-15; (22) FG 544/GB/OS/7/C11-4. (23) Archiasterella cf. A. hirundo Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990; FG 544/GB/LC/1/B3-7; scale bar 300 μm. (24, 25) Holotype of Microdictyon montezumaensis n. sp.; FG 544/GB/M/5/C8-8; scale bar=100 μm: (24) ventral view, (25) lateral view of (24). (26, 27) Holotype of Microdictyon cuneum n. sp.; FG 544/GB/M/5/C8-17: (26) ventral view; scale bar 100 μm, (27) detail of (26); scale bar 50 μm. (28, 29) Microdictyon rhomboidale Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986; scale bar=100 μm: (28) FG 544/GB/M/5/C8-9, (29) oblique lateral view of (28). (30–32) Microdictyon sp.; scale bar=100 μm: (30) ventral view; FG 544/GB/M/5/C6-8; (31) ventral view; FG 544/GB/M/6/B10-3; (32) ventral view; FG 544/GB/M/6/B10-2. (33, 34) Archaeooides cf. A. granulatus Qian, Reference Qian1977; scale bar=100 μm: (33) FG 544/GB/SM/15/B8-20; (34) FG 544/GB/SM/14/A1-5. (35–37) Pelagiella aff. P. subangulata (Tate, Reference Tate1892); scale bar=200 μm: (35) FG 544/GB/M/5/C8-13, lateral view; (36) FG 544/GB/M/5/C8-12, lateral view; (37) FG 544/GB/M/5/C8-11, lateral view.
1892 Ophileta subangulata Reference TateTate, p. 184, pl. 2, fig. 8a–b.
1984 Pelagiella emeishanensis He in Reference Xing, Ding, Luo, He and WangXing et al., p. 167, pl. 13, figs. 1–5.
1986 Pelagiella sp.; Reference LaurieLaurie, p. 447, fig. 10D–E.
1990 Pelagiella subangulata; Runnegar in Reference Bengtson, Conway Morris, Cooper, Jell and RunnegarBengtson et al., p. 254, figs. 167, 168A–D, 169A–F, H–L.
1994 Pelagiella emeishanensis; Reference ElickiElicki, p. 71, fig. 4.8.
1994 Pelagiella lorenzi Kobayashi, Reference Kobayashi1939; Reference ElickiElicki, p. 71, fig. 4.6, 4.7.
1996 Pelagiella emeishanensis; Reference ElickiElicki, p. 155, pl. 7, figs. 6, 7.
1996 Pelagiella lorenzi; Reference ElickiElicki, p. 154, pl. 7, figs. 1–5.
1996 ?Pelagiella aff. adunca He and Pei in He, Pei, and Fu; Reference ElickiElicki, p. 155, pl. 8, figs. 1–4.
1996 ?Pelagiella sp.; Reference ElickiElicki, p. 156, pl. 8, figs. 5–8.
2001 Pelagiella subangulata; Parkhaev in Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and ZhuravlevGravestock et al., p. 193, pl. 44, figs. 1–14, pl. 45, figs. 1–10.
2002 Pelagiella subangulata; Reference ElickiElicki, p. 23, pl. 1, figs. 1–18.
2003 Pelagiella subangulata; Reference ElickiElicki, p. 57, pl. 2, fig. 1.
2003 Pelagiella subangulata; Reference Elicki, Hamann and MünzbergerElicki, Hamann, and Münzberger, p. 33, pl. 5, figs. 3, 4.
2004 Pelagiella subangulata; Reference SkovstedSkovsted, p. 30, pl. 8, figs. a, b.
2006 Pelagiella subangulata; Reference WotteWotte, p. 151, fig. 5.n–5.p.
2007 Pelagiella subangulata; Reference Steiner, Li, Qian, Zhu and ErdtmannSteiner et al., p. 83, fig. 7I, 7J.
2014 Pelagiella subangulata; Reference ParkhaevParkhaev, p. 374, pl. 3, figs. 5, 6.
2016 Pelagiella subangulata; Reference Betts, Paterson, Jago, Jacquet, Skovsted, Topper and BrockBetts et al., p. 183, fig. 18A–18H.
Holotype
Ophileta subangulata Tate, Reference Tate1892 (p. 184, pl. 2, fig. 8a, 8b); “Cambrian limestone at Parara, near Ardrossan,” South Australia.
Occurrence
About one hundred internal molds from the Grassy Spring (GS 13), Groom Range (GR 11), Log Cabin Mine (LC 6), Oak Spring Summit (OS 5), and Split Mountain (SM 14) sections; Dyeran Stage. Three internal molds from the Montezuma Range section (M 5); basal Montezuman Stage.
Description
Small univalve internal molds up to 1 mm long and 0.4 mm high. Turbospiral and dextrally coiled with 1–1.5 rapidly expanding whorls. Last whorl wide; cross section irregular oval/trapezoidal to sub-triangular. Aperture often broken. Near convex right side of the apertural margin the residual of a projecting ear. Spire slightly submerged culminates in a plane or slightly concave left flank. Protoconch often hook-shaped. Surfaces of the molds without ornamentation.
Remarks
Due to strong corrosion and lack of the aperture in any of the specimens, an affiliation to a definite species of Pelagiella Matthew, Reference Matthew1895 or Costipelagiella Horný, Reference Horný1964 is difficult. Pelagiella is characterized by a high morphological variation, resulting in a multitude of nominated species, often with unclear differences. Even within a species, variation in morphology and ornamentation is large, as it is for P. subangulata (Tate, Reference Tate1892) (Parkhaev in Gravestock et al., Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001; Skovsted, Reference Skovsted2004). According to Parkhaev in Gravestock et al. (Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001), it is most probable that P. subangulata and P. medianensis (Zhou and Xiao, Reference Zhou and Xiao1984) represent morphologic variations of the same species. However, differences between both species are often only observable from adult forms with well-preserved shell material. According to Parkhaev in Gravestock et al. (Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001), P. medianensis is regarded as the junior synonym of P. adunca (He and Pei in He et al., Reference He, Pei and Fu1984), which thus replaces the former species name. On the other hand, it seems that P. subangulata continuously shifts morphologically into P. adunca, thus suggesting both species represent a morphological continuum within a species. Therefore, P. medianensis and P. adunca have to be revised, critically. Shell ornamentation of P. subangulata and P. primaeva (Billings, Reference Billings1872 [1871]) shows comparable V-shaped ridges on the shell periphery (Runnegar in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990; Landing and Bartowski, Reference Landing and Bartowski1996; Landing et al., Reference Landing, Geyer and Bartowski2002). Thus, P. primaeva needs a careful revision as well (Skovsted, Reference Skovsted2004).
Differences between Pelagiella and Costipelagiella are subtle and only visible on shell morphology and ornamentation. Costipelagiella nevadense Skovsted, Reference Skovsted2006a originally derives from the basal Emigrant Formation of the Split Mountain section (Skovsted, Reference Skovsted2006a), and occurs in our samples SM 14 and SM 15 as well (Fig. 5.14–5.16). Without preserved shell material, an affiliation of our internal molds to C. nevadense could not be excluded with certainty.
Considering the poor preservation of our material and the taxonomic discrepancies mentioned above, we interpret our specimens as having an affinity to P. subangulata, characterized by a wide range of variability. However, it should be kept in mind that species identification is questionable when exclusively based on internal molds (Skovsted, Reference Skovsted2004; Topper et al., Reference Topper, Brock, Skovsted and Paterson2009).
Pelagiella subangulata is known from lower and middle Cambrian strata worldwide. Its first occurrence was recently discussed as a potential marker for defining the base of the Cambrian Series2/Stage 3 (e.g., Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtmann2007).
Helcionellid gen. and sp. indet. 1
Occurrence
Three specimens from the middle part of the Combined Metals Member (Dyeran Stage) of Oak Spring Summit section (OS 3).
Description
Large, weakly cyrtoconic, cap-shaped shells. Apex blunt and rounded, probably located in a central position; exact position uncertain due to the complete disappearance of the aperture. Ventral cross-section of incomplete specimen OS 3/B2-3 (Fig. 7.7, 7.8) sub-circular to elliptical. Width and length ~1.3 mm and ~2.6 mm, respectively. More-complete specimens OS 3/B2-4 (Fig. 7.11, 7.12) and OS 3/B2-5 (Fig. 7.9, 7.10) with a long, slightly concave posterior field. Approximate length of these specimens is 2.2 mm and 1.2 mm, respectively. Maximum height ~1.3 mm. Anterior field gently convex. Surface with distinct radial lirae (Fig. 7.7–7.12).
Remarks
Specimens show similarities to several helcionelloid molluscs, such as Trenella Parkhaev, Reference Parkhaev2001, Mellopegma Runnegar and Jell, Reference Runnegar and Jell1976, Stenotheca Salter in Hicks, Reference Hicks1872, and Helcionella Grabau and Shimer, Reference Grabau and Shimer1909. The long posterior field of our specimens is similar to Trenella or Mellopegma. According to Parkhaev (Reference Parkhaev2001) the posterior field of T. bifrons Parkhaev, Reference Parkhaev2001 is rather short and continues into a well-developed parietal train. The posterior field of the figured holotype (Parkhaev, Reference Parkhaev2001, pl. 3, fig. 1a–1c) seems to be more concave than that of our specimens. In addition, the shell of Trenella shows a significant lateral compression without radial lirae and the apex is more spoon-shaped. Species of Mellopegma are characterized by a long, slightly concave posterior field without parietal train. The elongated shell has faint comarginal rugae. The apex is blunt to slightly hooked (see Vendrasco et al., Reference Vendrasco, Kouchinsky, Porter and Fernandez2011). However, in comparison to our specimens, Mellopegma shows a significant lateral compression. There are also similarities of our material to Stenotheca pojetai Runnegar and Jell, Reference Runnegar and Jell1976, showing a blunt apex and fine radial lirae. The convexity of the anterior side is similar to that of our material, but the posterior side of S. pojetai is more steep and short. Species of Stenotheca are also characterized by a strong lateral compression. A morphological similarity to Helcionella is given by the radial lirae and the oval cross section of the apertural region. Even if species of Helcionella show a wide morphological range (Jacquet and Brock, Reference Jacquet and Brock2016), the absence of large concentric rugae and a blunter apex in our material make an affiliation to this genus questionable. Considering the sub-circular cross-section, similarities to Miroconulus Parkhaev in Gravestock et al. (Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001) or Anuliconus Parkhaev in Gravestock et al. (Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001) are probable. However, both taxa are characterized by concentric rugae, the apex of Miroconulus is slightly displaced and hooked posteriorly, and Anuliconus is highly conical with a posteriorly hooked apex. Therefore, it seems most probable that our Helcionellid gen. and sp. indet. 1 represents either a new species of Helcionella or a new genus of helcionelloid molluscs. However, the incompleteness of the material hinders a certain taxonomic affiliation.
Helcionellid gen. and sp. indet. 2
Occurrence
Two specimens from the middle part of the Combined Metals Member (Dyeran Stage) of Oak Spring Summit section (OS 3).
Description
Large, weakly cyrtoconic, cap-shaped shells with well-developed concentric rugae (Fig. 7.13, 7.15). Apex blunt and rounded; however, specimen OS 3/B2-8 slightly hooked (Fig. 7.13). Aperture is absent, but specimens show a sub-circular to elliptical outline. Height and length of the broken specimens OS 3/B2-8 and OS 3/B2-9 are ~0.9 mm and ~1.9 mm, and ~1.5 mm and ~2.9 mm, respectively.
Remarks
Specimens are similar to Helcionellid gen. and sp. indet. 1, but with well-developed concentric rugae and without radial lirae. Rugae, the sub-circular cross-section and the slightly hooked apex indicate an affiliation to Helcionella, but our material shows a blunter apex and the concentric rugae are less prominent. Again, poor preservation prevents a secure taxonomic placement.
Phylum uncertain
Class Hyolitha Marek, Reference Marek1963
Order Hyolithida Sysoiev, Reference Sysoiev1957
Family Nelegerocornidae Meshkova, Reference Meshkova1974
Genus Microcornus Mambetov, Reference Mambetov1972
Type species
Microcornus parvulus Mambetov, Reference Mambetov1972 (p. 268, fig. 1a–1e); Rhombocorniculum cancellatum Zone, Geres Member, basal Shabakty Formation (lower Cambrian; upper Atdabanian Stage; correlated with the lower Cambrian Stage 3); Ushbas River, Malyi (Lesser) Karatau, Kazakhstan.
Microcornus sp.
Occurrence
Several poorly preserved internal molds of the Indian Springs Canyon and Montezuma Range sections (I 1, I 2, I 3, I 7, I 12, I 16, M 5); Montezuman Stage. Several internal molds or shells from the Echo Canyon (E 10, E 12), Grassy Spring (GS 13, GS 17), Groom Range (GR 5, GR 11), Log Cabin Mine (LC 6, LC 7), Oak Spring Summit (OS 5, OS 7), and Split Mountain (SM 14) sections; Dyeran Stage.
Description
Slender shells or internal molds. Aperture and apex often incomplete. Incomplete specimens normally ~2 mm long (~3.3 mm maximum; Fig. 5.27). Dorsal side with distinct, rounded median ridge. Ventral side flat to gently convex. Lateral sides rounded. Cross-section sub-triangular. Protoconch absent, but probably separated from the mature conch by a shallow constriction (see Fig. 5.23). Surface sculpture not preserved.
Remarks
Microcornus differs from Parkula Bengtson in Bengtson et al. (Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990) by a more prominent dorsal median ridge and a sub-triangular cross-section. The flat to gently convex ventral side and the sub-triangular cross-section of the conch indicate affiliation to M. eximius Duan, Reference Duan1984 or M. petilus Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990. Microcornus eximius is characterized by a flat ventral side, whereas M. petilus has a convex ventral side (Demidenko in Gravestock et al., Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001). However, the absence of opercula prevents an assignment to a species.
Family unassigned
Genus Parkula Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990
Type species
Parkula bounites Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990 (p. 223, figs. 149–151); Abadiella huoi Zone, Parara Limestone (lower Cambrian; correlated with the Cambrian Series 2); Kulpara, Yorke Peninsula, South Australia.
Parkula sp.
Occurrence
Poorly preserved internal molds from the Montezuma Range section (M 6); Montezuman Stage. Several internal molds or shells from the Antelope Canyon (AC 1, AC 6), Echo Canyon (E 10, E 12, E 15), Grassy Spring (GS 13, GS 17), Groom Range (GR 5, GR 11), Log Cabin Mine (LC 6, LC 7), Oak Spring Summit (OS 1, OS 4, OS 5, OS 6, OS 6/2, OS 7, OS 11), and Split Mountain (SM 14, SM 15) sections; Dyeran–Delamaran stages.
Description
Conchs with lenticular cross-section. Dorsal side with faint median ridge. Ventral side less convex than dorsal side. Aperture and apex often incomplete. Most specimens incomplete and 1 mm in length (with a maximum of 2.7 mm; Fig. 7.21). Angle of divergence 12–20°. Aperture perpendicular to the long axis of the conch. Apex slightly bulbous (Fig. 7.17, 7.19). Surface generally smooth, but a few specimens with faint transverse lines and irregularly spaced depressions of ~6 μm in diameter (Fig. 7.19, 7.25).
Remarks
Parkula differs from Microcornus by having a faint dorsal median ridge and a lenticular to sub-triangular cross-section. Cross-section is similar to P. esmeraldina Skovsted, Reference Skovsted2006a. However, poor preservation and the absence of opercula hinder a certain taxonomic affiliation.
Phylum and class uncertain
Order Hyolithelminthida Fisher, Reference Fisher1962
Family Hyolithellidae Walcott, Reference Walcott1886
Genus Hyolithellus Billings, Reference Billings1872 (1871)
Type species
Hyolithes micans Billings, Reference Billings1872 (p. 215, figs. 3a, 3b); Bonnia-Olenellus Zone, Dyeran Stage; Troy, New York State, USA.
Hyolithellus? sp.
Occurrence
Several fragments of internal molds from the Montezuma Range (M 5) and Indian Springs Canyon (I 2, I 3) sections; Montezuman Stage. Few fragments from the Echo Canyon section (E 12), Grassy Spring (GS 13, GS 17), Groom Range (GR 4, GR 5, GR 11), Indian Springs Canyon (I 12), Log Cabin Mine (LC 6, LC 7), Oak Spring Summit (OS 5, OS 6), and Split Mountain (SM 15) sections; Dyeran Stage. Several fragments from the Antelope Canyon (AC 3), Groom Range (GR 8), Log Cabin Mine (LC 1), and Oak Spring Summit (OS 11) sections; Delamaran Stage.
Description
Straight (Fig. 6.24, 6.25, 6,27, 6.28) to gently curved (Fig. 6.26) fragments with circular cross-section. Tube fragments slightly expanding. Length and width of tube fragments up to 2.4 mm and 200 μm, respectively. Internal molds smooth.
Remarks
Classification of hyolithelminthids is primarily based on cross-section, ornamentation, and degree of tapering of the phosphatic tubes, which has resulted in a large variety of generic and specific names of often unclear differences (Landing, Reference Landing1988; Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990; Skovsted, Reference Skovsted2006b; Paterson et al., Reference Paterson, Skovsted, Brock and Jago2007; Topper et al., Reference Topper, Brock, Skovsted and Paterson2009; Skovsted and Peel, Reference Skovsted and Peel2011; Smith et al., Reference Smith, Brock and Paterson2015). Following Bengtson in Gravestock et al. (Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001) and Elicki (Reference Elicki2011), we use the formal classification into hyolithellid and torellellid hyolithelminths. Internal molds described have a circular cross-section and are thus referred with some uncertainty to the hyolithellid genus Hyolithellus Billings, Reference Billings1872 (1871).
Phylum uncertain
Class Coeloscleritomorpha Bengtson and Missarzhevsky, Reference Bengtson and Missarzhevsky1981
Order Chancelloriida Walcott, Reference Walcott1920
Family Chancelloriidae Walcott, Reference Walcott1920
Remarks
Several samples have produced isolated rays that cannot be assigned to a particular genus within this family. These are listed as chancelloriid spicules even though they may belong to co-occurring taxa listed below.
Genus Allonnia Doré and Reid, Reference Doré and Reid1965
Type species
Allonnia tripodophora Doré and Reid, Reference Doré and Reid1965 (p. 20, fig. 1); Carteret Formation (lower Cambrian; correlated with the Cambrian Series 2); Carteret, Cotentin Peninsula, Normandy, France.
Allonnia sp.
Occurrence
Several spicules from the Antelope Canyon (AC 0, AC 2, AC 4, AC 5), Echo Canyon (E 6, E 10, E 12, E 16), Grassy Spring (GS 1, GS 2, GS 8, GS 13, GS 16, GS 17, GS K), Groom Range (GR 4, GR 5, GR 8, GR 11), Indian Springs Canyon (I 12), Log Cabin Mine (LC 1, LC 2, LC 3, LC 5, LC 6, LC 7), Montezuma Range (M 5), Oak Spring Summit (OS 1, OS 2, OS 4, OS 5, OS 6, OS 6/2, OS 7, OS 7.0, OS 11, OS 12), and Split Mountain (SM 14, SM 15) sections; Montezuman–Delamaran stages.
Description
Poorly preserved spicules with 4+0 rays slightly diverge from the basal plane.
Remarks
Chancelloriids with 2+0, 3+0, and 4+0 rays are referred to the genus Allonnia (see Qian and Bengtson, Reference Qian and Bengtson1989; Moore et al., Reference Moore, Li and Porter2013). Orientation and arrangement of our four-rayed spicules indicate a systematic affiliation to A. tetrathallis (Jiang in Luo et al., Reference Luo, Jiang, Wu, Song and Ouyang1982).
Genus Chancelloria Walcott, Reference Walcott1920
Type species
Chancelloria eros Walcott, Reference Walcott1920 (p. 329–331, pl. 86, figs. 2, 2a–c; pl. 88, figs. 1, 1a–f); Ogygopsis Zone, Burgess Shale Member, Stephen Formation (middle Cambrian; correlated with the Cambrian Series 3); northeast of Burgess Pass, British Columbia, Canada.
Chancelloria sp. 1
Occurrence
Hundreds of spicules from the Log Cabin Mine (LC 6) and Oak Spring Summit (OS 4) sections; Dyeran Stage.
Description
Poorly preserved spicules with 6+0 rays. Rays slightly bent upwards from the basal plane. Foramen on the lower side rounded to oval (Fig. 8.14).
Remarks
Detailed systematic affiliation is difficult due to poor preservation. Shape and organization of spicules suggest an affiliation to Chancelloria. However, an affiliation to Archiasterella Sdzuy, Reference Sdzuy1969 (e.g., A. elegans Demidenko in Gravestock et al., Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001) could not be excluded.
Chancelloria sp. 2
Occurrence
Thousands of spicules and large number of isolated rays from the Antelope Canyon (AC 0, AC 2, AC 4, AC 5), Echo Canyon (E 6, E 10, E 12, E 16), Grassy Spring (GS 1, GS 2, GS 8, GS 13, GS 16, GS 17, GS K), Groom Range (GR 4, GR 5, GR 8, GR 11), Indian Springs Canyon (I 12), Log Cabin Mine (LC 1, LC 2, LC 3, LC 5, LC 6, LC 7), Montezuma Range (M 5, M 6), Oak Spring Summit (OS 1, OS 2, OS 4, OS 5, OS 6, OS 6/2, OS 7, OS 7.0, OS 11, OS 12), and Split Mountain sections (SM 14, SM 15); Montezuman–Delamaran stages.
Description
Spicules with 5+1 broken rays. The vertical ray is more robust and shorter than the lateral rays. Rays slightly bent upwards from the basal plane.
Remarks
Spicules show a flat base and an almost radial symmetry. Number and arrangement of the rays indicates a systematic affiliation to Chancelloria.
Genus Archiasterella Sdzuy, Reference Sdzuy1969
Type species
Archiasterella pentactina Sdzuy, Reference Sdzuy1969 (p. 134–137, pl. 15, fig. 4–12, 13?, text figs. 2d, 3, 4); Andalusiana cornuta-Termierella sevillana band (lower Cambrian; middle Marianian Stage; correlated with the uppermost Terreneuvuian/Cambrian Stage 2); basin of Guadalcanal, Sierra Morena, southern Spain.
Archiasterella cf. A. hirundo Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990
Occurrence
One spicule from the Log Cabin Mine section (LC 1); Delamaran Stage.
Description
Spicule with 4+0 rays. Specimen strongly recrystallized.
Remarks
Configuration and arrangement of rays suggests a similarity with A. hirundo Bengtson in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990.
Phylum Echinodermata Klein, Reference Klein1734
Indeterminate echinoderm ossicles
Occurrence
Several ossicles from the Echo Canyon (E 12, E 15), Log Cabin Mine (LC 6), and Split Mountain (SM 14, SM 15) sections; Dyeran Stage.
Remarks
Echinoderm ossicles with preserved stereome microstructure. Morphology ranges from plates, irregular segments, to barrel-shaped segments, typical for eocrinoids and edrioasteroids. However, no assignment to a particular taxon is possible.
Phylum Tardipolypoda Chen and Zhou, Reference Chen and Zhou1997
Class Xenusia Dzik and Krumbiegel, Reference Dzik and Krumbiegel1989
Order Scleronychophora Hou and Bergström, Reference Hou and Bergström1995
Family Eoconchariidae Hao and Shu, Reference Hao and Shu1987
Genus Microdictyon Bengtson, Matthews, and Missarzhevsky in Missarzhevsky and Mambetov, Reference Missarzhevsky and Mambetov1981
Type species
Microdictyon effusum Bengtson, Matthews, and Missarzhevsky in Missarzhevsky and Mambetov, Reference Missarzhevsky and Mambetov1981 (p. 78, pl. 13, figs. 3, 5); Rhombocorniculum cancellatum Zone, Geres Member, basal Shabakty Formation (lower Cambrian; upper Atdabanian Stage; correlated with the lower Cambrian Series 2); Ushbas River, Malyi (Lesser) Karatau, Kazakhstan (see Mambetov and Missarzhevsky, Reference Mambetov and Missarzhevsky1972).
Microdictyon montezumaensis new species
Holotype
Specimen FG 544/GB/M/5/C8-8 from sample M 5 from the upper Fallotaspis Zone of the Montenegro Member; middle Montezuman Stage; 5 m below the 127 m aluminum tag; Montezuma Range section.
Diagnosis
Microdictyon with simple, smooth nodes.
Description
One fragment of ~180 μm thickness (Fig. 8.25). Sclerite composed of a dense crystalline layer (capping, sensu Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986) forming its surface and the walls/bases of holes, and a coarser crystalline layer hosting the holes (framework, sensu Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986). Holes surrounded by a prominent ridge (Fig. 8.24, 8.25). Cross-section of holes clearly shows the relationship between holes and ridges, offering a barrel-shaped structure (Fig. 8.25). At the capping, hole diameters constricting from ~80 μm to ~50 μm, extending into bulbous cavities (width of 120 μm in their central part) in the framework. Holes surrounded by six regularly arranged weak nodes (Fig. 8.24, 8.25).
Etymology
Named after Montezuma Range.
Remarks
The exact determination of Microdictyon species is primarily based on the morphology of the nodes surrounding the holes. A further aspect is the hole diameter and a common basal closure of the holes. The last feature is a typical characteristic of M. effusum (Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986, p. 101, fig. 3). However, the absence of such a basal closure in all other described species of Microdictyon may be an artifact of preservation. According to the original description, the nodes of M. effusum have a mushroom-like shape, although this and the basal closure are not observable on the figured material (Bengtson et al. in Missarzhevsky and Mambetov, Reference Missarzhevsky and Mambetov1981, pl. 13, figs. 3, 5). Bengtson et al. (Reference Bengtson, Matthews and Missarzhevsky1986) described a distinct brim and a sub-centrally placed apex for M. effusum. Nodes of our sclerite show no prominent relief (Fig. 8.24, 8.25). However, the good preservation of the surface layer indicates no or only minor erosion, thus excluding a destruction of prominent nodes. Therefore, the gentle morphology on the sclerite is considered as representing the original shape. Because the structure of the nodes is an essential criterion for species definition, it is necessary to assign this fragment to the new species M. montezumaensis.
It should be kept in mind that individual complete plates of Microdictyon could combine features (e.g., node morphology) diagnostic for a range of species (Chen et al., Reference Chen, Hou and Lu1989; Topper et al., Reference Topper, Brock, Skovsted and Paterson2011). The simple, hump-like nodes of Microdictyon montezumaensis n. sp. are singular for this species and not known from other fragments and complete plates of Microdictyon. However, it couldn’t be excluded that the generally applied diagnostic characteristics result in a multitude of different species of Microdictyon, probably overestimating the real taxonomic diversity, and thus have to be critically revised.
Most Microdictyon sclerites are found in lower Cambrian successions worldwide. Few specimens are known from the middle Cambrian (Ptychagnostus gibbus Zone) of Utah and Bornholm, both probably representing reworked lower Cambrian material (Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986; Berg-Madsen, Reference Berg-Madsen1981).
Microdictyon cuneum new species
Holotype
Fragmented specimen FG 544/GB/M/5/C8-17 from sample M 5 from the upper Fallotaspis Zone of the Montenegro Member; middle Montezuman Stage; 5 m below the 127 m aluminum tag; Montezuma Range section.
Diagnosis
Microdictyon with short, wedge-shaped nodes.
Description
Fragment with fully preserved, dense crystalline capping and partly preserved, coarse crystalline framework. Thickness ~50 μm. Hole diameter nearly uniform, ~75 μm. Nodes wedge shaped, gradually develop from ridges that surround the holes. One side of the nodes forms an angle of ~30° with the ridge surface forming the wedge shape. Other side of nodes forms an overhang or an acute angle to the ridge. Wedge-shaped nodes are oriented into the same direction.
Etymology
Latin cuneus, meaning wedge. Referring to the wedge-shaped appearance of the nodes.
Remarks
Only two species of Microdictyon are characterized by spike-shaped nodes: Microdictyon robisoni Bengtson, Matthews, and Missarzhevsky, Reference Bengtson, Matthews and Missarzhevsky1986 shows tall and narrow nodes ending in slight expansions. These expansions are clearly offset from the basal socket, which is not present in our specimen. Nodes of M. sphaeroides Hinz, Reference Hinz1987 develop from a smaller base to a wide, flat rim, finally culminating in a sloped spine. Therefore, node morphology of both species is completely different compared to M. cuneum n. sp. Nodes of M. cuneum n. sp. develop continuously from the rim of the capping showing no offset or rim. They are also shorter than the spiny nodes of M. robisoni.
Microdictyon rhomboidale Bengtson, Matthews, and Missarzhevsky, Reference Bengtson, Matthews and Missarzhevsky1986
1986 Microdictyon rhomboidale Reference Bengtson, Matthews and MissarzhevskyBengtson, Matthews, and Missarzhevsky, p. 102, figs. 4–6.
1987 Microdictyon sp.; Reference Voronova, Drozdova, Esakova, Zhegallo, Zhuravlev, Rozanov, Sayutina and UshatinskayaVoronova et al., p. 56, pl. 24, figs. 6–7.
1992 Microdictyon rhomboidale; Reference Bengtson and Conway MorrisBengtson and Conway Morris, p. 461, fig. 2F.
2007 Microdictyon aff. rhomboidale; Reference Zhang and AldridgeZhang and Aldridge, p. 405, fig. 2N–2R.
2013 Microdictyon rhomboidale; Reference Bengtson and Conway MorrisBengtson and Conway Morris, p. 461, fig. 2F.
2015 Microdictyon cf. rhomboidale; Reference Kouchinsky, Bengtson, Clausen and VendrascoKouchinsky et al., p. 481, fig. 55.
2015 Microdictyon sp.; Reference Kouchinsky, Bengtson, Clausen and VendrascoKouchinsky et al., p. 481, fig. 56.
Holotype
Microdictyon rhomboidale Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986 (figs. 4–6); lower Cambrian (upper Atdabanian or lower Botoman stages; correlated with the middle to upper Cambrian Stage 3); north of Bograd village, Batney Hills, Kuznetskij Alatau Range, Republic of Khakassia, Russia (see Zadorozhnaya et al., Reference Zadorozhnaya, Osadchaya and Repina1973).
Occurrence
One fragment from sample M 5 from the upper Fallotaspis Zone of the Montenegro Member; middle Montezuman Stage; 5 m below the 127 m aluminum tag; Montezuma Range section.
Description
Holes are circular to sub-circular, decreasing in size towards the rim, range of 115 μm to 14 μm near margin. Nodes slightly mushroom-shaped with distinct brim.
Remarks
The capping of the fragment is completely preserved, whereas the major part of the framework is corroded. The fragment represents the periphery of a complete sclerite with a steep rim (Fig. 8.28). The shape of the nodes is typical for M. rhomboidale Bengtson et al., Reference Bengtson, Matthews and Missarzhevsky1986. The fragment compares well to Microdictyon n. sp. 1 of Bengtson et al. (Reference Bengtson, Matthews and Missarzhevsky1986) described from the region 42 km south of Goldfield (Esmeralda County, Nevada; Albers and Stewart, Reference Albers and Stewart1972). The material is derived from the lower Nevadella Zone and is thus stratigraphically slightly younger than the fragments described herein. According to Bengtson et al. (Reference Bengtson, Matthews and Missarzhevsky1986) their Nevadan material could be most probably referred to M. cf. rhomboidale. Due to the stratigraphic and regional closeness of both settings, an affiliation of our fragment to M. rhomboidale is most probable.
Microdictyon sp.
Occurrence
Several fragments from samples M 5 and M 6 from the upper Fallotaspis Zone of the Montenegro Member; middle Montezuman Stage; M 5 and M6 are located 5 m and 6 m, respectively, below the 127 m aluminum tag; Montezuma Range section.
Description
Thin phosphatic plate fragments with hexagonal meshwork. The plates represent only the upper capping. Hole diameter ranges from 85 μm and 180 μm on the slightly convex surface and decreases to <9 μm towards the periphery. Fragments strongly corroded, obliterating any prominent surface and probably enlarging hole diameters.
Remarks
Due to the insufficient preservation of our fragments no exact determination is possible.
Genus Archaeooides Qian, Reference Qian1977
Type species
Archaeooides granulatus Qian, Reference Qian1977 (pl. 2, fig. 21); Meishucunian Stage (correlated with the Cambrian Stage 2); central and southwest China.
Archaeooides cf. A. granulatus Qian, Reference Qian1977
Occurrence
Two specimens from samples SM 14 and SM 15 from the lowermost Emigrant Formation of the Split Mountain section; Samples SM 14 and SM 15 are derived 1.0 m respectively 0.5 m above the base of the Emigrant Formation; Dyeran Stage.
Description
Well-rounded to flattened on one side, hollow, ranging from 250 μm (Fig. 8.33) to 365 μm (Fig. 8.34) in diameter. Surface with crystalline texture and covered with circular to oval pits 15–23 μm in diameter.
Remarks
The spherical specimen from sample SM 15 (Fig. 8.33) is similar to the ‘perforated sphere’ published by Skovsted (Reference Skovsted2006a, fig. 4C) from the same locality but from a slightly higher stratigraphic position (~1.4 m above the base of the Emigrant Formation). The absence of a flattened area suggesting an encrusting lifestyle of the organism excludes an affiliation of both spheres to Aetholicopalla Conway-Morris in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990. There is also no indication for a double-walled surface, even if an erosion of the outer wall could not be excluded. The spheres show clear similarities to Archaeooides granulatus Qian, Reference Qian1977, which are single-walled. The surface of A. granulatus and related synonyms (e.g., A. kuanchuanpuensis Qian, Reference Qian1977, A. acuspinatus Qian, Reference Qian1977, Gaparella porosa Missarzhevsky in Missarzhevsky and Mambetov, Reference Missarzhevsky and Mambetov1981) is characterized by porous tubercles with pore diameters ranging from 10 μm to 30 μm (Missarzhevsky and Mambetov, Reference Missarzhevsky and Mambetov1981; Missarzhevsky, Reference Missarzhevsky1989; Parkhaev and Demidenko, Reference Parkhaev and Demidenko2010). Surface ornamentations of the specimen of sample SM 15 and that figured by Skovsted (Reference Skovsted2006a) are probably corroded.
The specimen of sample SM 14 (Fig. 8.34) shows a slightly convex area, which could be interpreted as a zone of attachment of the organism on the substrate, typical for Aetolicopalla granulata Conway Morris in Bengtson et al., Reference Bengtson, Conway Morris, Cooper, Jell and Runnegar1990. However, the occurrence of pores, even on this area, points against an encrusting life mode of the hemisphere. There is further no indication for a double-wall that necessary for defining this subsphere to Aetolicopalla. The porous structure of the surface indicates an association to Archaeooides granultus, even if a prominent sculpture/ornamentation does not occur. Archaeooides granulatus is characterized by a wide morphology, ranging from spheres, ellipsoids, and hemispheres to spheres flattened on two opposite sides (see Parkhaev and Demidenko, Reference Parkhaev and Demidenko2010). The specimen of sample SM 15 fits into this morphological range. The pores of the Laurentian specimens are fewer than known from Archaeooides. However, based on their (hemi)spheroidal morphology and the single wall, the Laurentian organisms from samples SM 14 and SM 15 and the specimen of Skovsted (Reference Skovsted2006a) are referred to Archaeooides cf. A. granulatus Qian, Reference Qian1977.
The general stratigraphic occurrence of Archaeooides and Aetholicopalla is the Tommotian–Botoman interval of the Siberian nomenclature, which is the Meishucunian–Nangaoan stages of the Chinese nomenclature. The record of the Laurentian Archaeooides slightly below the Dyeran-Delamaran boundary most probably represents the youngest occurrence of these organisms worldwide.
Discussion
Fossil distribution patterns are most probably an artifact of the chemical preparation that eliminated portions of the calcareous microfossils. Helcionelloid molluscs, hyoliths, and hyolithelminths occur in almost all sections investigated for the inner, middle, and outer shelf environments of Nevada and California. Sclerites of sponges and chancelloriids are almost absent at Grassy Spring section (inner shelf), whereas echinoderm ossicles only occur at Split Mountain (outer shelf), Echo Canyon, and Log Cabin Mine sections (both inner shelf; Figs. 3, 4).
Occurrences of Pelagiella aff. P. subangulata and several species of Microdictyon in the lower part of the Montezuman Stage in the Montezuman Range section are most probably important for biostratigraphic correlation. Taxa such as Pelagiella subangulata, Microdictyon effusum, and the tooth-like sclerite Rhombocorniculum cancellatum (Cobbold, Reference Cobbold1921) are characterized by an almost worldwide distribution and are thus useful for correlation of Cambrian Series 2/Stage 3 (Li et al., Reference Li, Zhu and Steiner2003; Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtmann2007; Rozanov et al., Reference Rozanov, Zhu, Pak and Parkhaev2008). Well-established biozonations based on SSF assemblages including these taxa were used in Siberia (the so-called Tommotian fauna; e.g., Khomentovsky and Karlova, Reference Khomentovsky and Karlova1993), Australia (e.g., Gravestock et al., Reference Gravestock, Alexander, Demidenko, Esakova, Holmer, Jago, Lin, Melnikova, Parkhaev, Rozanov, Ushatinskaya, Zang, Zhegallo and Zhuravlev2001; Jago et al., Reference Jago, Sun and Zang2002, Reference Jago, Zang, Sun, Brock, Paterson and Skovsted2006), and South China (e.g., Qian, Reference Qian1999). Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtmann2007) using the P. subangulata and the R. cancellatum taxon-range zones for the base of Cambrian Series 2 have provided detailed correlation between several regions of the Yangtze Platform. Both zones contain additional important faunal elements (e.g., M. effusum, hyoliths, bradoriids, and brachiopods). According to Steiner et al. (Reference Steiner, Li, Qian, Zhu and Erdtmann2007), the P. subangulata range Zone of the Qiongzhusian of South China appears to correlate with the P. lorenzi Zone of the middle–late Atdabanian of the Siberian Platform. Furthermore, the occurrence of P. subangulata, R. cancellatum, and M. effusum indicates a correlation with the Camenella baltica Zone of Avalonia and Newfoundland (Landing et al., Reference Landing, Nowlan and Fletcher1980; Hinz, Reference Hinz1987; Landing, Reference Landing1988). The occurrence of Pelagiella aff. P. subangulata and species of Microdictyon from the lower Montezuman Stage of the Montezuma Range section most probably corresponds with the bases of the taxon-range zones of South China, Siberia, and Avalonia. It therefore most probably identifies the base of Cambrian Series 2/Stage 3 in Nevada and enables the correlation of western Laurentia with these regions. However, the verification of SSF associations suitable for a global correlation of the basal Cambrian Series 2 needs further critical and comprehensive evaluation, as indicated by Landing et al. (Reference Landing, Geyer, Brasier and Bowring2013).
Acknowledgments
We are grateful to P.G. Scholten (Show Low, Arizona) for his field assistance in July 2014. Special thanks to S. Niemeyer (University of Cologne) for her assistance in the lab and for microfossil hunting; H. Cieszynski (also Cologne) is thanked for SEM photography. We gratefully acknowledge the constructive comments of E. Landing (New York State Museum, Albany) and C.B. Skovsted (Swedish Museum of Natural History, Stockholm) and the editorial guidance of N.J. Butterfield (University of Cambridge). The research was financially supported by the German Research Foundation (WO 1215/6).










