INTRODUCTION
Streptococcus agalactiae, also called Group B Streptococcus (GBS), is a facultative Gram-positive coccus distinguished from other types of Streptococcus by the presence of a type B-specific carbohydrate antigen known as the C substance [Reference Edwards, Baker, Remington and Klein1, Reference Manning2]. There are nine known serotypes, the distribution of which vary with geographic location, although serotypes Ia, II, III and V predominate in invasive disease [Reference Harrison3]. S. agalactiae is a common colonist of the gastrointestinal and genitourinary tracts, and can be found in up to 40% of the female population and 30% of the male population [Reference Pickering4, Reference Bliss5]. It was first noted in the 1970s as a leading cause of neonatal disease but over the last two decades the species has emerged as an invasive pathogen in pregnant women and the elderly [Reference Schrag6, Reference Schuchat7]. While the incidence of early-onset neonatal disease and infection in pregnant women has declined over the past decade due to prenatal screening, that of late-onset neonatal disease has remained stable, and the incidence of disease in the elderly has increased [Reference Schrag6, Reference Blancas8–Reference Borchardt12].
S. agalactiae is a genetically diverse organism. When characterized using pulsed-field gel electrophoresis (PFGE) multiple PFGE types often share the same serotype [Reference Benson13, Reference Skjaervold, Bergh and Bevanger14]. However, the level of genetic diversity within the various molecular capsular types, as characterized by differing PFGE types, remains unclear. Previous studies have used PFGE types as a surrogate for serotyping, presuming that isolates from a single individual with the same PFGE type have the same serotype [Reference Bliss5, Reference Manning15]; however, this presumption has been based on the examination of a small number of isolates [Reference Gordillo16]. Given the importance of correctly characterizing capsular types, both for understanding transmission dynamics and vaccine formulation, there is a need to validate the presumption that isolates from one individual with the same PFGE type have the same capsular type.
Over time, the proportion of S. agalactiae isolates that are non-typable using the Lancefield serotyping method has increased [Reference Farley10], suggesting that the use of PFGE to infer capsular type in different individuals may be desirable. However, it is noteworthy that one study reported a single mother–child pair of isolates that were identical by PFGE but with different serotypes [Reference Benson13]. The capsular serotype of a strain could potentially be altered by as little as the horizontal transfer of a single gene [Reference Chaffin17, Reference Luan18] which might not result in a change in PFGE type. Therefore the reliability of using PFGE profiles as a predictor of capsular type for isolates from different individuals, even from within the same geographic area, warrants investigation.
We examined a large collection of S. agalactiae isolates from individual cases in order to describe the genetic diversity of these isolates and test the hypotheses that S. agalactiae PFGE types correspond to a specific serotype within an individual, and between different individuals from the same geographic area.
METHODS
Study protocol
As described previously [Reference Manning19, Reference Foxman20], 738 male and female University of Michigan students were recruited to provide specimens from throat and mouth, vagina, anal orifice, and urine samples. In total, 913 S. agalactiae isolates were collected over a 12-week period, a subset of which was from students who provided multiple samples over the study period. Isolates were serologically confirmed with the Slidex Strepto B kit (bioMérieux, USA).
PFGE
Of the 913 original isolates, 882 were typed using PFGE and patterns were analysed for relatedness by visual inspection and using the BioNumerics software (Applied Maths, Belgium) as described previously [Reference Bliss5, Reference Foxman20]. A subset of the isolates either failed to yield restriction fragments with SmaI (13), or were not analysed with BioNumerics (6), or were not viable at the time of the present study (12).
Capsular typing using library on a slide hybridization
Sixteen of the isolates from the original collection were not viable. The remaining 897 isolates were grown in 9 ml Todd–Hewitt broth (THB) (BD, USA) at 37°C in 5% CO2 for 36 h. Genomic DNA was extracted and purified using sonication and heat treatment as previously described [Reference Zhang21]. The DNA from each isolate was arrayed in duplicate on Vivid Gene Array slides with nylon membranes (Pall, USA) using the VersArray ChipWriter Compact system (Bio-Rad, USA).
PCR amplification of probe sequences was performed as follows: DNA lysates were prepared from 3 ml S. agalactiae overnight culture in THB. Each reaction mixture (48 μl) included 1 μl of each primer (25 pmol), 1 μl DNA lysate, and 45 μl Platinum PCR SuperMix (Invitrogen, USA). The primers and PCR conditions used to amplify capsular types Ia, Ib, II, III, IV,V and VI–VIII genes were as described previously [Reference Borchardt12, Reference Wen22], with the following modifications: 94°C for 30 s, 49°C (type V) or 55°C (type III) for 30 s, and 72°C for 25 s, for 35 cycles. PCR product (10 μl) was analysed by electrophoresis on a 1% agarose gel, stained with 0·02 μg ethidium bromide/ml, and visualized by UV transillumination.
PCR amplification products were purified using the QIAquick gel extraction kit and the QIAquick PCR purification kit (Qiagen Inc., USA) and fluorescein labelled with 2·5 μl of 1 mm fluorescein-12-dCTP (PerkinElmer, USA) using a modification of the BioPrime DNA Labeling System (Invitrogen).
The probes were hybridized to genomic DNA arrayed on slides in duplicate using previously described methods [Reference Zhang23] and hybridization signals were detected using a ArrayIt Microarray SpotWare Colorimetric Scanner (TeleChem International Inc., USA) and analysed with IconoClust software (Clondiag Chip Technologies, Germany). The signal intensity of the spots was adjusted for background and normalized to the signal intensity of a quantification probe which consisted of a mixture of S. agalactiae housekeeping genes (adhP, glcK, glnA, pheS). These values were used to account for differences in genomic DNA concentrations at different spots. Cut-offs for probe positive strains were established using previously published methods [Reference Zhang24].
A hierarchical strategy for capsular-type confirmation was employed, such that at least one isolate with a unique PFGE pattern was assigned a capsular type via traditional dot-blot hybridization, in addition to the Library on a Slide dot-blot hybridization. Isolates with non-confirmatory capsular-type results in the Library on a Slide assay or that were discrepant with both traditional and Library on a Slide were confirmed by PCR and dot-blot hybridization methods using the primer sequences and methods described earlier [Reference Borchardt12, Reference Wen22]; 892/897 isolates originally printed on the Library on a Slide were confirmed using the hierarchal typing strategy and were assigned a unique capsular-type designation.
RESULTS
Of the 892 isolates assigned a unique capsular type and 882 isolates assigned a PFGE pattern, a total of 872 S. agalactiae isolates from 152 individuals had both PFGE pattern data and a unique capsular-type assignment. These 872 isolates were used for the final analyses. Overall, an average of six isolates were available from each individual.
Capsular type
The capsular-type distribution of the S. agalactiae isolates was examined in the final collection. In order to account for the possibility that an individual was persistently colonized with the same organism over the course of the study, the capsular-type distribution of the collection was also examined by choosing one isolate from each unique PFGE pattern from a given individual (168). The capsular-type distributions were very similar whether analysing one isolate or all isolates per person, suggesting few individuals were colonized with more than one serotype (Fig. 1).
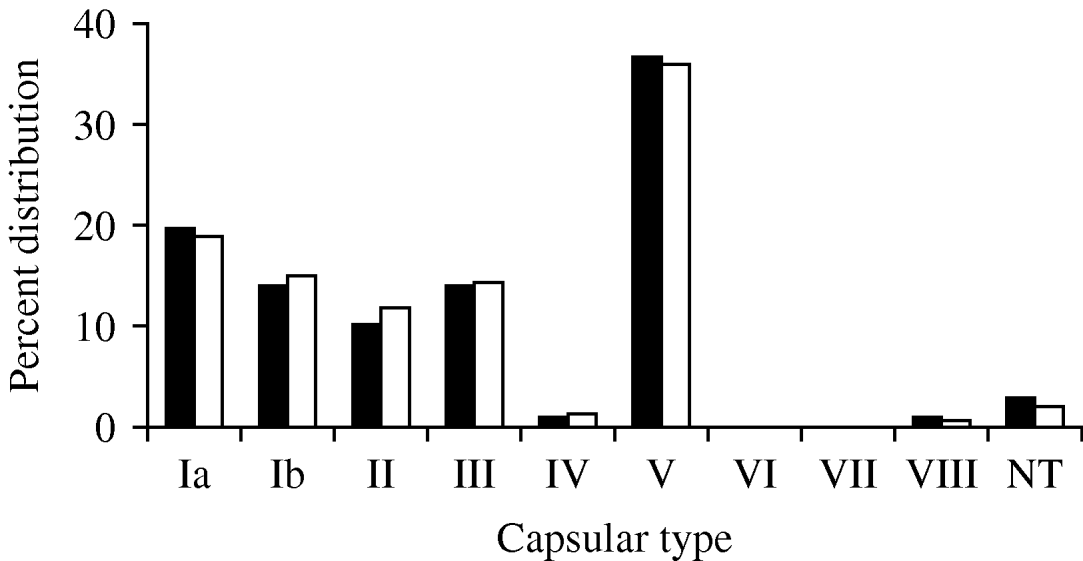
Fig. 1. Capsular-type distribution of S. agalactiae isolates collected between January and April 2001, from healthy male and female University of Michigan undergraduates based on the total collection (▪, n=872) and a subgroup collection of one isolate for each unique PFGE pattern from an individual (□, n=168). NT, Non-typable.
As reported previously, based on results of traditional dot-blot hybridization to determine capsular type [Reference Foxman20], there was a predominance of type V isolates (322, 36·9%). Additionally the collection included type Ia (170, 19·4%), type III (124, 14·2%), type Ib (123, 14·1%), type II (88, 10·1%), type VIII (10, 1·1%) and type IV (8, 0·9%). Only 27 isolates from the entire collection were non-typable (3%) by all molecular capsular-typing strategies. Interestingly, while a majority of the isolates from a given individual had the same capsular type, nine (5·9%) of the 152 individuals were concurrently colonized with two different capsular types; an additional two individuals were colonized with two different capsular types over the course of the study.
Diversity within capsular type
Fifty-two PFGE patterns were identified in the 872 isolates. To explore genetic diversity we chose one representative isolate for each PFGE pattern from each individual, resulting in a total of 168 isolates with both a PFGE type and a capsular type. Based on Simpson's diversity index, which indicates the probability of randomly choosing two isolates of a given capsular type with a different PFGE type [Reference Hunter and Gaston25, Reference Grundmann, Hori and Tanner26], capsular types III, II and Ib exhibited the greatest heterogeneity; while capsular type V isolates were the most homogeneous (Table 1).
Table 1. Simpson's diversity index of PFGE within capsular serotypes of S. agalactiae
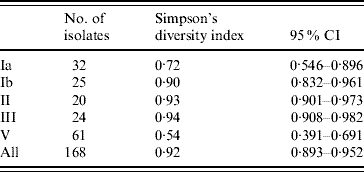
CI, Confidence interval.
Data not shown for capsular types IV (n=2) and VIII (n=1) and non-typable isolates (n=3) [Reference Hunter and Gaston25, Reference Grundmann, Hori and Tanner26].
PFGE type within individuals
Fourteen of the 152 individuals (9·2%) carried isolates with more than one PFGE type. In three of these individuals, all isolates, which included an average of seven isolates per person, were of the same capsular type. In the remaining 11 individuals, one had three isolates with unique PFGE patterns, representing two distinct capsular types, nine persons had two PFGE types representing two distinct capsular types (Fig. 2), and one individual had 11 isolates representing two PFGE types and two serotypes. Two of the isolates sharing PFGE types had different serotypes. There was a 2·0% probability of an individual having more than one unique PFGE type but representing a single capsular type and a 6·6% probability of an individual having more than one unique PFGE type each representing a distinct capsular type. We observed one individual (1/152) where both these phenomena occurred (0·7%). Therefore, within a given individual, while differing PFGE patterns potentially represented identical capsular types, the probability that all isolates with a unique PFGE pattern represent the same capsular type was 99%, which may reflect technical rather than true biological variability.

Fig. 2. Representative PFGE pattern of 12 colonizing S. agalactiae isolates recovered from two healthy individuals: Group B Streptococcus recovered from throat, rectal and urine samples of study participant no. 760 are shown in lanes 2–7 and 9–10, and from study participant no. 774 in lanes 11–14. Lanes 2–5, 7 and 9–10 from participant no. 760 were capsular type III, while lane 6 was capsular type II. Lanes 11–14 from participant no. 774 were capsular type V. Lanes 1, 8, 15 are control strain A909.
PFGE type in individuals
When examining the distribution of capsular types and PFGE patterns across individuals, 10/52 (19·2%) identified PFGE patterns occurred in more than one capsular type. Seven PFGE patterns included two capsular types, while three PFGE patterns included three capsular types. Based on these results, there could be between an 8·3% and 15·4% error rate in capsular-type assignment if PFGE was used as a surrogate for capsular typing in these individuals from similar geographic areas.
DISCUSSION
DNA sequencing of various strains of S. agalactiae has demonstrated the genetic diversity of the species and isolates of different serotypes have been noted to share more sequence similarities than isolates of the same serotype [Reference Tettelin27, Reference Tettelin28]. Tettelin et al. suggest that the level of S. agalactiae genetic diversity and evolutionary patterns are not adequately reflected with serotype classification [Reference Tettelin27]; thus the level of heterogeneity within serotype classification could vary widely. We describe the heterogeneity of S. agalactiae and the impact of this heterogeneity on scientific inference by examining the PFGE patterns and capsular types of 872 isolates from 152 individuals. S. agalactiae is highly heterogeneous but the heterogeneity varies by capsular type, with the greatest heterogeneity occurring in capsular types III, Ib and II. Within an individual, isolates with the same PFGE patterns had identical capsular types, while across individuals PFGE types appeared in more than one capsular type. Therefore, capsular type alone was not sufficient to characterize epidemiological relatedness. Although PFGE types appear to be a valid surrogate for capsular typing of isolates from the same individual, it is not a valid surrogate for serotype in isolates from different individuals.
The heterogeneity we observed is consistent with previous reports describing much smaller collections [Reference Skjaervold, Bergh and Bevanger14, Reference Perez-Ruiz29], and as observed in these earlier studies, capsular type V was the most homogeneous [Reference Skjaervold, Bergh and Bevanger14, Reference Savoia30]. These studies did not report a diversity index score and so we cannot make a direct comparison with our study findings. Interestingly, the relative homogeneity of type V isolates is consistent with the emergence of a predominant capsular type V clonal lineage [Reference Elliott, Farmer and Facklam31].
Assuming that a common PFGE type represented the same capsular type across individuals would have resulted in an error rate in capsular-type assignment of as much as 15%. Thus, using 2–3 band differences in PFGE pattern might not be sufficiently stringent for outbreak investigations [Reference Tenover32], as geographically and temporally linked isolates with distinct serotypes shared almost identical PFGE patterns, providing a strong potential for linking epidemiologically unrelated strains and obscuring the role and routes of transmission in colonization and disease.
Multiple researchers have demonstrated that S. agalactiae isolates sharing identical multilocus sequence types (MLST) can possibly represent differing capsular types [Reference Luan18, Reference Davies33, Reference Jones34]. Jones et al. described the increased potential of S. agalactiae sequence type 17 to cause invasive disease within neonates when compared to other sequence tyoes independent of capsular type [Reference Jones35]. Given that S. agalactiae capsule is a key determinant of virulence and vaccine development is based on capsular type, it is important to recognize that MLST typing is likely to encounter similar problems as PFGE typing if used as a surrogate for capsular typing.
Our study collection was large, enabling us to characterize the genetic diversity more precisely than had been done previously. Furthermore, we quantified the degree of diversity using Simpson's diversity index. The consistent effect across a large number of isolates strongly supports the hypothesis that PFGE typing distinguishes between capsular types isolated from a single individual. Using the hierarchical capsular-typing strategy we employed of dot-blot hybridization and PCR, only 27 isolates (3%) of our entire collection could not be assigned a capsular type, consistent with previous reports [Reference Harrison3, Reference Hickman36]. The non-typable isolates might contain a defective cps operon not detected using a genotype-based strategy. It is also possible that these few non-typable isolates represent the newly described serotype IX, although the few reported serotype IX isolates were collected from a geographic region distant from our study population [Reference Slotved37]. An additional potential limitation of our study is the omission of 20 isolates (2·2%) which had corresponding serotype data but could not be digested and characterized by PFGE. It is possible that exclusion of these isolates could have resulted in a decreased Simpson's diversity index for some of the capsular types. However, given the small fraction of undigested isolates, it is unlikely that their inclusion would significantly alter the relative heterogeneity of capsular types.
In summary, by PFGE, S. agalactiae is a genetically diverse organism, with diversity varying within capsular types. Neither S. agalactiae capsular-type nor PFGE-type designation alone appear sufficiently discriminatory to determine the relatedness of S. agalactiae isolates, given that either typing method alone can link epidemiologically unrelated isolates or fail to identify epidemiologically related isolates. Thus, a combined typing strategy such as PFGE or MLST with capsular typing should be considered to more adequately understand the epidemiology of S. agalactiae.
ACKNOWLEDGEMENTS
We gratefully acknowledge Shannon Manning and Sandra McCoy for their work on typing the S. agalactiae isolates by PFGE. We also acknowledge the work of Sheng Li, Elizabeth Levin, Sara McNamara, Anne Styka, Suhael Momin and Caitlin Murphy who assisted with DNA isolation. This work was supported, in part, by R01 AI051675 (B.F.) and T32 HD007513 from the National Institutes of Health.
DECLARATION OF INTEREST
None.





