CVD is one of the leading causes of morbidity and mortality worldwide. It is suggested that low levels of HDL-cholesterol constitute an independent risk factor for CVD(Reference Miller1–Reference Castelli, Anderson and Wilson3). Also, a recently concluded clinical intervention trial supports the idea that increasing the levels of HDL-cholesterol can protect against clinical CVD(Reference Rubins, Robins and Collins4). Based on these observations and current concepts regarding the anti-atherogenic roles of HDL in promoting reverse cholesterol transport(Reference Tall5) and as an antioxidant(Reference Parthasarathy, Barnett and Fong6), interventions involving an increase in HDL concentrations are expected to constitute an important therapeutic option for CVD prevention. For example, consumption of polyphenol-rich fruit juice or cocoa chocolate has been shown to increase plasma HDL-cholesterol concentrations(Reference Kurowska, Spence and Jordan7–Reference Mursu, Voutilainen and Nurmi9). Additionally, dietary supplementation with concentrated tomato juice, which has a high antioxidant capacity due to its high content of lycopene, significantly increased lycopene and HDL-cholesterol levels(Reference Madrid, Vasques and Leyton10).
Ripe paprika fruit (Capsicum annuum) is widely used as a vegetable and food additive, as this fruit is considered to be a good source of carotenoid pigments. Capsanthin is the major carotenoid present in paprika, and is present in an acylated form with fatty acids(Reference Gregory, Chen and Philip11, Reference Biacs, Daood and Pavisa12). This carotenoid, which does not possess provitamin A activity, has been shown to be effective as a free-radical scavenger(Reference Matsufuji, Nakamura and Chino13).
Oshima et al. (Reference Oshima, Sakamoto and Ishiguro14) studied the accumulation and clearance of capsanthin in the plasma of human males after ingestion of paprika juice. These studies revealed that dietary capsanthin was absorbed into the body and distributed to plasma lipoproteins. Furthermore, it was confirmed that xanthophylls, including capsanthin, are distributed to HDL in larger amounts than to LDL, when compared with hydrocarbon carotenoids. Xanthophylls can act as antioxidants against free radical attack and exposure to singlet oxygen in plasma lipoproteins(Reference Boey, Nagao and Terao15, Reference Ojima, Sakamoto and Ishiguro16). Therefore, dietary xanthophylls seem to participate in the primary defence mechanism of HDL against oxidative stress, and may also be expected to affect lipid metabolism and/or maintain favourable blood lipid profiles. In fact, some reports have suggested that certain carotenoids may affect HDL-cholesterol concentrations(Reference Brady, Mares-Perlman and Bowen17, Reference Slattery, Jacobs and Dyer18) and alter adipocytokine levels(Reference Sugiura, Matsumoto and Kato19, Reference Hussein, Nakagawa and Goto20) or hepatic gene expression(Reference Jeyakumar, Vajreswari and Giridharan21). Therefore, it is reasonable to speculate that ingestion of paprika, which possesses abundant capsanthin, may modulate lipid metabolism.
The purpose of the present study was to evaluate the effect of capsanthin, the main carotenoid in paprika, on in vivo lipid metabolism in rats.
Materials and methods
Animals
Known-pathogen-free male Crlj:WI rats (Wistar rats, aged 4 weeks) were purchased from Charles River Laboratories Japan, Inc. (Yokohama, Japan) and maintained on a normal CE-2 (CLEA, Tokyo, Japan) diet for 1 week before starting the experiment. All rats were housed individually in stainless-steel cages under controlled conditions (temperature 23 ± 1°C, humidity 55 ± 5 %, lights on from 07.00 to 19.00 hours). At the end of the experiments, the rats were anaesthetised with sodium pentobarbital and blood and liver samples were taken. Plasma samples were stored at − 80°C until analysed. Livers were immediately frozen and kept at − 80°C until analysed. All animals were treated in accordance with guidelines established by the Japanese Society of Nutrition and Food Science (Law 105 and Notification 6 of the Japanese government). The experimental protocol was approved by the Kagome Animal Use Committee.
Diets and preparation of samples
The basic diet contained the following ingredients (per kg): 250 g casein, 453 g maize starch, 200 g sucrose, 50 g maize oil, 35 g American Institute of Nutrition (AIN)-93 mineral mix, 10 g AIN-93 vitamin mixture and 2 g choline chloride. The diet ingredients were purchased from Oriental Yeast Co. (Tokyo, Japan). The composition of the other experimental diets resembled the basic diet, except that paprika powder, paprika extract, residue of paprika extract or purified capsanthin replaced an equivalent weight of maize starch.
Paprika powder was obtained by lyophilising paprika paste (TAT, Istanbul, Turkey). Paprika extract and its residue were prepared by organic solvent extraction. Briefly, paprika powder (20 g) was extracted by hexane–acetone–ethanol–toluene (10:7:6:7, by vol.) three times to separate the soluble organic components, including carotenoids(Reference Horwitz and Latimer22). The extracts were combined and evaporated to produce the paprika extract (0·5 g). In addition, paprika extract residue (approximately 19·5 g) was acquired by the removal of organic solvents from the extraction residue. Capsanthin concentrations in the paprika powder, paprika extract and residue of paprika extract were determined by HPLC(Reference Aizawa and Inakuma23) at 2·43, 64·97 and 0·62 mmol/kg, respectively.
Purified capsanthin for animal feed was extracted from commercially available non-acylated free capsanthin-containing powder (Capsanthal; BASF Japan Ltd, Tokyo, Japan), and purified by a chromatographic method. Briefly, Capsanthal was extracted by methanol and the extracts were applied to preparative HPLC (GL Science Inc., Tokyo, Japan) with a Soken pack octadecylsilyl (ODS-ST-C) column (Soken Chemical & Engineering Co., Ltd, Tokyo, Japan) and eluted with methanol. All fractions containing capsanthin were collected and evaporated. Crude capsanthin extracts were subjected to silica gel column chromatography (Wako-gel C-200; Wako, Tokyo, Japan) and sequential elutions of a solvent mixture of dichloromethane and methanol. The fractions containing capsanthin were pooled, and the solvent was removed in vacuo to obtain purified capsanthin. Capsanthin purity (>90 %) was determined by absorption spectroscopy and HPLC methodology(Reference Aizawa and Inakuma23).
Experimental design
In the present study, the main objective was to examine the effect of paprika or capsanthin ingestion on lipid metabolism. The feeding period of 2 weeks was determined by previous reports, which showed remarkable changes in lipid profiles and hepatic gene expression after ingestion of food ingredients(Reference Sugiyama, Yamakawa and Kawagishi24–Reference Shimada, Yamakawa and Morita26). Furthermore, the maximum dose of capsanthin (0·49 mmol/kg diet) was chosen to produce an appreciable accumulation in the liver, according to previous experiments(Reference Ito, Kurabe and Ishiguchi27, Reference Narisawa, Fukaura and Hasebe28).
In experiment 1, after a maintenance period of 1 week, rats were divided into five groups of six animals each, with similar average body weights. The control group was fed a basal diet (BD), and the experimental groups were fed a basal diet with paprika powder (PAP), paprika extract (EXT), residue of paprika extract (RES) or purified capsanthin (CAP), as shown in Table 1. The diets and water were given ad libitum for 2 weeks. Faeces were collected and measured for 3 d at the end of the experiment.
Table 1 Composition of experimental diets in experiment 1 (g/kg diet)
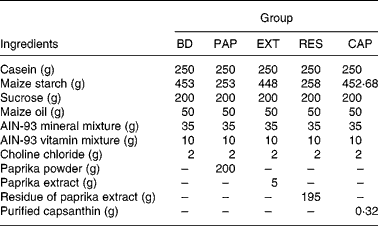
BD, basal diet (capsanthin concentration 0·00 mmol/kg diet); PAP, basal diet with paprika powder (0·49 mmol/kg diet); EXT, basal diet with paprika extract (0·32 mmol/kg diet); RES, basal diet with residue of extract (0·12 mmol/kg diet); CAP, basal diet with purified capsanthin (0·49 mmol/kg diet); AIN, American Institute of Nutrition.
In experiment 2, rats were divided into three groups of eight animals. The groups were fed either a basal diet (control) or a basal diet supplemented with one of two different capsanthin concentrations (low-capsanthin diet (LOW), 0·16 g purified capsanthin/kg diet; high-capsanthin diet (HIGH), 0·32 g purified capsanthin/kg diet). The diet capsanthin concentrations were 0·25 and 0·49 mmol/kg diet, respectively. The diets and water were provided ad libitum for 2 weeks. The concentration of liver capsanthin and relative mRNA concentrations of hepatic genes were measured in this experiment.
All blood samples were obtained from non-fasted rats. The concentrations of plasma total cholesterol, HDL-cholesterol and TAG were measured manually using commercial in vitro enzymic test kits (Wako, Tokyo, Japan).
The previously described method(Reference Ojima, Sakamoto and Ishiguro16) was modified and used for measuring carotenoids in liver. Briefly, the tissue was homogenised and saponified by the addition of 60 % KOH and 3 % butyrated hydroxytoluene in ethanol, heated at 40°C for 30 min, and subsequently extracted (twice) with hexane–dichloromethane (4:1, v/v). The supernatant fraction was dried and reconstituted in a hexane–acetone–ethanol–toluene solvent. Analyses were performed using a Shimadzu SPD-M10 vp diode array detector (Shimadzu, Kyoto, Japan) and C30 carotenoid column (5 μm, 250 × 4·6 mm; YMC, Wilmington, NC, USA) with a flow rate of 1·0 ml/min.
Experimental diets were stored at − 30°C until required, with fresh food provided daily. Food intake was measured daily, and body weight and water intake were measured three times per week.
Real-time quantitative PCR analysis of gene expression
Hepatic gene expression (relative mRNA concentrations) was measured in the rats from experiment 2. For analysis of gene expression, total RNA was extracted from rat liver samples using Trizol reagent (Invitrogen, Tokyo, Japan) according to the manufacturer's instructions. RNA was spectrophotometrically quantified (A260) and its integrity verified by agarose gel electrophoresis using ethidium bromide for visualisation. Total RNA was reverse transcribed with a PrimeScript RT reagent kit (Takara Bio Inc., Shiga, Japan) for cDNA synthesis. The relative mRNA quantities of ATP-binding cassette transporter A1 (ABC-A1), apoA1, apoA5, apoC3, hepatic lipase, lecithin cholesterol acyltransferase (LCAT), lipoprotein lipase (LPL) and scavenger receptor class B type 1 (SR-B1) were measured by real-time quantitative PCR using SYBR Green I (Takara Bio Inc.) and the ABI PRISM 7000 Sequence Detection System (Applied Biosystems Japan Ltd, Tokyo, Japan). Real-time quantitative PCR was performed using a ninety-six-well PCR plate and a reaction mixture (50 μl) containing 25 μl SYBR Premix Ex Taq™ II (2 × ), 1 μl ROX Reference Dye (10 × ), 16 μl Rnase-free water (all from Takara Bio Inc.), 2 μl forward primer (10 μm), 2 μl reverse primer (10 μm) and 4 μl template. The primer oligonucleotides were selected using the online primerselect system (Takara Bio Inc.) and Primerpairs software (Applied Biosystems Japan Ltd) from database sequences. The primer sequences used for RT-PCR were as follows: 5′-CAG CAA CTA CAG TGG CGG TAA CA-3′ (forward) and 5′-AAT GCT TAG GGC ACA ATT CCA CA-3′ (reverse) for rat ABC-A1 (NM_178095); 5′-ATC TAA AGG TTG TGG CCG AGG A-3′ (forward) and 5′-CTC GAT CAG GGT AGG GTG GTT C-3′ (reverse) for rat apoA1 (NM_012738); 5′-GGA GTG TGT ACA TCC CTG CCA GT-3′ (forward) and 5′-ACT GCA GAG GGC TCA GTT CCT TAT T-3′ (reverse) for rat apoA5 (NM_080576); 5′-ATC CTT GCT GCT GGG CTC TAT G-3′ (forward) and 5′-TTC AGG GAT TTG AAG CGA TTG TC-3′ (reverse) for rat apoC3 (NM_012501); 5′-GGC ACA GTC AAG GCT GAG AAT G-3′ (forward) and 5′-ATG GTG GTG AAG ACG CCA GTA-3′ (reverse) for rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH; NM_017008); 5′-ACC CGG AAA CAC TGC AGG AG-3′ (forward) and 5′-GTT GGG ACT GTC GGG ACT TCA-3′ (reverse) for rat hepatic lipase (NM_012597); 5′-CCC AAG GCT GAA CTC AGT AAC CA-3′ (forward) and 5′-CGG TAG CAC AGC CAG TTT ACC A-3′ (reverse) for rat LCAT (NM_017024); 5′-CCA ATC GTT AGC ATT TCG TTT GAG-3′ (forward) and 5′-TTG CGC AGT GCA GAA TTT GA-3′ (reverse) for rat LPL (NM_012598); 5′-GTT CCG TGA AGA TGC AGC TGA G-3′ (forward) and 5′-AAC CAC AGC AAT GGC AGG ACT AC-3′ (reverse) for rat SR-B1 (NM_031541). For determination of mRNA concentration, a threshold cycle (Ct) and amplification efficiency were obtained from each amplification curve using the Applied Biosystems software 1.1 (Applied Biosystems Japan Ltd). The expression signal of the housekeeping gene GAPDH served as an internal control for normalisation.
Statistical analysis
Results are expressed as mean values and standard deviations for six or eight rats. Data were analysed by one-way ANOVA. If significance was observed, post hoc pairwise comparisons were conducted using Tukey's test. The correlations were investigated by Pearson's coefficient test. All the analyses were performed with the SPSS 15.0J software computerised statistical analysis program (SPSS Japan Inc., Tokyo, Japan). P < 0·05 was considered statistically significant.
Results
Effects of paprika extracts on body weight and plasma lipids in rats (experiment 1)
Diets containing different paprika fractions, paprika powder (PAP), paprika extract (EXT), residue of paprika extract (RES) and purified capsanthin (CAP), were examined in this experiment. There were no noticeable differences in body-weight gain, food intake and liver weight among the groups (Table 2). The wet faecal weights in the PAP and RES groups were significantly (P < 0·05) higher than those in the basal diet (BD), EXT and CAP groups. There were no detectable differences in plasma total cholesterol and TAG concentrations. However, the HDL-cholesterol concentration in the CAP group was significantly (P < 0·05) higher than that in the BD group. Significant differences in HDL-cholesterol concentration were not observed between the PAP, EXT and RES groups, and the BD and CAP groups. However, using Pearson's correlation analysis, a statistically significant correlation (r 0·567; P < 0·001) was found between the capsanthin concentration in the diet and the plasma HDL-cholesterol concentration.
Table 2 Body weight, food intake, liver weight, faecal weight and plasma lipid contents in rats fed basal and experiment 1 diets for 2 weeks
(Mean values and standard deviations for six rats per group)

BD, basal diet; PAP, basal diet with paprika powder; EXT, basal diet with paprika extract; RES, basal diet with residue of extract; CAP, basal diet with purified capsanthin.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Body weight at the end of the 2-week feeding period.
† Wet faecal weight for 3 d at the end of the 2-week feeding period.
Effect of capsanthin on body weight, plasma lipid and hepatic mRNA levels (experiment 2)
Two different concentrations of capsanthin were compared in this experiment. HDL-cholesterol levels tended to increase (17 % increase) when capsanthin was administered at a low dose (LOW group) and were significantly raised when administered at a high dose (HIGH group; P < 0·01; 30 % increase). No significant differences were observed in body-weight gain, food intake, liver weight, and plasma total cholesterol and TAG concentrations among the groups (Table 3). There was no capsanthin content in the liver of animals fed the BD, but it was increased markedly with the administration of capsanthin-containing diets (P < 0·001 between each group). Statistically significant relationships were observed between plasma HDL-cholesterol concentrations and capsanthin concentrations in the diet (r 0·597; P < 0·005) or liver (r 0·583; P < 0·005) (Fig. 1).
Table 3 Body weight, food intake, liver weight, plasma lipid contents and liver capsanthin concentration in rats fed basal and experiment 2 diets for 2 weeks
(Mean values and standard deviations for eight rats per group)
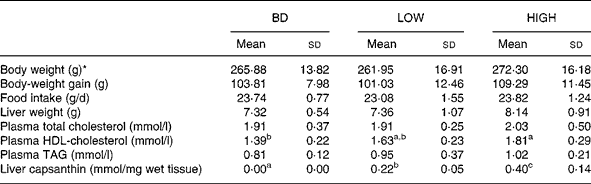
BD, basal diet; LOW, basal diet with purified capsanthin (0·16 g/kg diet); HIGH, basal diet with purified capsanthin (0·32 g/kg diet).
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Body weight at the end of the 2-week feeding period.

Fig. 1 Correlation (r 0·583; P < 0·005) between plasma HDL-cholesterol concentration and capsanthin concentration in the liver of rats fed the basal diet (○), the low capsanthin-containing diet (△) and the high capsanthin-containing diet (□) for 2 weeks.
Real-time quantitative PCR analyses were performed to measure mRNA levels in rat liver from the three dietary groups (Fig. 2). Administration of capsanthin caused a significant increase (P < 0·05) in the relative quantity of apoA5 mRNA levels in LOW and HIGH groups. Those levels were positively correlated with the capsanthin concentration in liver (r 0·514; P < 0·01). Also, the relative quantity of LCAT mRNA in the HIGH group was significantly higher than that of the BD group. No significant differences or correlations were observed for relative quantities of other mRNA levels related to HDL-cholesterol metabolism in this experiment.

Fig. 2 Quantitative analysis of liver mRNA of rats fed the basal diet (BD; □), the low capsanthin-containing diet (![]() ) or the high capsanthin-containing diet (
) or the high capsanthin-containing diet (![]() ) for 2 weeks. For each gene, the mRNA level is shown relative to its level in the BD group (set at 1·0). ABC, ATP-binding cassette transporter; HL, hepatic lipase; LCAT, lecithin cholesterol acyltransferese; LPL, lipoprotein lipase; SR-B1, scavenger receptor class B type 1. Values are means for eight rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the BD group (P < 0·05).
) for 2 weeks. For each gene, the mRNA level is shown relative to its level in the BD group (set at 1·0). ABC, ATP-binding cassette transporter; HL, hepatic lipase; LCAT, lecithin cholesterol acyltransferese; LPL, lipoprotein lipase; SR-B1, scavenger receptor class B type 1. Values are means for eight rats per group, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the BD group (P < 0·05).
Discussion
Previously, preliminary experiments revealed that administration of paprika increased plasma HDL-cholesterol in rats (K Aizawa and T Inakuma, unpublished results). The present study was conducted to examine what constituent of paprika (C. annuum) principally affected lipid metabolism in vivo in rats, hypothesising that capsanthin, the main carotenoid in paprika, might be responsible. As shown in Table 2, administration of purified capsanthin significantly increased (P < 0·05; 44 % increase) plasma HDL-cholesterol levels. Furthermore, administration of two different capsanthin concentrations resulted in a dose-dependent increase in plasma HDL-cholesterol in experiment 2 (Table 3). Consequently, we concluded that the main active component was capsanthin. This conclusion was supported by the results from experiment 1, which showed that a significant correlation (r 0·567; P < 0·001) was found between diet capsanthin concentrations and plasma HDL-cholesterol concentrations.
In experiment 1, it was observed that faecal weight in the PAP and RES groups was significantly higher (P < 0·05; 202 and 213 % increase compared with the BD group, respectively) than that of the BD, EXT and CAP groups. These effects were attributed to the dietary fibre component of the experimental diets. It is reported that the administration of dietary fibre results in a dose-dependent increase in faecal weight(Reference Haack, Chester and Vollendorf29, Reference Cummings and Speiller30). It was estimated that paprika powder (PAP) and residue of paprika extract (RES) are composed of 51·8 and 53·1 % carbohydrate (including dietary fibre), respectively. These factors raised the dietary fibre content by approximately 10 % in PAP and RES experimental diets. Additionally, it has been indicated that certain kinds of dietary fibre influence total cholesterol or TAG levels in blood, and increase bile acid or neutral sterol excretions(Reference Jenkins, Kendall and Axelsen31, Reference Marlett32). In the present study, we did not delve deeper into the effect of dietary fibre, because we were particularly intrigued by the ability of capsanthin to increase HDL-cholesterol levels. The cholesterol-lowering effects of dietary fibre are primarily observed in the LDL portion, and dietary fibre did not produce substantial changes in HDL-cholesterol levels(Reference Gallaher, Bowman and Russell33). We may be able to observe blood lipid changes induced by the addition of paprika-derived dietary fibre in the experimental diet if the experimental duration is extended.
Quantitative analysis of liver mRNA in the capsanthin-administered group showed a significant increase in apoA5 and LCAT expression, without notable changes in ABC-A1, apoA1, apoC3, hepatic lipase, LPL, and SR-B1 (Fig. 2). HDL is synthesised through a complex pathway(Reference Zannis, Chroni and Kypreos34). HDL assembly initially involves cell surface ABC-A1 transporter-mediated transfer of phospholipids and cholesterol to extracellular lipid-poor apoA1. This is followed by remodelling of the plasma compartment of HDL particles, by the esterification of cholesterol by the enzyme LCAT, the exchange between HDL and other lipoproteins of both apolipoproteins (apoA1 and other less abundant apolipoproteins) and lipids, and the putative transfer of additional cellular cholesterol to the growing particles by SR-B1(Reference Krieger35). Finally, HDL lipid hydrolysis is mediated by various lipases (LPL, hepatic lipase and endothelial lipase) and exchange of lipids by the cholesteryl ester transfer protein and by the phospholipid transfer protein. The increase in LCAT and apoA1 gene expression would normally contribute to an increase in HDL-cholesterol concentrations. In the present study, the hepatic apoA1 mRNA level among all groups was not significantly different, but LCAT mRNA was significantly increased by capsanthin administration. Therefore, one reason for the higher concentration of HDL-cholesterol in rats fed capsanthin diets could be due to alterations in the expression and/or activity of LCAT.
The role that LCAT plays in HDL metabolism has been established in both patients and animals with LCAT deficiency, as well as in animals over-expressing human LCAT. LCAT deficiency is associated with severely reduced concentrations of HDL, whereas transgenic animals over-expressing LCAT show markedly higher plasma HDL levels(Reference Hoeg, Santamarina-Fojo and Bérard36–Reference Mehlum, Staels and Duverger38). However, a number of studies point to a relationship between LCAT and apoA1 expression(Reference Ettinger, Miller and Albers39, Reference Auerbach and Parks40).
The reason for the discrepancy in the present study that hepatic apoA1 mRNA levels were not correlated with LCAT mRNA levels is unclear. On the other hand, although the plasma apoA1 concentration was drastically reduced in LCAT knockout mice, there was no reduction in hepatic apoA1 mRNA(Reference Forte, Oda and Knoff41), suggesting that both genes may not be regulated in a coordinated manner. At present, the molecular mechanisms regulating LCAT are not well understood. However, capsanthin may only regulate LCAT mRNA expression, without affecting apoA1 mRNA expression.
Additionally, a significant increase in hepatic apoA5 mRNA levels was observed by administration of capsanthin (Fig. 2). ApoA5 is a newly discovered apolipoprotein, which was identified independently by two groups(Reference Pennacchio, Olivier and Hubacek42, Reference van der Vliet, Sammels and Leegwater43). Disruption of the apoA5 gene in mice resulted in hypertriacylglycerolaemia, whereas overexpression led to decreased plasma TAG concentrations, thus establishing an important role for this protein in TAG homeostasis(Reference O'Brien, Alborn and Sloan44, Reference Pennacchio and Rubin45). Marçais et al. (Reference Marçais, Verges and Charrière46) reported that a mutation in the apoA5 gene led to severe hypertriacylglycerolaemia by exerting a dominant-negative effect on the plasma lipolytic system for TAG-rich lipoproteins. They suggested that apoA5 accelerated lipolysis by facilitating the interaction of TAG-rich lipoproteins with heparin sulfate-proteoglycan-bound LPL. In the present study, plasma TAG levels did not change appreciably, in spite of the increase in the apoA5 mRNA level. One possibility is that the LPL mRNA level did not change (Fig. 2), indicating that LPL activity was not enhanced by administration of capsanthin. Also, only a small amount of capsanthin was supplemented into the normal diet for a short period of time (2 weeks). Alteration of the quantity and duration of capsanthin administration may clarify these remarkable observations.
Qu et al. (Reference Qu, Perdomo and Su47) indicated that apoA5 exerted an effect on HDL-cholesterol metabolism in APOC3 transgenic mice. They reported that increased apoA5 production promoted α-HDL formation, resulting in significant increases in both the number and size of HDL particles. In addition, increased apoA5 levels were associated with enhanced LCAT activity. It appears that HDL particles with increased apoA5 content were associated with increased cholesterol-loading capacity. This hypothesis is consistent with the present results. Capsanthin may be thought to act in a similar manner, increasing cholesterol efflux to HDL particles by increasing apoA5 levels and/or enhancement of LCAT activity, thereby resulting in significant increases in plasma HDL-cholesterol.
In order to test this possibility in human subjects in an exploratory open study, we administered 320 g paprika juice (made from paprika paste (TAT, Istanbul, Turkey) diluted with water), containing 38·9 μmol capsanthin per d for 2 weeks to nine healthy males. This treatment resulted in a significant (P < 0·05) increase (26 %) in plasma HDL-cholesterol concentrations (from 1·63 (sd 0·10) mmol/l before paprika juice supplementation to 2·05 (sd 0·15) mmol/l after 2 weeks of consumption). There was a concomitant significant increase in plasma capsanthin levels (from 0·008 (sd 0·001) to 0·136 (sd 0·090) μmol/l; P < 0·0005) and a tendency towards increased LCAT activity (from 18·86 (sd 10·37) to 26·48 (sd 8·10) U; P = 0·086). In addition, there were no detectable differences in cholesteryl ester transfer protein levels and other lipid levels during the experiment (data not shown). This study was conducted as a pilot study by using a small number of subjects without a control group. Further, it is not feasible to transpose doses used in animal studies for use in human studies, because the bioavailability of carotenoids is distinctly different among species. Therefore, it is difficult to predict effective dosages due to species differences. However, the results are encouraging and justify further, more elaborate studies. Notably, these results do not contradict the results of the animal studies presented here, and they provide some indication as to the possible expectation that human subjects would exhibit responses similar to those in rats.
It has not been established whether other carotenoids in food, such as lycopene, α-carotene, β-carotene, lutein and zeaxanthin, have the same effect as capsanthin. A number of reports have shown that blood concentrations of certain carotenoids were significantly correlated to HDL-cholesterol level(Reference Brady, Mares-Perlman and Bowen17, Reference Slattery, Jacobs and Dyer18). However, this same report(Reference Brady, Mares-Perlman and Bowen17) and another recent report(Reference Sluijs, Beulens and Grobbee48) indicated that dietary carotenoid intake was not associated with HDL-cholesterol level. Interestingly, Waters et al. (Reference Waters, Clark and Greene49) reported that there was a positive correlation between HDL size and plasma lutein level. This finding suggests that lutein may affect the construction or function of HDL. Furthermore, xanthophylls, including capsanthin and lutein, are distributed to HDL in larger amounts than to LDL(Reference Oshima, Sakamoto and Ishiguro14). Thus, it is possible that not only capsanthin, but also other xanthophylls, might affect plasma HDL-cholesterol level.
In conclusion, the present study shows that capsanthin, the main carotenoid in paprika, has a plasma HDL-cholesterol-raising effect accompanied by a significant increase in hepatic apoA5 and LCAT mRNA. Additionally, we think that it is normal and reasonable that the small amount of capsanthin added to a normal diet, administered for a short period in rats, would not produce drastic changes in plasma lipid profiles and hepatic mRNA levels. Meanwhile, it is interesting and meaningful to be able to report that dietary intake of paprika can improve plasma lipid profiles.
Acknowledgements
The present study was partially supported by the New Food Creation Technology Research Association, a project of the Ministry of Agriculture, Forestry and Fisheries of Japan.
K. A. designed all experiments, carried out the main experimental work and wrote the manuscript. T. I. supported the idea of the manuscript and, as the director of the Biogenics Research Department, provided a number of suggestions. The authors also wish to thank the staff of the Biogenics Research Department, Kagome Co., Ltd, for their help with sample and experiment preparation.
None of the authors had any personal or financial conflict of interest.







