Almost 20% of newborns with simple or complex CHDs might have a duct-dependent pulmonary circulation after birth, Reference Bernier, Stefanescu, Samoukovic and Tchervenkov1 with a need for an intravenous prostaglandin E1 administration to ensure patency of ductus arteriosus. Reference Reddy and Saxena2 In duct-dependent pulmonary circulation, surgical or interventional manoeuvres are needed to create stable and reliable systemic-to-pulmonary shunt within a few days from birth. Surgical Blalock–Taussig shunt warrants an adequate pulmonary perfusion for several months. Reference Wespi, Callegari and Quandt3–Reference McMullan, Permut, Jones, Johnston and Rubio5 However, morbidity and mortality hazards remain high, >10% in high-risk patients. Reference Dave6
In the past two decades, patent arterial duct stenting emerged as a valuable alternative to surgical palliative procedures. Reference Aggarwal, Petit, Glatz, Goldstein and Qureshi7,Reference Gibbs, Rothman, Rees, Parsons, Blackburn and Ruiz8 Nowadays, it is an established and reliable alternative to surgical shunts, in newborns with both simple of complex types of CHD with a duct-dependent pulmonary circulation.
A multicentre comparison of palliative patent arterial duct stent and Blalock–Taussig shunt for infants with duct-dependent pulmonary circulation found that there was no statistical difference between treatment strategies in the hazard of a composite outcome of death or unplanned reintervention to treat cyanosis; however, those treated with a patent arterial duct stent has lower procedural complications, fewer necessity of mechanical ventilation, and a shorter neonatal ICU and hospital length of stay. Reference Tseng, Truong and Peck9–Reference Santoro, Gaio and Giugno11 Furthermore, patent arterial duct stenting promotes a significant and balanced pulmonary artery and pulmonary vascular bed growth. Reference Glatz, Petit and Goldstein10,Reference Santoro, Gaio and Giugno11 In several centres, arterial duct stenting, when feasible, is the first line choice in infants with duct-dependent pulmonary circulation. Reference Bentham, Zava and Harrison12 On the other hand, tortuous patent arterial duct anatomies represent a challenge, and, sometimes, a contraindication to patent arterial duct stenting. Reference Qureshi, Goldstein and Glatz13 Thus, several techniques were proposed to overcome anatomical challenges.
In this paper, we reported the early experience with a small 4 Fr guiding catheter, TorqVueTM LP, that might be considered to approach tortuous anatomies.
History of presentation
Case 1 neonatal pulmonary valve stenosis
The patient was female, born at 39 weeks’ gestation, her weight was 3.3 kg, natural onset pregnancy and vaginal birth.
She was evaluated at 3 hours of life for persistent oxygen saturation <80% and cyanosis onset. Cardiac physical examination was performed, and it highlighted a 3/6 heart murmur in the left sternal border; echocardiography showed isolated critical pulmonary valve stenosis (pulmonary annulus 6 mm Z score –2.61), severe tricuspid regurgitation with a max velocity of 5.7 m/s, a hypertrophic and mildly reductive right ventricle, and a tortuous patent arterial duct with a total length of 18 mm. Prostaglandin E1 infusion was started (15 ng/kg/min) to achieve cardiac malformation target oxygen saturation.
The patient was admitted to our cath lab at 2 days of life, and prostaglandin E1 infusion was maintained during the procedure.
Under general anaesthesia, after administration of antibiotic prophylaxis (Cefazolin 25 mg/kg) and un-fractioned heparin (100 IU/kg), the haemodynamic study confirmed a right ventricle/aortic pressure ratio of 1.8.
An effective balloon valvotomy was performed with a 6x20 mm Tyshak Mini® balloon (NuMED, Inc. Hopkinton, NY). Residual, infundibular, and invasive pressure gradient was 15 mmHg. Thus, metoprolol (1mg/kg/dose 3 times a day) was started to improve right ventricle diastolic compliance.
Prostaglandin E1 infusion was kept for a further 7 days. Finally, we tried, unsuccessfully, to withdraw prostaglandin E1. The oxygen saturation dropped below 75%. Thus, the infusion was restarted and a percutaneous patent arterial duct stenting implantation was planned due to a persisting ductal-dependent pulmonary circulation.
The patient was re-admitted to our cath lab. Prostaglandin E1 infusion was stopped some hours before the procedure to achieve a better stent grip when deployed in the patent arterial duct. Under general anaesthesia after administration of antibiotic prophylaxis and un-fractioned heparin administration, through a venous femoral route using a 4 Fr vascular introducer.
Patent arterial duct was crossed from the pulmonary side, with a 0.014” coronary guidewire. The TorqVueTM LP catheter was advanced up to the aorta, where the angiographies showed a tortuous patent arterial duct with knees, one on the proximal side and the other one on the distal side (Figure 1). The total duct length was of 18 mm. A 4x20 mm bare coronary stent (RebelTM Boston Scientific, Marlborough, MA, USA) was advanced directly inside the TorqVueTM LP catheter and successfully deployed. Post-implant angiography showed a good stent placement, as did the echocardiography, and a mild protrusion, not haemodynamically significant, in left pulmonary artery. A 5mg/kg aspirin therapy was started.
At a 2-month follow-up, the patient’s oxygen saturation was 94%, the weight gain was adequate, and the echocardiography showed a good stent position and an adequate intra-luminal flow.
Case 2 complex neonatal heart disease
A male was born at 32 + 2 weeks’ gestation by a caesarean section due to a, natural onset mono-chorionic mono-amniotic twin pregnancy. His birth weight was 1.6 kg.
The fetal echocardiography performed in our institution diagnosed a complex congenital heart malformation: double outlet right ventricle with malposition of great vessels mitral and pulmonary atresia.
At the delivery, oxygen saturation was in normal range for a newborn. Soon after, the patient was admitted to our neonatal ICU to monitor oxygen saturation. Echocardiography confirmed the diagnosis of double outlet right ventricle with malposition of great vessels, mitral atresia, normal tricuspid valve, tricuspid aortic valve, pulmonary atresia, and a patent ductus arteriosus with fetal, tortuous anatomy, and 3.4 mm of narrow diameter.
Due to decreasing values of oxygen saturation in the first hour of life prostaglandin E1 infusion 15 ng/kg/min was started, with an immediate relief of the oxygen saturation. After the first period of stable oxygen saturation, following increased desaturation crisis, prostaglandin E1 infusion was increased up to 25 ng/kg/min. Thus, when the patient reached 2 kg of weight, a patent arterial duct stenting was planned.
Under general anaesthesia after administration of antibiotic prophylaxis and un-fractioned heparin administration, through a surgical left carotid 4 Fr access, angiography was performed directly through the sheath to visualise the patent arterial duct. The fluoroscopy image confirmed echocardiographic findings: a tortuous patent arterial duct with a stenotic section in the third distal end (Figure 2). A MaverickTM (Boston Scientific, Marlborough, MA, USA) coronary balloon was advanced and inflated inside the duct to size the patent arterial duct length and to get straighten out the intricacies; however, it proved too short to cover the total patent arterial duct length. Thus, we first tried with a 4x24 mm bare coronary RebelTM stent. Unfortunately, during the positioning manoeuvres, the patent arterial duct changed deeply in shape and the stent resulted to be too long.
We removed the stent. Angiography from the sheath showed a widespread spasm of both pulmonary branches, and especially the left lung had not contrast enhancement as the right had and the patent arterial duct appeared to be curled on the stent.
Thus, a shorter (3.5x16 mm) bare RebelTM coronary stent placement was attempted. It failed due to the patent arterial duct morphology (patent arterial duct spasm associated to the tortuous anatomy). Finally, to improve the trackability and to make the patent arterial duct as straight as possible, we advanced a TorqVueTM LP 4 Fr catheter with a 2.7 Fr microcatheter, using the “mother in child” technique. Once the TorqVueTM LP catheter was in the left branch of the pulmonary artery, the 3.5x16 mm RebelTM coronary stent was advanced in the catheter and successfully deployed. Final angiography was performed by using the same TorqVueTM LP catheter.
Aspirin 5mg/kg was started immediately after the procedure; in addition, Heparin 10/UI/kg/h, intravenous was administrated for the first 24h after stent placement.
Seven days after the procedure, the patient was discharged from neonatal ICU.
No major vascular access complication was registered.
Discussion
Patent arterial duct stenting is a neonatal palliative procedure used in both simple and complex heart diseases. To implant a coronary stent, a 4 Fr sheath or a 5 Fr guiding catheter is needed. The vascular approach can be arterial or venous. The venous approach is preferable because it allows the operator to use larger sheath and a wide range of stent and catheters. However, the feasibility of retrograde approach is tied up to the presence of an antegrade pulmonary flow, and to a relatively straight course between inferior vena cava and pulmonary artery. In all the other cases, the arterial approach should be preferred.
Various techniques were described in literature for patent arterial duct stenting: the “bare” technique allows the use of smaller sheaths but, at the same time, precludes the execution of angiographies during the stent deployment. Alternatively, a long sheath catheter (Flexor 4 Fr), or a 5 Fr guiding catheter can be used to have additional support during stent placement and to perform angiograms during implantation, with a higher risk of vascular injury. This approach can be considered in challenging anatomies because of the risk of spasm or dissection is high, like in case #2, where we faced a severe duct spasm despite a straight access to the arterial duct by a carotid approach.
TorqVueTM LP 4 Fr catheter was designed for percutaneous patent arterial duct closure in premature babies. This catheter can be used in a 4 Fr sheath, either from venous or from arterial approach. To improve the handling, the trackability, and to reduce the discrepancies between catheter’s lumen and the guidewire, a microcatheter can be used. Furthermore, the use of the same catheter both for the diagnostic and the interventional manoeuvres reduces the procedural time, the number of catheter’s changes, and the risk of a guidewire displacement. In summary, TorqVueTM LP catheter offers higher support that increases the feasibility of stent deployment also in case of long stent and/or tortuous patent arterial duct anatomies, as shown the case #2. This technique might be useful to improve the procedural success, reduce the risk of vascular injuries, and minimise the sheath size and vascular access complications. This catheter was approved for low weight newborns (up to 700 g). Thus, it might widespread the pool of patients eligible for this procedure.
Conclusion
Percutaneous patent arterial duct stenting is a well-established procedure in clinical practice. TorqVueTM LP catheter might be helpful in challenging anatomies to increase the feasibility of the procedure and treduce procedural times, guidewire displacement during the stent deployment, and the risk of vascular injuries.
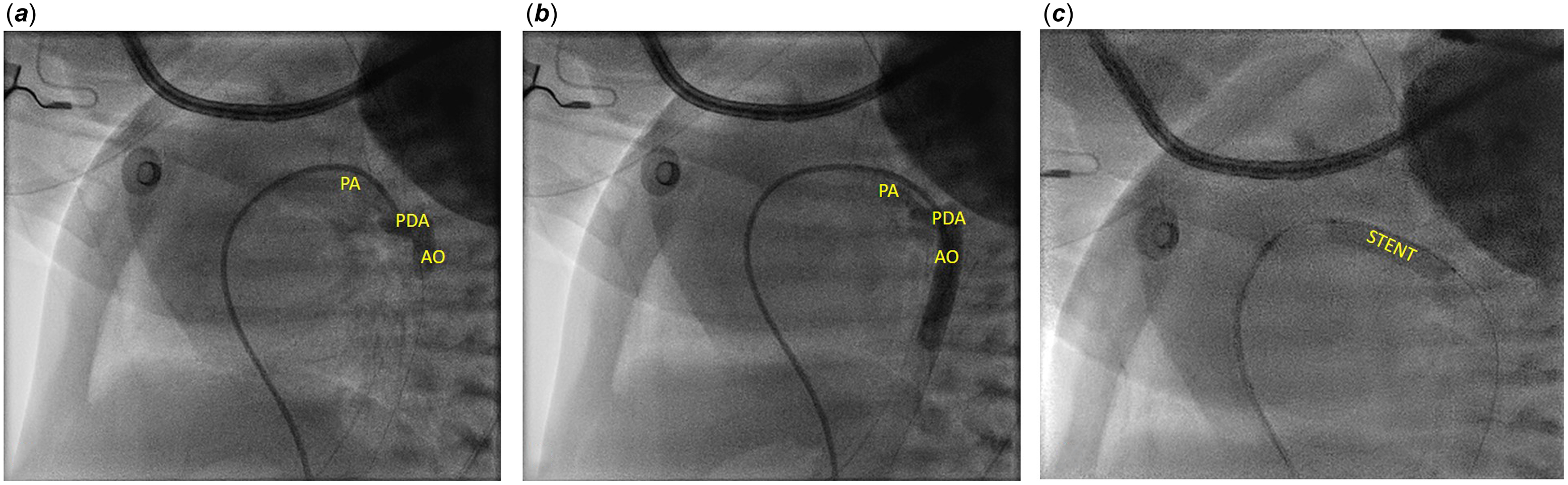
Figure 1. A. and B. Angiography performed with 4 Fr TorqVueTM LP catheter. C. Coronary Rebel stent implanted using 4 Fr TorqVueTM LP catheter. AO = aorta; PDA = patent ductus arteriosus; PA = pulmonary artery; STENT = stent.
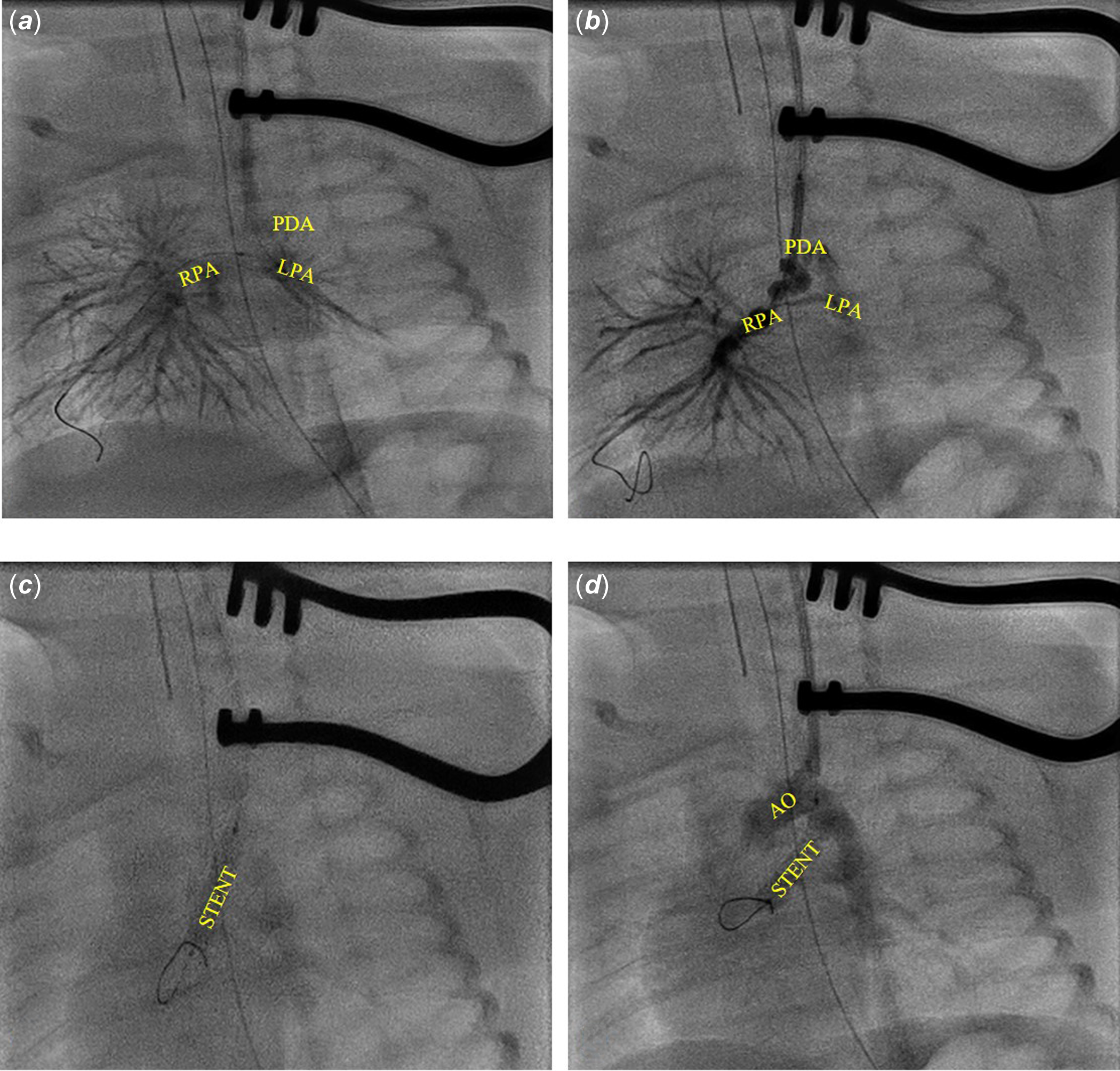
Figure 2. A. Angiography by the left carotid access. B. 3.5x 24 RebelTM stent protruding in left carotid and the PDA curled on the stent. C. 3.5x 16 mm RebelTM stent deployment. D. Angiography after the 3.5x16 mm RebelTM stent deployment. AO = aorta; PDA = patent ductus arteriosus; RPA = right branch pulmonary artery; LPA = left branch pulmonary artery; STENT = stent.
Competing interests
None.





