CVD is the leading cause of morbidity and mortality worldwide(Reference Wang, Naghavi and Allen1). Annually, more than 17 million deaths are attributable to CVD, with these deaths anticipated to increase by 30 % over the next decade(Reference Smith, Collins and Ferrari2). Most CVD are potentially preventable, and it is important to design and implement effective strategies to prevent CVD.
Observational studies have shown fruit consumption was associated with a lower risk for developing total CVD, CHD and stroke(Reference Aune, Giovannucci and Boffetta3). Among kinds of fruits, strawberries are well known for being rich in polyphenol, vitamins and minerals(Reference Giampieri, Forbes-Hernandez and Gasparrini4,Reference Giampieri, Alvarez-Suarez and Battino5) . In fact, strawberries have been ranked as a top source of polyphenol and antioxidant capacity among foods consumed in the USA(Reference Halvorsen, Carlsen and Phillips6). Strawberry polyphenols have been shown to have direct and indirect antimicrobial, anti-allergy and antihypertensive properties, inhibit the activities of some physiological enzymes and receptors and protect from oxidative stress-related diseases(Reference Giampieri, Forbes-Hernandez and Gasparrini4).
The biological and functional properties of strawberries have been studied in animal models(Reference Giampieri, Forbes-Hernandez and Gasparrini4) and extended to humans in a few epidemiological studies(Reference Gaziano, Manson and Branch7–Reference Sesso, Gaziano and Jenkins9). In the past decade, emerging interventional trials have been conducted to examine the health effects of strawberries in humans(Reference Zunino, Parelman and Freytag10–Reference Amani, Moazen and Shahbazian20). However, most of these trials had small sample sizes, which might have resulted in insufficient statistical power. Additionally, the results varied across studies, with some showing strawberries had a beneficial effect on cardiovascular risk factors whereas others did not. Therefore, we aimed to evaluate the treatment effects of strawberry interventions on cardiovascular risk factors by conducting a meta-analysis of randomised controlled trials (RCT).
Methods
Literature search
This meta-analysis was conducted and reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guideline for reporting meta-analyses of RCT(Reference Moher, Liberati and Tetzlaff21). Two authors (Q. G. and J.-Y. D.) independently searched PubMed, Web of Science and Scopus for relevant studies published before 19 May 2019. The search was updated on 20 February 2020. The endpoints of interest were blood pressure, total cholesterol (TC), LDL-cholesterol, HDL-cholesterol, TAG, fasting blood glucose, endothelial function and inflammatory factors. The keywords used for the literature search were ‘strawberry’, ‘hypertension’, ‘blood pressure’, ‘lipid’, ‘cholesterol’, ‘LDL’, ‘HDL’, ‘triglyceride’, ‘blood glucose’, ‘endothelial function’, ‘inflammation’ and ‘trial’. The search strategy is shown in the online Supplementary material. We also conducted a manual review of reference lists of the identified studies. No effort was made to retrieve unpublished studies, and there was no restriction to publication date.
Study selection
Studies were selected for analysis if they (1) were RCT (either parallel or crossover); (2) used strawberries as the intervention and had a control group; (3) measured blood pressure, lipid profiles, blood glucose, endothelial function or inflammatory factors at baseline and follow-up and (4) had an intervention longer than 1 week. Studies were excluded if they examined the acute effects of a strawberry intervention within several hours, or when the intervention included fruits other than strawberries.
Data extraction
We extracted the characteristics of each trial included in this meta-analysis. The extracted data included: name of first author, study area, publication year, intervention duration, study design, dose of intervention and control group, sample size, mean age or age range, sex, as well as the mean and standard deviation values for each endpoint at baseline and post-intervention. For studies with more than two follow-up measurements, data from the last follow-up were used. Similarly, for studies with more than two intervention groups, data from the highest dose group were used.
Quality assessment
The quality of the included trials was evaluated using the revised tool for assessing risk of bias in randomised trials(Reference Higgins, Sterne and Savovic22). Each included trial was judged as ‘low risk’, ‘high risk’ or ‘some concerns’ for six aspects: randomisation process, deviations from the intended interventions, missing outcomes, outcome measurement and selection of reported results. An overall risk of bias judgement was then made. Disagreements during study selection, data extraction and quality assessment were resolved by discussion among the authors.
Statistical analysis
For each endpoint, mean net changes between the baseline and follow-up values in the intervention and control groups/periods were calculated. The standard deviations for the net changes were obtained from the original studies or computed using a standard formula(Reference Higgins, Eldridge and Li23). The effect size of the intervention was calculated as the mean difference in the net changes in the intervention and control groups/periods. Heterogeneity between studies was assessed using Cochran’s Q test (P < 0·10 as statistically significant) and the I 2 statistic, which is considered a measure of the inconsistency between studies(Reference Higgins, Thompson and Deeks24). A random-effects model(Reference DerSimonian and Laird25) was used to perform the pooled analysis when P for heterogeneity <0·10; otherwise, a fixed-effects model was selected. We conducted pre-specified stratified analyses and meta-regression analyses by baseline endpoint values to examine whether baseline levels could modify the effects of the intervention. Sensitivity analyses were also performed to test whether individual studies had a considerable impact on the overall results. The risk for publication bias was assessed using Egger’s test(Reference Egger, Davey Smith and Schneider26). A ‘trim and fill’ method(Reference Duval and Tweedie27) was used to correct results when such bias was detected. All analyses were performed using Stata version 12.0.
Results
The flow of the literature search is shown in Fig. 1. The initial search of the electronic databases identified 129 records, but most of these were excluded after scanning the title and abstract. This left twenty-two articles for full-text review. Eleven articles were further excluded because they examined acute effects of a strawberry intervention (n 6), had no outcome of interest (n 2), used cranberries or mixed berries as the intervention (n 2) or had no control group (n 1). Finally, eleven RCT were included in our analysis (blood pressure n 6, lipid profile n 7, blood glucose n 7, C-reactive protein (CRP) n 6). The RCT that used oat bran bread as a control(Reference Jenkins, Nguyen and Kendall13) was not included in the analysis for lipid profile because there is a body of evidence supporting a lipid-lowering effect of oat bran(Reference Hui, Liu and Lang28), and using oat bran as a control may mask the true effect of a strawberry intervention on participants’ lipid profile.

Fig. 1. Flow chart of study selection.
Table 1 shows the characteristics of each included RCT. These eleven trials were published between 2008 and 2017. Seven studies were double-blind RCT, two were single-blind RCT and two were open-label RCT. Six trials used cross-over designs, whereas the others used parallel designs. The interventions included freeze-dried strawberry beverage or powder (n 10) or fresh strawberries (n 1). RCT with a single- or double-blind design used a placebo similar in colour and flavour to strawberries as the control, and the two open-label RCT used oat bran bread or water as a control. The sample size of each RCT was 17–60 participants, with a total of 357 participants in the eleven trials. The intervention duration ranged from 1 to 12 weeks, with a median of 6 weeks. All RCT enrolled middle-aged individuals, except for one trial that enrolled adolescents aged 14–18 years(Reference Djurica, Holt and Ren16). The results of quality assessment of the included RCT are shown in online Supplementary Table S1. In general, most RCT had low risk for overall bias.
Table 1. Characteristics of included randomised controlled trials examining the effects of strawberry intervention on cardiovascular risk factors in men and women
(Mean values and standard deviations; ranges)

X, crossover; SB, single-blind; FDS, freeze-dried strawberries; DB, double-blind.
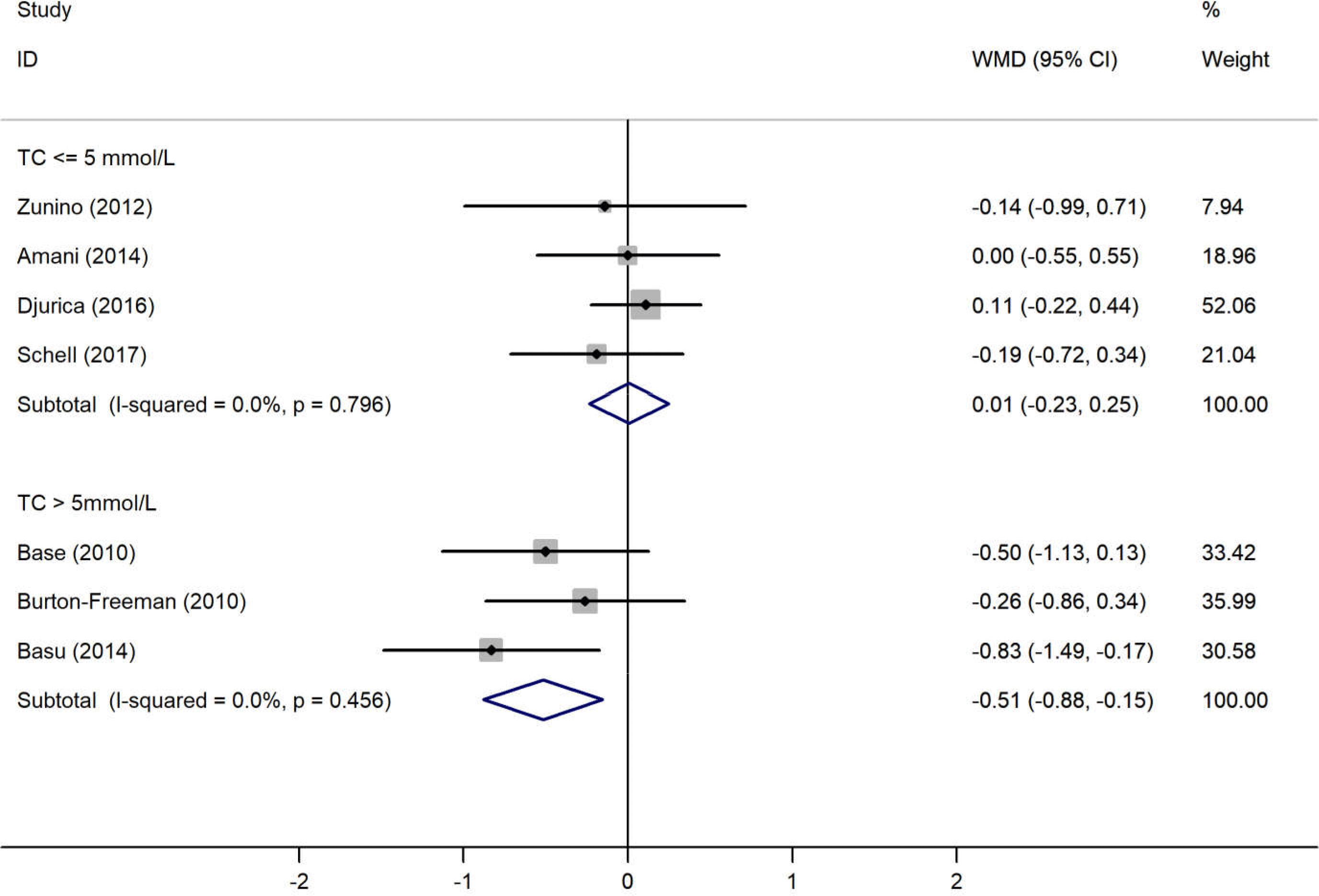
The results of the pooled analysis examining the effects of the strawberry interventions on blood pressure, lipid profile, blood glucose and CRP levels are shown in Table 2. The main analyses showed that the strawberry interventions significantly reduced CRP levels by 0·63 (95 % CI −1·04, −0·22) mg/l but did not affect blood pressure, lipid profile or fasting blood glucose. There was little evidence of heterogeneity throughout the analyses. The analysis stratified by baseline endpoint levels showed the strawberry interventions led to a significant decrease in TC levels among those with baseline TC levels >5 mmol/l (weighted mean difference −0·52 (95 % CI −0·88, −0·15) mmol/l) (Fig. 2). Similarly, the strawberry interventions significantly reduced LDL-cholesterol among those with baseline LDL-cholesterol levels >3 mmol/l (weighted mean difference −0·31 (95 % CI −0·60, −0·02) mmol/l) (Fig. 3). Tests for publication bias using Egger’s regression showed no evidence of such bias in all endpoints, though the test for TC was borderline significant (Table 2). Correcting the potential bias by the ‘trim and fill’ method obtained the same results for TC.
Table 2. Meta-analysis of randomised controlled trials examining the effects of strawberry intervention on cardiovascular risk factors
(Weighed mean differences and 95 % confidence intervals)


Fig. 2. Meta-analysis on the effects of strawberry intervention on total cholesterol (TC) by the baseline levels. WMD, weighted mean difference.

Fig. 3. Meta-analysis on the effects of strawberry intervention on LDL-cholesterol by the baseline levels. WMD, weighted mean difference.
The results of the meta-regression showed that the baseline values of TC and LDL-cholesterol were associated with the magnitude of the treatment effects (online Supplementary Figs. S1 and S2). In the sensitivity analyses, no single RCT showed a substantial impact on the overall pooled results for each endpoint.
Discussion
This meta-analysis of RCT indicated that the strawberry interventions significantly reduced CRP levels but did not affect blood pressure, lipid profile or fasting blood glucose compared with placebo. However, in the sub-group analysis, the strawberry interventions significantly lowered TC and LDL-cholesterol levels in those with high baseline levels. The results of meta-regression indicated that baseline levels of TC and LDL-cholesterol were associated with the treatment effect of strawberry interventions.
The potential cardio-protective effects of strawberry consumption may be attributed to its richness in polyphenols, folate, vitamin C and minerals(Reference Giampieri, Alvarez-Suarez and Battino5,Reference Giampieri, Tulipani and Alvarez-Suarez29) . Anthocyanins are the best-known polyphenolic compounds in strawberries, which have been shown to have antioxidant, anti-inflammation and cardio-protective properties(Reference Afrin, Gasparrini and Forbes-Hernandez30). Studies in animal and cell models showed that gastric lipase(Reference McDougall and Stewart31) and cholesteryl ester transfer protein(Reference Qin, Xia and Ma32) are necessary for lipid generation. One clinical trial involving 120 patients with dyslipidaemia aged 40–65 years showed that anthocyanins could increase cellular cholesterol efflux to serum and decrease the mass and activity of plasma cholesteryl ester transfer protein(Reference Qin, Xia and Ma32). There was also evidence that anthocyanins may enhance ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux in macrophages, which in turn can improve lipid profiles(Reference Xia, Hou and Zhu33). Furthermore, a recent meta-analysis of seventeen RCT showed that anthocyanin supplementation led to significant reductions in TAG (−0·10 (95 % CI −0·16, −0·05) mmol/l), LDL-cholesterol (−0·23 (95 % CI −0·29, −0·52) mmol/l) and apoB (−0·14 (95 % CI −0·17, −0·11) μmol/l)(Reference Shah and Shah34).
It was uncertain why baseline levels of TC and LDL-cholesterol were associated with the treatment effects of strawberry interventions. One explanation may be that individuals with higher baseline levels of these lipids could have more room for improvement compared with those with lower baseline levels. However, the results of the sub-group analyses should be interpreted with caution because they were based on a limited number of RCT.
Several prospective cohort studies have examined the association of anthocyanin intakes with risk for CVD. Cassidy et al. reported that a high intake of anthocyanins was associated with a decreased risk for myocardial infarction among 93 600 women (aged 25–42 years) in the Nurses’ Health Study II (hazard ratio (HR) 0·68; 95 % CI 0·49, 0·96)(Reference Cassidy, Mukamal and Liu35). In addition, those authors detected an 8 % decrease in risk for hypertension (HR 0·92; 95 % CI 0·86, 0·98) in the highest quintile of anthocyanin intake compared with the lowest quintile based on three cohorts: Nurses’ Health Study I with 121 700 female nurses aged 30–55 years, Nurses’ Health Study II with 116 430 women aged 25–42 years and Health Professionals Follow-Up Study with 51 529 men aged 40–75 years(Reference Cassidy, O’Reilly and Kay36). The inconsistent results of RCT and cohort studies on blood pressure may be due to biases involved in cohort studies, in particular confounding bias and measurement errors. Mink et al. showed that dietary intake of anthocyanidins was associated with a reduced risk for mortality from CHD, CVD and all-cause death (for any v. no intake: HR 0·88 (95 % CI 0·78, 0·99); HR 0·91 (95 % CI 0·83, 0·99) and HR 0·90 (95 % CI 0·86, 0·95), respectively) in the Iowa Women’s Health Study(Reference Mink, Scrafford and Barraj8). A recent meta-analysis by Kimble et al. showed that intake of dietary anthocyanins reduced the risk for incident CHD (HR 0·91; 95 % CI 0·83, 0·99) and CVD mortality (HR 0·92; 95 % CI 0·87, 0·97)(Reference Kimble, Keane and Lodge37). However, no associations were observed between anthocyanin intake and risk for incident stroke, myocardial infarction or total CVD(Reference Kimble, Keane and Lodge37).
Conversely, evidence from observational studies directly examining the association between strawberry consumption and risk for CVD is limited. The Women’s Health Study that involved 38 176 middle-aged women showed consuming ≥2 servings/week of strawberries compared with no consumption had a non-significant association with a higher risk for CVD throughout a 10 year follow-up (HR 1·27; 95 % CI 0·94, 1·72)(Reference Sesso, Gaziano and Jenkins9). In addition, a cross-sectional analysis of baseline variables in that study showed a slightly reduced likelihood (14 % lower) of having elevated CRP levels among high strawberry consumers(Reference Sesso, Gaziano and Jenkins9). In the Iowa Women’s Health Study (34 489 postmenopausal women aged 55–69 years), higher strawberry consumption was not associated with CHD mortality (HR 0·95; 95 % CI 0·83, 1·08) compared with lower consumption(Reference Mink, Scrafford and Barraj8). Among 1299 older adults that participated in the Massachusetts Health Care Panel study, the consumption of ≥1 serving of fresh strawberries or melons per d v. an intake of <1 serving per d was not associated with risk for CVD mortality (HR 0·70; 95 % CI 0·10, 4·79); however, it is worth mentioning that only 1·2 % of the participants in that study consumed ≥1 serving of fresh strawberries/melons per d(Reference Gaziano, Manson and Branch7). Given the limited evidence regarding strawberry consumption and risk for CVD, large-scale prospective cohort studies are still warranted.
The main strength of our meta-analysis was that all included studies were RCT, which minimised the risk for confounding and recall biases involved in observational studies. However, limitations of our meta-analysis should also be noted. First, the number of included RCT and the sample size of individual trials were limited. Despite the RCT design, there might have been considerable differences in baseline characteristics between the treatment and control groups in cases with a small sample size. For example, in the study by Amani et al. (Reference Amani, Moazen and Shahbazian20), the baseline LDL-cholesterol levels were 2·46 and 3·00 mg/l in the treatment (n 19) and control (n 17) groups, respectively, although the difference was not significant (P = 0·13). Second, the individual trials used various forms of strawberries and different doses as interventions. It remains uncertain whether such differences could result in different treatment effects. However, there was little evidence of heterogeneity across studies for all endpoints. Third, the treatment durations were relatively short (all ≤12 weeks). The long-term effects of the strawberry interventions were therefore not determined. Fourth, publication bias could be a threat to the validity of our findings. Test for such bias in TC was borderline significant, but correcting the bias changed the results little.
In conclusion, the findings of the present meta-analysis of RCT indicated that strawberry interventions significantly reduced CRP levels and may lower TC and LDL-cholesterol in those with higher baseline levels. Because of the limited number of included RCT and small sample sizes, it is premature to recommend strawberries as a dietary therapy for dyslipidaemia. Large-scale RCT are needed to verify our findings.
Acknowledgements
We thank Jennifer Carr for language editing.
This study was supported by Japan Society for the Promotion of Science KAKENHI grant number A18H063910 and T19K214700 to J.-Y. D.
The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit the manuscript for publication.
Q. G. collected the data, analysed the data and wrote the manuscript. L.-Q. Q., A. A. and E. S. E. conducted the technique review and edited the manuscript. J.-Y. D. designed the study, collected the data, analysed the data and edited the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452000121X