Electroconvulsive therapy (ECT) is a safe and effective treatment for major depressive disorder (MDD).1,2 Early studies on the mechanisms of action of ECT demonstrated that the improvement in depression primarily was accomplished with the seizure and not the electrical stimulus itself.Reference Cronholm and Ottosson3 Thus, inducing a generalised seizure is crucial to achieve adequate MDD treatment results with ECT.4 In its initial form, ECT was used without anaesthesia and muscle relaxation, which today is referred to as ‘unmodified ECT’. However, because of the risk of injury such as vertebrae fractures and joint dislocations during treatment, muscle relaxants and anaesthesia were introduced to the treatment, termed ‘modified ECT’.Reference Andrade, Shah and Tharyan5 Modified ECT is currently the recommended practice, but unmodified ECT is still used in some parts of the world.Reference Leiknes, Jarosh-von Schweder and Høie6 The seizure threshold elevating effect of several anaesthetic agents is well known. Thiopental and propofol are commonly used to treat status epilepticus.Reference Prabhakar and Kalaivani7 Other anaesthetic agents, such as ketamine and etomidate, have less effect on seizure quality during ECT.Reference Hoyer, Kranaster, Janke and Sartorius8 The effects of thiopental and propofol on seizure threshold seem to be dose-dependent.Reference Gálvez, Hadzi-Pavlovic, Smith and Loo9 Several studies have examined the effects of different anaesthetic agents and dose on seizure threshold and seizure lengths.Reference Bauer, Hageman, Dam, Báez, Bolwig and Roed10–Reference Malsch, Gratz, Mani, Backup, Levy and Allen13 It has been suggested that a light anaesthesia is of importance to increase remission rates.Reference Bundy, Hewer, Andres, Gass and Sartorius14 However, until now, no report has addressed the relationship between anaesthetic dose during ECT and MDD treatment outcomes.Reference Lihua, Su, Ke and Ziemann-Gimmel15 Recent studies suggest that light anaesthesia during surgical procedures does not come with increased short- or long-term risks.Reference Short, Campbell, Frampton, Chan, Myles and Corcoran16 The purpose of this study is to examine the effect of different dose intervals of anaesthetic agents on response and remission after ECT for MDD. We hypothesise that low doses of anaesthetic agents are associated with increased likelihood of MDD treatment effect.
Method
Study design
This was a nationwide observational study. Data on ECT procedures and outcomes were gathered from the Swedish National Quality Register for ECT (Q-ECT). Data for this register is gathered prospectively. The Q-ECT had an inclusion rate of 90% during 2018.Reference Elvin and Nordenskjöld17 Data on comorbidities and pharmacotherapy were gathered from the Swedish National Patient Register and the Swedish Prescribed Drug Register. This study was approved by the regional ethical review board in Uppsala, Sweden (Ethical permit 2014/174). No informed consent was required for this register-based study using anonymised data.
Study population
We included patients aged >18 years with MDD, who received treatment during 2012–2018 and were included in the Q-ECT. Only index series of ECT were included; maintenance and booster sessions were excluded. Only the first registered ECT series for each patient was included in the study. An additional inclusion criterion was complete data on anaesthetic medication. With our initial inclusion criteria, we found 10 815 patients. Of these, 2500 patients were excluded because of incomplete data on anaesthetics. The vast majority of patients were anaesthetised with either thiopental or propofol. Because of their infrequent use in this material, we excluded 42 patients with ketamine listed as their primary anaesthetic agent and 46 patients with remifentanil listed as their primary anaesthetic agent; 310 patients who received a combination of at least two anaesthetic medications were also excluded. After these exclusions, 7917 patients remained. Among these patients, 5078 received treatment with thiopental and 2839 received treatment with propofol. After calculating age- and gender-adjusted dose intervals there were 3455 patients in the low-dose interval group, 2430 patients in the medium-dose interval and 2032 patients in the high-dose interval (Fig. 1).
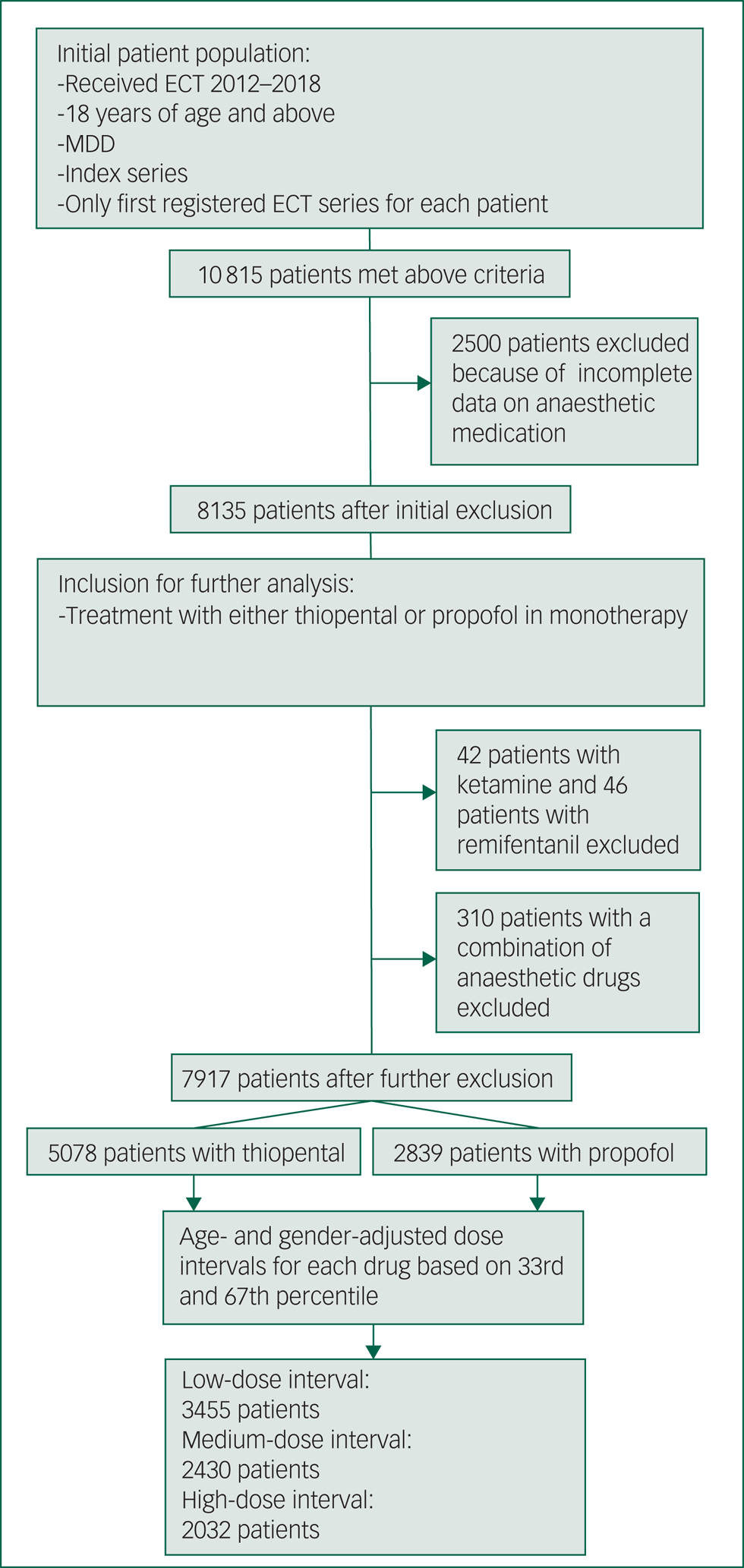
Fig. 1 Flow chart of study. Illustrates each step of inclusion and exclusion for the study population and the size of the study population for each step. ECT, electroconvulsive therapy; MDD, major depressive disorder.
Exposure
The exposure of this study was anaesthetic dose given during ECT, and the dose was defined as a low, medium and high interval. The anaesthetic dose for each drug was registered in the Q-ECT for the last treatment in the series. If several anaesthetic agents were used, then all of them were reported and listed as primary, secondary, third, etc. The continuous dose variable was adjusted for age and gender. For each drug, we calculated dose intervals for men and women separately, for the following age categories: 18–34, 35–54, 55–74 and ≥75 years. The low-, medium- and high-dose intervals for each of these categories were calculated with the 33rd and 67th percentiles. Doses up to the 33rd percentile were included in the low-dose interval, doses up to the 67th percentile were included in the medium-dose interval and doses above the 67th percentile were included in the high-dose interval. As an example, a 76-year-old woman receiving 275 mg of thiopental would be considered to be in the high-dose interval, whereas a 30-year-old man receiving the same dose would be considered to be in the low-dose interval. All dose intervals for each category can be seen in Supplementary Appendix 1 available at https://doi.org/10.1192/bjo.2021.31.
Primary outcome and measurements
The primary outcome of our study was response to treatment based on the Clinical Global Impression – Improvement scale (CGI-I), and was defined as a score of 1 or 2.Reference Riedel, Möller, Obermeier, Schennach-Wolff, Bauer and Adli18 The scale comprises the following seven ratings: 1, ‘Very much improved’; 2, ‘Much improved’; 3, ‘Minimally improved’; 4, ‘No change’; 5, ‘Minimally worse’; 6, ‘Much worse’ and 7, ‘Very much worse’. These measurements are recorded by the evaluating doctor in the ECT evaluation form and then reported to the Q-ECT. Evaluation is routinely done the day after the last ECT treatment.
In addition to our primary outcome, we also included the following secondary outcomes: distinct response and remission according to the CGI, response and remission according to the Montgomery–Åsberg Depression Rating Scale (MADRS-S), and subjective memory worsening. Distinct response was defined as a score of 1 with the CGI-I. Remission based on clinical rating was defined as a score of 1 on the Clinical Global Impression – Severity scale (CGI-S).Reference Riedel, Möller, Obermeier, Schennach-Wolff, Bauer and Adli18 The CGI-S is a seven-point rating scale that rates severity of the patient's illness as follows: 1, ‘Normal, not at all ill’; 2, ‘Borderline mentally ill’; 3, ‘Mildly ill; 4, ‘Moderately ill’; 5, ‘Markedly ill’; 6, ‘Severely ill’ and 7, ‘Among the most extremely ill patients’.Reference Guy19 The MADRS-S was used for self-rating depressive symptoms, which measures nine different items for depression, each rating 0–6 points.Reference Svanborg and Asberg20 Remission based on MADRS-S was defined as a score of <10 and response as ≥50% reduction on the MADRS-S scale.Reference Hawley, Gale and Sivakumaran21
Subjective memory complaints were measured with a seven-point scale based on the ‘failing memory’ item of the Comprehensive Psychopathological Rating Scale (CPRS-M).Reference Asberg, Montgomery, Perris, Schalling and Sedvall22 The scale ranges from 0 (‘Memory as usual’) to 6 (‘Complaints of complete inability to remember’). Subjective memory worsening was defined as a two-point increase on this scale after ECT.Reference Brus, Nordanskog, Båve, Cao, Hammar and Landén23
Comorbidities and pharmacotherapy
Data on the following categories of somatic and psychiatric comorbidities were gathered from the Swedish National Patient Register: diabetes mellitus type 2, heart disease, chronic obstructive pulmonary disease, kidney failure, anxiety, substance misuse and personality disorder. A patient was considered to have a comorbidity based on ICD-10 codes (see Supplementary Appendix 2 for all codes for each category). A comorbidity was considered to be present if the code was registered within 3 years before the first date of ECT. Considering that ICD-10 codes are recorded in the register at the end of a hospital stay, ICD-10 codes registered within a week after concluded treatment or at discharge after the current ECT series were also included.
Data on pharmacotherapy was gathered from the Swedish Prescribed Drug Register. Data is recorded based on the Anatomical Therapeutic Chemical Classification System (ATC). Data on the following prescription categories was gathered based on ATC codes (see Supplementary Appendix 3): anti-epileptics, antipsychotics, antidepressants, benzodiazepines and lithium. A patient was considered to be on treatment if the patient had filled a prescription within 100 days before the date of the first ECT in the series.
Statistical analysis
In addition to using age- and gender-adjusted dose interval, we applied crude and adjusted logistic regression models, yielding odds ratios and 95% confidence intervals, to associate anaesthetic dose intervals with our outcomes. We adjusted for the following potential confounders in our final regression models: age, gender, number of sessions in the series, psychiatric comorbidity (anxiety disorders, substance misuse and personality disorder) and psychiatric pharmacotherapies (antidepressants, anti-epileptics, antipsychotics, benzodiazepines and lithium). The choice of covariates in our model was based on a variable being a known confounder, such as age,Reference O'Connor, Knapp, Husain, Rummans, Petrides and Smith24–Reference Hascilowicz, Hamaguchi, Nakata, Yamamoto, Kiyama and Uezono27 or a potential confounder based on clinical reasoning.
Initially, we included choice of anaesthetic drug in our logistic regression model to rule out potential effects of this variable. There was no statistically significant difference between CGI-I score for thiopental and propofol (odds ratio 1.06, 95% CI 0.95–1.19, P = 0.298). We also included an interaction term for dose interval and choice of anaesthetic drug, which showed no statistical significance. With our calculations of age- and gender-adjusted dose interval combined with the above results, choice of drug was left out of further analysis owing to it being an unlikely confounder between dose interval and our outcomes.
All analyses were conducted with SPSS version 26.0 for Windows 10 (IBM Corp, Armonk, USA).
Missing data
In our initial material, 23.1% of patients had missing data on the exposure. The patients with missing data on anaesthetic agent and dose were, on average, older than those with complete data (55.9 v. 53.1 years, P = 0.001). There was a larger proportion of women in the group with missing data compared with the group with available data on the exposure (61.0% v. 57.8%, P = 0.006). For our covariates (age, gender, psychiatric pharmacotherapy, psychiatric comorbidity, number of sessions) used in our logistic regression model, the data was fully available for all patients in our study. For the outcome, a total of 7211 (91%) patients had available data on CGI-I score and 6800 (86%) had data on CGI-S score. For self-rated scales, MADRS-S score before treatment was available for 5252 patients (66%), and after treatment for 4042 patients (51%). Data for CPRS-M to calculate subjective memory worsening was available for 4497 patients (57%). There was no significant difference in terms of missing data regarding our primary outcome (CGI-I) for age and gender, or for the CGI-S and CPRS-M. However, patients with missing data on the self-rated scale, MADRS-S, were on average younger than patients with available data on the self-rated scale (52.5 v. 53.7 years, P = 0.019).
Results
Sociodemographic, clinical and treatment characteristics for each of the dose-interval groups can be seen in Table 1. On average, patients who received high-dose intervals required more sessions in a series compared with patients who received low-dose intervals (7.5, 7.8 and 8.1 sessions, on average, for low-, medium- and high-dose intervals, respectively; P ≤ 0.001). Patients who received low-dose intervals had longer seizures, on average, than patients who received high-dose intervals (low-dose interval, 38.7 s; medium-dose interval, 37.1 s; high-dose interval, 36.0 s; P ≤ 0.001) (Table 2). For our primary outcome, response measured with CGI-I, we found that low-dose intervals were associated with increased chance of response compared with high-dose intervals (odds ratio 1.22, 95% CI 1.07–1.40, P = 0.004) (Table 3). For our secondary outcomes, we found that low-dose intervals were associated with increased likelihood of response (based on MADRS-S score reduction) and remission (based on CGI-S and MADRS-S scores) compared with high-dose intervals (response based on MADRS-S score: odds ratio 1.31, 95% CI 1.09–1.56, P = 0.004; remission based on CGI-S score: odds ratio 1.37, 95% CI 1.17–1.60, P ≤ 0.001; remission based on MADRS-S score: odds ratio 1.31, 95% CI 1.10–1.54, P = 0.002). For distinct response (score of 1 on CGI-I), we found that both low- and medium-dose intervals were associated with increased chance of distinct response compared with high-dose interval (low-dose interval: odds ratio 1.51, 95% CI 1.31–1.73, P ≤ 0.001; medium-dose interval: odds ratio 1.32, 95% CI 1.14–1.52, P ≤ 0.001). Furthermore, for distinct response we also investigated a possible dose-dependent relationship by calculating odds ratios with medium-dose interval as a reference, which showed an association with higher chance for distinct response with low-dose intervals compared with medium-dose intervals, and a lower chance for distinct response with high-dose intervals compared with medium-dose intervals (low-dose interval: odds ratio 1.14, 95% CI 1.01–1.29, P ≤ 0.028; high-dose interval: odds ratio 0.76, 95% CI 0.75–0.87, P ≤ 0.001). Low-dose intervals were associated with higher risk of subjective memory worsening compared with high-dose intervals (odds ratio 1.32, 95% CI 1.09–1.60, P = 0.004).
Table 1 Sociodemographic, clinical and treatment characteristics for each of the dose interval groups
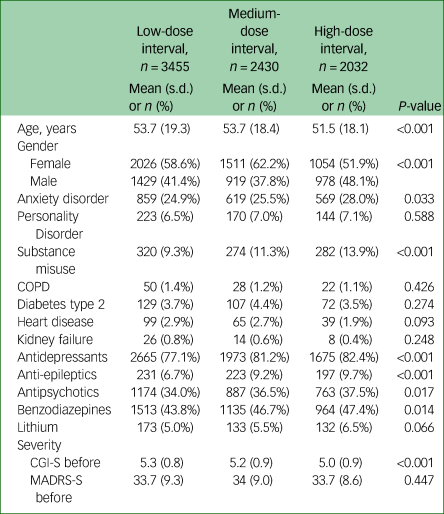
P-values were calculated using one-way ANOVA for mean and Pearson chi-squared for proportions. COPD, chronic obstructive pulmonary disease; CGI-S, Clinical Global Impression – Severity Scale, MADRS-S, Montgomery–Åsberg Depression Rating Scale, self-rated version.
Table 2 ECT treatment characteristics
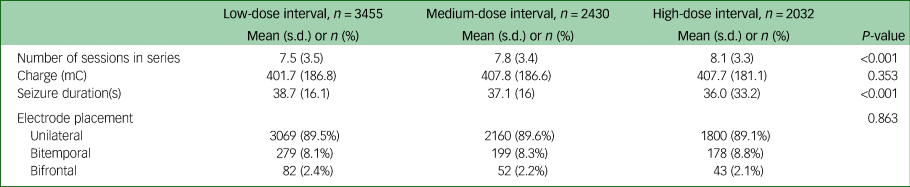
The table shows treatment characteristics for the ECT series of each group, including the total number of sessions in the series, charge given for the last treatment in each series, seizure duration for the last treatment in the series and electrode placement. P-values were calculated using one-way ANOVA for mean and Pearson chi-square for proportions. ECT, electroconvulsive therapy; mC, millicoulomb.
Table 3 Proportion and odds ratios of response, remission and subjective memory worsening
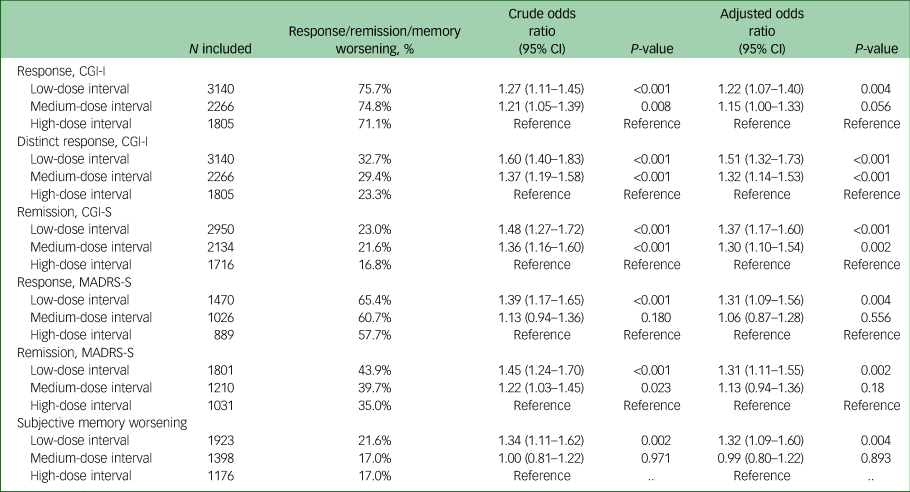
For each outcome, the table shows the number of included patients (if patients had missing data on the outcome they were excluded from the logistic regression models). The proportion of each outcome. Crude odds ratios and their 95% confidence intervals were calculated by correlating the age and gender-adjusted dose intervals and the outcomes without any further variables. A regression model adjusted for age, gender, number of treatments, psychiatric comorbidity and psychiatric pharmacotherapy was used to calculate adjusted odds ratios and their 95% confidence intervals. P-values are shown for both crude and adjusted odds ratios separately. CGI-S, Clinical Global Impression – Severity Scale; CGI-I, Clinical Global Impression – Improvement Scale; MADRS-S, Montgomery–Åsberg Depression Rating Scale, self-rated version.
Discussion
Main findings
In this study, patients with MDD who received low-dose anaesthetic during ECT had higher response and remission rates compared with patients who received high-dose anaesthetic. All of our outcomes pointed in the same direction, with superior MDD treatment outcomes, measured with both clinical assessments and self-rated scales, for low- compared with high-dose intervals. For one of our secondary outcomes, distinct response, there was a dose-dependent relationship. Furthermore, treatment series were shorter and seizure durations were longer for patients who received low-dose intervals. We found an increased risk of subjective memory worsening for patients who received low-dose intervals compared with patients who received high-dose intervals. The measurements of memory disturbance are routinely done the day after the last ECT session. With this in mind, it is more likely that the seizure activity itself was associated with the greater effect on memory the day after treatment rather than a direct effect of the anaesthetics. Previous studies have shown that objective negative effects on memory typically disappear within 15 days, and after this, the cognitive function improves.Reference Semkovska and McLoughlin28 The prolonged epileptic seizures in the low-dose interval group could explain the increased subjective memory worsening the day after the last ECT. Postictal agitation/post-ECT confusion is more frequent in unmodified ECT, which is an additional possible explanation to increased memory disturbances in the low-dose interval group.Reference Andrade, Shah, Tharyan, Reddy, Thirunavukarasu and Kallivayalil29 On the other hand, improved response and remission rates with lower doses of anaesthesia are likely to be associated with cognitive improvement in a longer perspective. However, a long-term follow-up of this finding is needed.
Comparison with previous findings
To our knowledge, this is the first study to investigate the effects of anaesthetic dose on response and remission rates after ECT for MDD. Our results are in line with previous findings of a dose-dependent relationship between anaesthetic dose and seizure thresholds and durations.Reference Gálvez, Hadzi-Pavlovic, Smith and Loo9
Strengths and limitations
This study has several strengths. It is the first study to investigate the association between dose of anaesthetic agents and MDD treatment outcome with ECT. We have access to high-resolution outcome data from the Q-ECT. Cross-matching this unique database with validated Swedish nationwide registers allowed us to adjust for comorbidities and pharmacotherapy. This study also has several limitations. Data on the anaesthetic agent was missing in 2500 patients in our initial cohort, and these were excluded from further analysis. The patients with missing data on anaesthetic agents were, on average, older than the patients who had complete data (patients with available data had a mean age of 53.1 years, patients with missing data had a mean age of 55.9 years; P ≤ 0.001). We believe that it is unlikely that age itself is the cause of the missing data. The mechanism of missing data on the exposure is most likely underreporting of this data to the register rather than unknown or unrecorded data for certain age groups. The reporting to the register is not carried out by the anaesthesiologist and thus, it is unlikely there would be a systematic bias between age and missing data on anaesthetic dose. However, older age is an established predictor of ECT response.Reference O'Connor, Knapp, Husain, Rummans, Petrides and Smith24,Reference Vlissides and Jenner25 Considering that older patients typically receive lower doses of anaesthetics, it could be difficult to disentangle the effect of age and anaesthetic on ECT response.Reference Homer and Stanski26,Reference Hascilowicz, Hamaguchi, Nakata, Yamamoto, Kiyama and Uezono27 To remove potential confounding from age, we used both age- and gender-adjusted dose intervals, and included a continuous age variable in our logistic regression models. An additional limitation was lack of data on weight, and thus, we cannot present administered anaesthetic dose per kilogram. Ideally, this information could have helped to construct our adjusted dose intervals. However, by adjusting dose intervals for both age and gender, we believe that weight is indirectly considered. We also lacked information on time from induction of anaesthesia to administered ECT, which could affect anaesthetic depth during ECT. As a consequence, we cannot rule out any systematic differences in time from induction to administered ECT being dependent on anaesthetic dose. Moreover, our three dose groups were different in size. This was because of the distribution of anaesthetic dose, with accumulation of the same doses in lower-dose intervals. Furthermore, because of the observational nature of this study, there could be residual confounding in our analyses.
In our study, we lack measurements of potential awareness during ECT. However, the induced seizure causes an immediate loss of consciousness, minimising the risk of awareness during the procedure. Furthermore, the general risks with light anaesthesia are low.Reference Short, Campbell, Frampton, Chan, Myles and Corcoran16 Nevertheless, further studies need to address if the risk of awareness and postictal agitation during the procedure increase with low-dose intervals of anaesthetics.
In conclusion, our study shows more favourable outcomes in terms of response and remission rates with low-dose intervals of anaesthetics for MDD after ECT, compared with high-dose intervals. For anaesthesia during ECT, anaesthetic depth is likely the main issue regarding MDD treatment effect rather than the actual dose. In our study anaesthetic dose likely corresponds to anaesthetic depth, although other factors also contribute. The clinical implications of our results would be that a light anaesthesia increases the likelihood of response and remission with ECT for MDD. This should be weighed against the risk of awareness during anaesthesia and the potential risk of postictal agitation. In conclusion, deep anaesthesia should be avoided during ECT, to improve treatment outcomes for MDD. Further studies need to explore if similar effects are achieved by increasing the time from induction to stimulation, as by lowering the anaesthetic dose. In addition, further studies on long-term effects of anaesthetic dose during ECT for MDD are needed.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2021.31.
Data availability
Requests for the data that supports the findings of this study can be sent to the corresponding author, A.K.. The data are not publicly available due to the privacy of research participants.
Author contributions
M.T., A.K., A.N., M.B. and E.M.-R. participated in study design. A.K. and R.A. carried out statistical analysis. A.K., M.T. and A.N. carried out initial preparations of the manuscript. A.K. had full access to the data in the study, and takes responsibility for the integrity of the data and the accuracy of data analysis. In addition, M.T. and R.A. verified the underlying data and the results. All authors reviewed and provided comments on the manuscript. All authors have read and approved the final version of the manuscript before publication.
Funding
M.T. has received funding from the Söderström-König Foundation. A.K. has received funding from Stiftelsen Professor Bror Gadelius Minnesfond. No funder had any role in study design, data collection, data analysis, data interpretation or writing of the manuscript.
Declaration of interest
None.








eLetters
No eLetters have been published for this article.