Gestational diabetes mellitus (GDM) is defined as a disorder in glucose tolerance with onset or first recognition during pregnancy(Reference Wang, Nie and Li1). It affects 5–10 % of Asian pregnant women, with an increasing trend in some developing countries(Reference Shaat and Groop2). Several contributing factors to GDM have been found, including severe obesity during pregnancy, strong family history of type 2 diabetes (T2D) and previous history of GDM, impaired glucose metabolism or glucosuria(Reference Metzger, Buchanan and Coustan3). Pregnant women with GDM are at increased risk of fetal macrosomia, prematurity, birth trauma, fetal death and respiratory distress syndrome, as well as maternal morbidity(Reference Bener, Saleh and Al-Hamaq4–Reference Cox8). Also, women with a history of GDM are at increased risk of developing T2D later in life(Reference Linne, Barkeling and Rossner9).
Although several strategies have been suggested, dietary intervention is the first-line therapy for maintaining maternal postprandial glucose levels and GDM management. Earlier studies have shown the efficacy of carbohydrate restriction on metabolic control(Reference Acheson10) and pregnancy outcomes(Reference Lim, Noakes and Norman11) in GDM. In addition, low-glycaemic-index (GI) diet has also been argued as a possible strategy in GDM management(Reference McGowan and McAuliffe12). Consumption of a low-GI diet among women with GDM significantly reduced the number needing to use insulin; however, no significant effects on pregnancy outcomes were found(Reference Moses, Barker and Winter13).
The Dietary Approaches to Stop Hypertension (DASH) eating plan is a low-GI low-energy-dense diet that was initially suggested for lowering blood pressure(Reference Vollmer, Sacks and Ard14); however, its effectiveness has also been reported in T2D(Reference Azadbakht, Fard and Karimi15) and the metabolic syndrome(Reference Azadbakht, Mirmiran and Esmaillzadeh16). We are aware of no study examining the effect of the DASH diet on metabolic profile in GDM. High contents of dietary fibre, phyto-oestrogens and isoflavones, as well as its low GI(Reference Azadbakht, Fard and Karimi15, Reference Azadbakht, Mirmiran and Esmaillzadeh16), might help GDM patients to control their metabolic profile. The present study was, therefore, performed to investigate the effects of the DASH eating plan on glucose tolerance and lipid profiles of pregnant women with GDM.
Subjects and methods
Participants
The present two-arm parallel randomised controlled clinical trial was carried out in Kashan, Iran, from April 2011 to December 2011. With the exception of the study dietitian (Z. A.), who provided the dietary education, all study personnel and participants were blinded to the dietary assignment. Pregnant women aged 18–40 years, diagnosed with GDM by a 100 g oral glucose tolerance test at 24–28 weeks of gestation, were recruited to the present study. Gestational age was assessed from the date of last menstrual period and concurrent clinical assessment(Reference Jehan, Zaidi and Rizvi17). Considering a type I error of 5 % (α = 0·05), study power of 80 % and serum HDL-cholesterol levels as a key variable, we reached a sample size of sixteen persons for each group. Pregnant women without a previous diagnosis of glucose intolerance were screened for GDM by two procedures. First, a 50 g glucose challenge test was used as preliminary screening. Individuals with 1 h plasma glucose concentrations of >7·77 mmol/l (1400 mg/l) were then asked to participate in a 100 g oral glucose tolerance test. Diagnosis of GDM was based on the criteria set by the American Diabetes Association(Reference Rossi18) and those in whom the plasma glucose levels met two of the following criteria were considered as having GDM: fasting >5·27 mmol/l (950 mg/l), 1 h ≥ 9·99 mmol/l (1800 mg/l), 2 h ≥ 8·60 mmol/l (1550 mg/l) and 3 h ≥ 7·77 mmol/l (1400 mg/l). A total of forty-five women who attended maternity clinics affiliated to the Kashan University of Medical Sciences, Kashan, Iran, were screened for GDM, of whom forty met the inclusion criteria. We excluded those with untreated hypothyroidism, smoking and kidney or liver diseases, as well as those taking oestrogen therapy. A total of forty pregnant women were recruited in the study, and after stratification for BMI ( < 30 and ≥ 30 kg/m2) and weeks of gestation ( < 26 or ≥ 26 weeks), they were randomly assigned to the control (n 20) or the DASH diet (n 20) for 4 weeks. Random assignment was done by the use of computer-generated random numbers. Among individuals in the control diet, three women (pre-eclampsia (n 2) or need to commence insulin therapy (n 1)) were excluded. A total of three women were excluded from the DASH diet (pre-eclampsia (n 2) and bed rest (n 1)). Finally, thirty-four participants (control diet (n 17) and the DASH diet (n 17)) completed the trial (Fig. 1). The study was conducted according to the guidelines laid down in the Declaration of Helsinki. The ethical committee of the Kashan University of Medical Sciences approved the study (no. 1384-90-5-18) and an informed written consent was obtained from all participants.

Fig. 1 Summary of patient flow.
Study design
Participants were randomly assigned to the control or the DASH diet for 4 weeks. They were asked not to alter their routine physical activity and not to receive any lipid-lowering medications during the 4-week intervention. All pregnant women consumed a supplement of Ca and ferfolic once a day. Compliance with the consumption of diets was monitored once a week through phone interviews. The compliance was also double-checked by the use of 3 d dietary records completed throughout the study. To obtain nutrient intakes of participants based on these 3 d food diaries, we used Nutritionist IV software (First, Databank) modified for Iranian foods.
Diets
The control diet was designed to contain 45–55 % carbohydrates, 15–20 % protein and 25–30 % total fat. This diet was planned as a 7 d menu cycle based on the usual practice in GDM. The macronutrient composition of the DASH diet was similar to the control diet; however, the DASH diet was rich in fruits, vegetables, whole grains, low-fat dairy products, and was low in saturated fats, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d (Table 1)(Reference Azadbakht, Mirmiran and Esmaillzadeh16).
Table 1 Constituents of the Dietary Approaches to Stop Hypertension (DASH) and control diets used in the study*
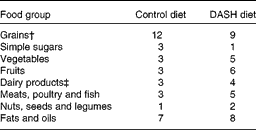
* Data are presented for a energy of 10 040 kJ (2400 kcal)/d
† At least four servings from whole grains in the DASH diet.
‡ Low-fat ( < 2 %) in the DASH diet.
Assessment of anthropometric measures
Maternal weight was assessed at baseline and after 4 weeks of intervention in maternity clinics by trained midwives. Body weight was measured in an overnight fasting state, without shoes and in a minimal clothing state, by the use of a digital scale (Seca) to the nearest 0·1 kg. Height was measured using a non-stretched tape measure (Seca) to the nearest 0·1 cm. BMI was calculated as weight in kg divided by height in m2. Pre-pregnancy weight and BMI were gathered from their earlier records.
Biochemical assessment
Fasting blood samples (10 ml) were taken at the baseline and after the 4-week intervention at the Kashan reference laboratory early in the morning after overnight fasting. Plasma glucose levels were quantified by the use of the glucose oxidase/peroxidase (GOD-POD) method with commercially available kits (Parsazmun Company). Plasma glycated Hb (HbA1c) levels were measured using Glycomat kits (Biocode Hycel) by the immunoassay method. Serum total cholesterol and TAG concentrations were assayed using commercial kits (Parsazmoon Company) by enzymatic colorimetric tests containing cholesterol oxidase/p-aminophenazone and glycerol phosphate oxidase, respectively. Serum HDL-cholesterol was measured after precipitation of the apoB-containing lipoproteins with phosphotungstic acid. Serum LDL-cholesterol levels were also measured using available kits. Plasma Hb levels were measured by colorimetery (Sysmex, Model XS 8001) using the enzyme kit (Solpholizer SLS). Participants underwent 3 h oral glucose tolerance tests, and blood samples were collected at 60, 120 and 180 min to measure plasma glucose levels.
Statistical analysis
To ensure the normal distribution of variables, histogram and Kolmogrov–Smirnov tests were applied. The independent samples Student's t test was used to detect differences in general characteristics and dietary intakes between the two groups. To determine the effect of the DASH diet on metabolic profiles, we applied repeated-measures ANOVA, where treatment × time interactions were tested by using Pillai's trace. In these analyses, the treatments (DASH and control) were regarded as between-subject factors and time with two time-points (baseline and week 4 of intervention) was considered as a within-subject factor. P< 0·05 was considered as statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 17 (SPSS, Inc.).
Results
Mean age of the study participants was not statistically different between the DASH and the control groups. Although pre-pregnancy weight (75·5 (sd 16·3) v. 67·4 (sd 9·3) kg, P= 0·08) and BMI (29·6 (sd 5·9) v. 26·7 (sd 3·0) kg/m2, P= 0·07) were higher in controls than that in the DASH diet group, the differences were not statistically significant (Table 2). Baseline weight and BMI, as well as their means after intervention, were not significantly different between individuals who consumed the DASH and control diets.
Table 2 General characteristics of the study participants (Mean values and standard deviations)

DASH, Dietary Approaches to Stop Hypertension.
* Obtained from independent samples Student's t test.
Based on the 3 d dietary records that participants provided throughout the study, no statistically significant difference was seen between the two groups in terms of dietary intakes of energy; however, significant differences were found in dietary intakes of SFA, PUFA, cholesterol, dietary fibre, simple sugars, Na and K between the two groups (P< 0·05) (Table 3).
Table 3 Dietary intakes of study participants throughout the study (Mean values and standard deviations)

* Obtained from independent samples Student's t test.
Adherence to the DASH eating pattern, compared with the control diet, resulted in improved glucose tolerance such that plasma glucose levels reduced at 60 ( − 1·86 v. − 0·45 mmol/l, P group= 0·02), 120 ( − 2·3 v. 0·2 mmol/l, P group= 0·001) and 180 min ( − 1·7 v. 0·22 mmol/l, P group= 0·002) after the glucose load (Table 4). Decreased HbA1c levels ( − 0·2 v. 0·05 %, P group= 0·001) were also seen in the DASH group compared with the control group. Mean changes for serum total ( − 0·42 v. 0·31 mmol/l, P group= 0·01) and LDL-cholesterol ( − 0·47 v. 0·22 mmol/l, P group= 0·005), TAG ( − 0·17 v. 0·34 mmol/l, P group= 0·01) and total:HDL cholesterol ratio ( − 0·6 (sd 0·9) v. 0·3 (sd 0·8), P group= 0·008) were significantly different between the two diets. Additionally, consumption of the DASH diet favourably influenced systolic blood pressure ( − 2·6 v. 1·7 mmHg, P group= 0·001). Mean changes of fasting plasma glucose ( − 0·29 v. 0·15 mmol/l, P group= 0·09) were non-significant comparing the DASH diet with the control diet. Although consumption of the DASH diet resulted in increased levels of serum HDL-cholesterol (0·12 mmol/l), we failed to find a significant difference comparing the two diets. The effect of time was significant for plasma glucose levels at 60 (P time< 0·0001), 120 (P time= 0·004) and 180 (P time =0·008) min after glucose load, as well as for HbA1C (P time =0·03) and diastolic blood pressure (P time= 0·009). No significant time–group interactions were found.
Table 4 Serum lipid profiles and glucose post-load at baseline and after the intervention (Mean values and standard deviations)

DASH, Dietary Approaches to Stop Hypertension; FPG, fasting plasma glucose; GTT, glucose test tolerance; HbA1C, glycated Hb; SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Obtained from repeated measures ANOVA.
Consumption of the DASH eating pattern, compared with the control diet, resulted in a lower rate of caesarean section (41·2 v. 88·2 %, P= 0·001) as well as a decreased need for insulin therapy after delivery (11·8 v. 58·8 %, P= 0·005). Mean birth weight of babies whose mothers received the DASH diet was significantly lower than those whose mothers received the control diet (3083 (sd 402) v. 3641 (sd 579) g, P= 0·006).
Discussion
We found that consumption of the DASH eating pattern for 4 weeks among pregnant women with GDM had beneficial effects on glucose tolerance, lipid profiles and systolic blood pressure compared with the control diet. However, the effects of the DASH eating pattern on serum HDL-cholesterol and diastolic blood pressure were not significantly different compared with the control diet. To our knowledge, the present study is the first to report the effect of the DASH diet on metabolic profiles of pregnant women with GDM.
Gestational diabetes is associated with several complications in maternal and offspring health, as well as in later stages of their lives(Reference Bener, Saleh and Al-Hamaq4–Reference Jelsema6, Reference Linne, Barkeling and Rossner9). We showed that the DASH eating pattern could significantly improve glucose tolerance and lipid profiles of pregnant women with GDM. Earlier studies have shown the beneficial effects of the DASH eating pattern on fasting plasma glucose and lipid profiles of patients with hypertension, CVD, the metabolic syndrome and T2D(Reference Azadbakht, Fard and Karimi15, Reference Azadbakht, Mirmiran and Esmaillzadeh16, Reference Champagne19, Reference Doyle and Cashman20). In a study by Azadbakht et al. (Reference Azadbakht, Fard and Karimi15), a significant reduction in body weight, waist circumference, fasting blood glucose levels, %HbA1C, serum LDL-cholesterol levels, and in systolic and diastolic blood pressure was found with the DASH diet that was consumed for 8 weeks among type 2 diabetic patients. Adherence to the DASH diet for 8 weeks among men and women with the metabolic syndrome resulted in similar findings(Reference Azadbakht, Mirmiran and Esmaillzadeh16). Harsha et al. (Reference Harsha, Sacks and Obarzanek21) found favourable effects of the DASH diet, compared with the control diet, on serum total and LDL-cholesterol levels in subjects with elevated blood pressure. They concluded that the beneficial effects of the DASH eating pattern on serum lipid profiles is independent of the dietary Na levels of this diet. The same findings on serum lipid profiles have also been reported among hypercholesterolaemic patients(Reference Obarzanek, Sacks and Vollmer22). The present findings are in line with previously reported ones on the beneficial effects of the DASH diet on metabolic profiles. It must be kept in mind that the control diet in the present study resulted in increased serum TAG and LDL-cholesterol levels, as well as systolic and diastolic blood pressure from baseline. One might assume that this was due to the design of the control diet or to some other reasons, including bias in advice. We think that the increments seen in serum TAG and LDL-cholesterol levels, as well as in SBP and DBP, are due to the design of the control diet which contains lower amounts of whole grains, vegetables and higher amounts of simple sugars compared with the DASH diet. However, it is very difficult to understand the exact reason for this finding. Although the control diet has been planned based on ‘usual advice’ given to GDM patients in clinical situations, the diet was delivered by the one who was responsible for delivering the DASH diet as well. The use of only one dietitian to deliver the control and intervention diets might be a source of potential bias, because that person can load the dice in favour of the intervention diet. Definitely, this clinical trial would have been better if the ‘usual advice’ given to patients with GDM in the control group had been delivered by a separate person. However, the components of the diets were different. Moreover, the dietitian was responsible for delivering the menus, and the 7 d menu cycle was planned by the study dietitian and re-checked by the study senior investigator. However, the importance of metabolic control in GDM patients is of great importance due to the unfavourable effects of GDM on pregnancy outcome.
Several mechanisms can explain the health benefits of the DASH eating pattern. High content of dietary fibre and phyto-oestrogens due to higher amounts of fruit and vegetables in the DASH diet might contribute to its beneficial effects on serum TAG, total and LDL-cholesterol levels(Reference Azadbakht, Mirmiran and Esmaillzadeh16, Reference Obarzanek, Sacks and Vollmer22, Reference Sacks and Katan23). Furthermore, the simple sugar content of the DASH diet was almost half that of the control diet. Earlier studies have reported that a high-sucrose diet increases serum glucose and lipid profiles(Reference Macan, Vrkic and Vrdoljak24–Reference Man and He26). Consumption of high-GI carbohydrates in the control diet pattern could result in increased levels of plasma glucose concentrations, whereas a low-GI DASH pattern would result in better metabolic control. Earlier studies have shown that consuming a high-carbohydrate diet that is mainly composed of low-GI varieties can maintain blood glucose concentrations within normal ranges(Reference McGowan and McAuliffe12, Reference Scholl, Chen and Khoo27, Reference Romon, Nuttens and Vambergue28).
Increasing dietary intakes of soluble fibre has also been shown to reduce serum total cholesterol concentrations, independent of dietary fat intake(Reference Van Horn29Reference Brown, Rosner and Willett30). High intake of legumes in the DASH diet, compared with the control diet, might also be responsible for its beneficial effects on glucose tolerance and lipid profiles(Reference Esmaillzadeh and Azadbakht31). In a study by Azadbakht et al. (Reference Azadbakht, Shakerhosseini and Atabak32), soya consumption in patients with T2D with nephropathy reduced total cholesterol, TAG and LDL-cholesterol levels. Such findings have also been reported in hypercholesterolaemic patients(Reference Hermansen, Hansen and Jacobsen33). Consuming higher amounts of non-hydrogenated vegetable oils in the DASH diet, compared with the control diet, might also contribute to favourable effects on glucose tolerance and improved lipid profiles. Several studies have reached modulation of blood pressure and serum lipid profiles with consumption of edible vegetable oils(Reference Sudhakar, Kalaiarasi and Al-Numair34, Reference Esmaillzadeh and Azadbakht35). In the present study, Na intake in the control diet was 2·8 times greater than that in the DASH diet. Several studies reported that high Na intake was associated with abnormal metabolic profiles and cardiovascular risks(Reference Alderman and Cohen36, Reference Gonzalez, Forcada and de Cavanagh37). High Na intake could result in abnormal metabolic profiles through activation of the renin–angiotensin–aldosterone system(Reference Huan, Deloach and Keith38), G972R polymorphism of the IRS-1 gene related to insulin resistance(Reference Dziwura, Binczak-Kuleta and Miazgowski39), increased signalling through the mineralocorticoid receptor and increased production of reactive oxygen species and oxidative stress, which in turn contribute to insulin resistance(Reference Lastra, Dhuper and Johnson40). Further beneficial effects of the DASH diet, compared with the control diet, could be explained by higher K intake in the DASH diet. K intake in the DASH group was almost 1·5 times greater than that in the control group. Recent studies have indicated that high K intake can result in improved insulin resistance and lipid profiles(Reference Ishikawa, Arai and Takano41, Reference Reungjui, Pratipanawatr and Johnson42). Although the precise mechanisms of beneficial effects of dietary K on metabolic profiles have not been fully elucidated, it could exert its effects through sympathetic nerve inhibition, suppressed salt-induced insulin resistance and decreased production of reactive oxygen species(Reference Ando, Matsui and Fujita43). High intake of Mg and Ca in the DASH diet, compared with the control diet, might also be responsible for improving glucose tolerance and decreased lipid profiles. Earlier studies have reported that increased dietary Mg intake ameliorates insulin resistance, serum lipid profiles and lowers inflammation, endothelial dysfunction, oxidative stress and platelet aggregability(Reference Champagne19, Reference Bo and Pisu44). Such findings have also been reached with high Ca intake. Higher Ca intake would lead to decreased fatty acid absorption and increased faecal fatty acid content(Reference Reid45). This reduced absorption of fatty acids could in turn result in decreased levels of metabolic profiles(Reference Vaskonen46). Furthermore, increasing dietary Ca intake can result in an elevation of liver intracellular Ca, which in turn would stimulate microsomal TAG transfer protein(Reference Cho, Kang and Choi47). Probably, microsomal TAG transfer protein can lower the formation and secretion of lipid profiles.
One of the limitations of the present study is the duration of this trial. We were unable to administer the diets for more than 4 weeks due to the particular condition of the pregnant women.
In conclusion, consumption of the DASH eating pattern for 4 weeks among pregnant women with GDM results in beneficial effects on glucose tolerance and lipid profiles compared with a control diet.
Acknowledgements
The present study was supported by a grant (no. 9013) from the Vice-chancellor for Research, KUMS, Kashan, Iran. The authors would like to thank the staff of Naghavi and Shaheed Beheshti Clinics (Kashan, Iran) for their assistance in the present project. Z. A. conducted the study, carried out the statistical analyses, wrote the manuscript and contributed in the interpretation of the findings. Z. T., M. S. and T. F. contributed in data collection and assisted in writing the manuscript. A. E. contributed in conception and design and also advised on statistical analyses, contributed in drafting the manuscript and assisted in interpretation of the findings. All authors approved the final version of the manuscript. The authors declare no conflict of interest.







