Introduction
Sellar masses are mostly benign growths arising within the sellar/parasellar region which account for up to 18% of all intracranial neoplasms. Reference Dolecek, Propp and Stroup1,Reference Shibui2 Despite recent advances in medical treatment, transsphenoidal surgery (TSS) remains the primary therapeutic option for certain hormone-producing pituitary adenomas and most non-hormone-producing sellar masses with evidence of a mass effect. While the overall risk of complications with TSS is low in experienced hands, Reference Casanueva, Barkan and Buchfelder3 either transient or permanent hormonal dysfunction has been reported in up to 25% of patients. Reference Prete, Corsello and Salvatori4 Previous studies have suggested that patients undergoing transsphenoidal pituitary surgery should be followed in-hospital post-operatively by a multidisciplinary team that includes endocrinology. Reference Inder and Alford5,Reference Dumont, Nemergut and Jane6 This approach has been proposed by some groups to reduce the length of stay in hospital after surgery; furthermore, since complications after surgery are often endocrine in nature, input from endocrinology is arguably crucial. Reference Carminucci, Ausiello and Page-Wilson7 Accordingly, recent endocrine and neurosurgical guidelines have emphasized the importance of coordinated care of TSS patients in the immediate post-operative period to minimize the risk of endocrine complications. Reference Prete, Corsello and Salvatori4,Reference Carminucci, Ausiello and Page-Wilson7–Reference Woodmansee, Carmichael and Kelly9
Although a dedicated multidisciplinary team comprising a subspecialized neurosurgeon, endocrinologist, and nurse is ideal, Reference Carminucci, Ausiello and Page-Wilson7 such a team is unlikely to be available for all TSS patients all of the time, even in select centers. In our own center, where all patients requiring routine TSS are assessed and followed by a subspecialized team dedicated to the care of patients with pituitary disorders, we frequently found that a patient’s discharge from the hospital was delayed while waiting for an in-hospital endocrinology consultation (IHEC) to be completed. In many cases, there was no change in the management plan based on the IHEC, leading us to question whether routine assessment of all TSS patients is necessary.
To assess the need for IHEC, we developed and instituted a “Physician’s Guide to Determine Whether In-Hospital Consultation is Required” (Figure 1). The IHEC Physician’s Guide was specifically designed to identify known pre-operative factors, as well as relevant intra-operative findings, that would mandate a post-operative IHEC. To assess the need for an IHEC, the Physician’s Guide includes the following questions: “A) Was the patient on DDAVP therapy pre-operatively?”; “B) Was all pituitary tissue removed?”; “C) Was there any aggressive stalk manipulation?”; and “D) Any other consideration – if so explain?” If the answer to any of these is “yes” then the neurosurgery team requests an IHEC; otherwise, the on-call endocrinology team is not consulted. In this study, we assessed the predictive value of our IHEC Physician’s Guide in identifying patients who truly required IHEC.

Figure 1. IHEC Physician’s Guide.
Methods
The Halifax Neuropituitary Program (HNP) was established in April 2001 and provides tertiary-level clinical care to patients with pituitary disorders living in the province of Nova Scotia, Canada; it also provides quaternary pituitary care for patients living in Atlantic Canada (total population: 2.3 million). A comprehensive prospective provincial computerized registry of all patients has been maintained since November 2005 and the program currently follows approximately 1800 patients with pituitary disorders. The HNP team includes a neurosurgeon (D.B.C.), otolaryngologist (E.M.), endocrinologist (S.A.I.), specialized endocrine nurse (L.T.), specialized neurosurgery nurse (A.L.O.H.), and program coordinator. All patients undergoing elective TSS are followed according to a specific protocol which includes a comprehensive assessment by the full HNP team (except otolaryngology) before surgery followed by an assessment by endocrinology and otolaryngology 2-week post-surgery. Subsequently, the patient is seen by the full HNP team (except otolaryngology) at 3 months, 9 months, annually for 5 years, and then every 2–3 years thereafter. A complete pituitary hormonal assessment is conducted at the pre-surgery visit and, if indicated, hormonal replacement is initiated. All surgical patients are routinely given glucocorticoid (GC) replacement therapy in the form of intravenous hydrocortisone intra-operatively, changing to oral hydrocortisone once the patient can swallow. All patients are monitored by neurosurgery for features of diabetes insipidus (DI) and, if there is evidence of DI, treatment is initiated. Post-operative TSS standardized pre-printed orders are shown in Appendix 1A and 1B in the Supplementary Material. Note that all transsphenoidal pituitary surgery patients are discharged home taking steroid (hydrocortisone or Cortef™) replacement. Following discharge, patients do a fasting 09:00 h blood test at 1 week, including serum sodium, potassium, cortisol, thyroid-stimulating hormone (TSH), and free thyroxin (fT4) after holding the dose of hydrocortisone the evening before the test and prior to taking the morning dose on the day of the test. The results of these tests are reviewed by endocrinology at the 2-week post-surgery visit. The testing protocol for cortisol has previously been published. Reference Munro, Elnenaei and Doucette10–Reference Watts and Tindall13 Briefly, if serum AM cortisol is <130 nmol/L then GC is continued, if 130–250 nmol/L then adrenocorticotrophic (ACTH) stimulation testing is done and if the post-ACTH cortisol is >500 nmol/L, or if serum AM cortisol is >250 nmol/L, then GC therapy is discontinued at the 2-week visit. Patients are asked specifically about symptoms of DI and, if present, appropriate investigations are conducted and management is initiated.
For this study, we retrospectively assessed all patients who underwent TSS for a sellar mass at our center between January 1, 2016 and December 31, 2019 and had a minimum follow-up of 3 months. All surgeries were conducted by the same neurosurgeon (D.B.C.) and all follow-up visits were conducted by the same HNP team. Prior to the implementation of the IHEC Physician’s Guide on January 1, 2016, the practice had been for endocrinology to be consulted on all patients undergoing TSS while still in the hospital. We assessed adherence to the protocols, positive and negative predictive value of the IHEC Physician’s Guide in identifying patients requiring IHEC, length of hospital stay, rate of readmission to hospital for endocrine complications prior to the 2-week endocrine assessment, and readmission to hospital for non-endocrine complications within 30 days of discharge. Tumor volume, using coronal and sagittal MRI + gadolinium images at the time of surgery, was calculated based on the following formula for an ellipsoid: (4/3)*pi*radius1*radius2*radius3 in mm3 and converted to mL. Categorical data were analyzed using chi-square and Fisher’s exact tests. Numerical data were analyzed by ANOVA or t-test. SPSS® and GraphPad Prism® were used for all statistical analyses.
The study was approved by the Nova Scotia Health Authority’s Ethics Review Board (http://www.nshealth.ca/research-ethics).
Results
Patient Demographics
A total of 116 patients (63 males and 53 females) underwent TSS, of which 109 had elective and 7 had emergency surgery. The overall mean age was 55.6 ± 2.6 years and the mean tumor volume was 7.5 ± 1.5 mL. Of these patients, 24 (14 females and 10 males) required IHEC and 92 (39 females and 53 males) did not require IHEC based on the IHEC Physician’s Guide. The mean age of the IHEC group was 53 ± 3.7 years and the no-IHEC group was 58.3 ± 1.4 years (p = 0.1). The mean tumor volumes were similar in both groups: 8.3 ± 2.1 mL in the IHEC group and 6.7 ± 0.8 mL in the no-IHEC group (p = 0.4). The pathology of sellar masses in the IHEC group included: non-functioning adenoma (NFA; n = 9), growth hormone (GH) adenoma (n = 5), Rathke’s cleft cyst (RCC; n = 2), craniopharyngioma (n = 1), meningioma (n = 1), thyroid-stimulating hormone (TSH) adenoma (n = 1), hypophysitis (n = 1), spindle cell oncocytoma (n = 2), metastatic thyroid cancer (n = 1) and metastatic breast cancer (n = 1). The pathology of sellar masses in the no-IHEC group included: NFA (n = 47), GH adenoma (n = 12), prolactinoma (n = 7), meningioma (n = 6), follicle-stimulating hormone (FSH) adenoma (n = 6), ACTH adenoma (n = 5), RCC (n = 3), clival chordoma (n = 2), craniopharyngioma (n = 1), hypophysitis (n = 1), metastatic lung cancer (n = 1), and myeloma (n = 1) (Table 1).
Table 1: Patient demographics, distribution of sellar masses and indications for surgery in IHEC and no IHEC patients based on the Physicians’ Guide
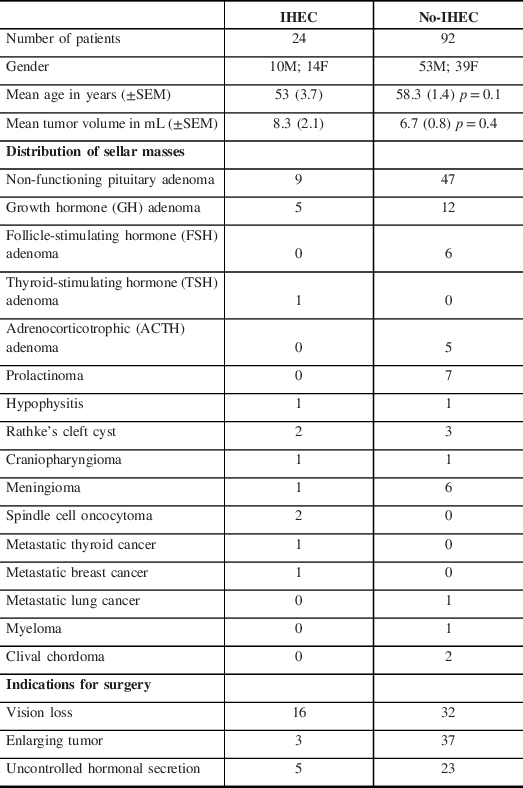
The indications for surgery in the IHEC group were vision loss (n = 16), uncontrolled hormonal secretion (n = 5), and enlarging tumor (n = 3). In the no-IHEC group, indications were enlarging tumor (n = 37), vision loss (n = 32), and uncontrolled hormonal secretion (n = 23) (Table 1). The indications for IHEC are summarized in Table 2; the most common indication for IHEC was aggressive stalk manipulation during surgery.
Table 2: Indications for IHEC based on the “Physician’s Guide to Determine Whether In-Hospital endocrinology Consultation is Required”
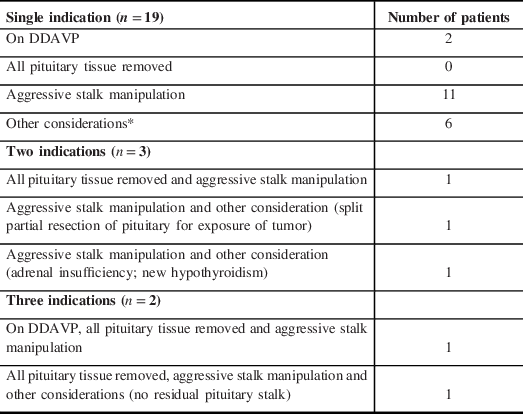
*Other considerations included split partial resection of pituitary for exposure of tumor, adrenal insufficiency, panhypopituitarism, hypogonadism, new hypothyroidism, no previous endocrinology evaluation prior to surgery.
Peri-operative Endocrine Complications and Readmissions
The most common peri-operative endocrinology concern that initiated a request for IHEC was the risk of central DI. Of the 24 patients in whom IHEC was requested, 19 (79%) developed symptoms of polyuria and polydipsia; 15 required desmopressin (DDAVP) therapy at discharge whereas 3 had transient symptoms which settled on their own without intervention and 1 patient received DDAVP at the first post-operative 2-week follow-up visit. Two other patients (12%) were managed for the syndrome of inappropriate secretion of anti-diuretic hormone (SIADH) during the hospital admission. Two patients had secondary hypothyroidism requiring replacement whereas no endocrine intervention was needed in the other patient. Following discharge from the hospital, one patient (who had in-hospital SIADH) required readmission within 30 days after discharge for significant hyponatremia. None of the remaining 23 patients required any endocrine intervention prior to their routine 2-week post-operative endocrine visit.
Of the 92 patients in the no-IHEC group, 2 (2%) developed transient polyuria and polydipsia during admission: one settled without any intervention and the other briefly required DDAVP that was later stopped at the 2-week post-operative endocrine visit. Two other patients developed symptoms of DI, one 2 weeks and the other 2 months after discharge; both were managed as outpatients. Five patients developed SIADH after discharge but prior to their routine 2-week post-operative endocrinology assessment, of which four required readmission for significant hyponatremia. All five patients had a full recovery.
Based on these data, the IHEC Physician’s Guide reliably predicted the need for IHEC in 23 out of 24 patients (sensitivity 0.96: 95% CI 0.7888–0.9989; positive predictive value 0.96: 95% CI 0.7888–0.9989) and reliably excluded 59 of 60 patients who did not require IHEC (specificity 0.99: 95% CI 0.9409–0.9997; negative predictive value 0.99: 95% CI 0.9409–0.9997); (Fisher’s exact test, p < 0.0001).
Pre-operatively 15 out of 24 IHEC patients required GC therapy and 29 out of 92 patients (no IHEC) required GC therapy. Serum AM cortisol levels before surgery were lower in patients requiring IHEC (223.3 ± SEM 41.1 nmol/L) compared with no-IHEC (308.6 ± SEM 19.6 nmol/L) (p < 0.05). While all patients were discharged home on GC therapy, persistent secondary adrenal insufficiency at the 2-week and 3-month post-operative visit occurred in 17 (75%) of 24 patients in the IHEC group and 16 (17.4%) of 92 patients in the no-IHEC group (p = 0.001).
In the IHEC group, 15 of 24 patients were adrenal insufficient prior to surgery (serum AM cortisol levels below 250 nmol/L), While 2 of these 15 patients regained adrenal function post-operatively at 2 weeks, 5 additional patients lost adrenal function post-operatively. Only 6 of 24 patients in the IHEC group had serum AM cortisol levels above 250 nmol/L at the 2-week and 3-month post-operative visit. In the no-IHEC group patients that required GC therapy pre-operatively (n = 29) 17 patients regained adrenal function 2-week post-operatively. However, 14 patients who did not require GC therapy pre-operatively required GC therapy for persistent (2-week and 3-month endocrinological evaluation) adrenal insufficiency. Sixty-six of 92 patients in the no-IHEC group had serum AM cortisol levels above 250 nmol/L at the 2-week and 3-month post-operative visit. Adrenal insufficiency was more likely post-operatively in patients requiring IHEC, with mean serum AM cortisol levels 141.8 ± 32.3 nmol/L compared to 304.1 ± 16.3 nmol/L in patients not requiring IHEC (p = 0.001).
Hospital Stay and Readmissions
As one would anticipate, patients in the IHEC group had more extensive/invasive surgical procedures: for example, 20 out of 24 (83%) IHEC group patients had fat/fascia grafts to repair intra-operative CSF leaks compared with 34 out of 92 (37%) in the no-IHEC group (p < 0.001). Consistent with this, the median length of stay in the hospital of the IHEC group was longer at 4 days (mean 6.9 ± 2.6 days) compared with 3 days (mean 2.7 ± 0.2 days) in the no-IHEC group patients (p < 0.01).
The rate of readmission for non-endocrine complications was also higher in the IHEC group (5 of 24; 20.8%) compared with the no-IHEC group (7 of 92; 7.6%; p = 0.01). The reasons for readmission in the IHEC group were cerebrospinal fluid (CSF) leak (n = 3), stroke (n = 1), and admission to another service for management of metastatic cancer (n = 1). Readmissions in the no-IHEC group were due to infection (n = 3: sepsis, sinusitis, and leg graft wound dehiscence), feeling unwell without an identifiable cause (n = 2), feeling unwell with a large nasal thrombus (n = 1), and CSF leak (n = 1).
Discussion
TSS accounts for approximately 20% of all intracranial surgeries Reference Black, Zervas and Candia14,Reference Jane, Sulton and Laws15 and, despite the refinements in the technique, the risk of endocrine complications remains significant including transient DI in 4–18%, permanent DI in 0.3–2% and new-onset anterior pituitary dysfunction in 5–25% patients. Reference Prete, Corsello and Salvatori4,Reference Agam, Wedemeyer and Wrobel16,Reference Nemergut, Zuo and Jane17 Consequently, current neurosurgery and endocrine guidelines recommend coordinated care for all post-operative TSS patients by a multidisciplinary team. Reference Prete, Corsello and Salvatori4,Reference Carminucci, Ausiello and Page-Wilson7–Reference Woodmansee, Carmichael and Kelly9 Following these best practice guidelines, all patients undergoing TSS in our center routinely underwent endocrine assessment prior to the initiation of the current study. Although having all post-operative TSS patients managed by a multidisciplinary team appears to intuitively make sense, the added value of having all TSS patients followed post-operatively while in hospital by an endocrinologist has not been formally assessed. Furthermore, we frequently found that a patient’s discharge from the hospital was delayed while waiting for an IHEC to be completed. The fact that most TSS patients do not develop post-operative endocrine dysfunction requiring in-hospital input from an endocrinologist, we questioned whether the routine assessment of all TSS patients is always necessary. The results of this study show that our IHEC Physician’s Guide, completed by the surgical team immediately at the end of surgery, can be used to determine who will, and who will not, need an IHEC.
Our study showed that while the overall risk of endocrine complications following TSS in the full cohort was similar to previous reports at 21%, most patients did not develop new-onset endocrine dysfunction after surgery. Reference Prete, Corsello and Salvatori4,Reference Agam, Wedemeyer and Wrobel16,Reference Nemergut, Zuo and Jane17 The predominant endocrine complication requiring IHEC was almost exclusively related to DI and/or SIADH. Previous studies have shown that the risk of endocrine complications after TSS is associated with the experience of the neurosurgeon, the extent of surgical manipulation, and the consistency of the tumor. Reference Ciric, Ragin and Baumgartner18,Reference Chee, Mathias and James19 The primary indications for IHEC in our study included aggressive stalk manipulation and an assortment of “other” patient-specific reasons (Table 2). Other studies have also reported a higher risk of endocrine complications after TSS in larger tumors, tumor with firm or hard consistency and where the stalk is aggressively manipulated during surgery. Reference Chee, Mathias and James19–Reference Shur, Lasry and Tewfik21 In our study, the size of the tumor and age of the patients did not differ between the two groups.
Delayed post-operative hyponatremia due to SIADH has been reported as being common after TSS, reported in a wide range from 2.3 to 53% of patients. Reference Hussain, Piper and Ludlam22–Reference Taylor, Tyrrell and Wilson25 The overall risk of delayed SIADH after discharge in our cohort was similar in the IHEC (4.2%) and no-IHEC (5.4%) groups. It is important to note that the IHEC Physician’s Guide was not designed for, and does not reliably predict, the development of delayed SIADH; therefore, all patients should be routinely assessed for hyponatremia after discharge.
It is noteworthy that we use standardized post-operative order sheets for managing GC therapy, monitoring fluid status, ordering laboratory investigations, and managing DI in all TSS patients (see Appendix 1 in Supplementary Material). Furthermore, we routinely treat all TSS patients with peri-operative GC therapy (Appendix 1A) and all patients are discharged on GC until assessed by endocrinology approximately 2 weeks after discharge. While some groups have advocated measuring immediate post-operative serum cortisol to assess the need for GC replacement, Reference Inder and Hunt26 in our center we found this difficult to implement due to inconsistent timings of the test and controversy regarding the most appropriate cutoff value of post-operative serum cortisol. We have previously published results from our practice of discharging patients on GC and assessing the need for ongoing GC therapy at the 2-week post-operative visit. Reference Munro, Elnenaei and Doucette10–Reference Yip, Stewart and Imran12 Our data also show that in the IHEC patients, persistent secondary adrenal insufficiency occurred in 75% of patients; 28.2% of patients in the no-IHEC group also had persistent secondary adrenal insufficiency at the initial 2-week post-operative visit. These data remind us that the IHEC guide was not designed for, and cannot be used as, a reliable predictor to determine whether or not a patient requires post-operative GC therapy.
Our study has important limitations, in that it is a single-center experience where all TSS were conducted by the same neurosurgery team. Standardized orders assist our in-hospital management team (including residents and nurses) who are confident in fluid management, including serum electrolyte disturbances; this may not be the case in all institutions. There is considerable subjectivity to the use of the IHEC Physician’s Guide: for example, it is conceivable that different neurosurgeons may perceive stalk manipulation differently and perhaps a more standardized classification may need to be developed to grade the degree of manipulation.
In conclusion, results from our study demonstrate that while endocrine dysfunction is commonly seen after TSS, most patients do not require IHEC. Furthermore, the necessity for IHEC can be predicted immediately at the end of surgery using the IHEC Physician’s Guide.
Acknowledgments
We wish to acknowledge Ms. Lisa Tramble RN for identifying and testing some of the suspected DI patients after discharge from the hospital.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
All authors (DBC, ALOH, EM, and SAI) contributed to data collection, analysis, drafting and revision of the manuscript. All authors approved the final submission.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.226.





