Introduction
Superficial lymph node abscesses in the neck are a common cause of hospital admission and emergency surgery in children. Conservative management is often sufficient for cases of minor cervical lymphadenopathy.Reference Çetin, Olgun, Özses and Erdağ1 However, when these progress to form pockets of pus, surgical incision and drainage may be required. Suppuration may occur in various tissue spaces in the neck, and so a superficial lymph node abscess must be distinguished from infection in a deeper location such as the retropharyngeal, parapharyngeal and prevertebral spaces. Depending on severity and location, complications may include bacteraemia, airway compromise and Lemierre's syndrome (septic thrombophlebitis of the internal jugular vein).Reference Cabrera, Deutsch, Eppes, Lawless, Cook and O'Reilly2 Definitive management usually involves intravenous antimicrobial therapy.Reference Morales-Angulo, Bezós Capelastegui, García Mantilla, Carrera Herrero and Pía Roiz3
Having up-to-date information on local patterns of microbiology and antibiotic sensitivity is helpful for initiating empirical antibiotic therapy. This is particularly important given the rapidly growing rates of resistance among commonly encountered isolates.
Our hospital published a report detailing the microbiology of neck abscesses in children between January 1996 and December 2000.Reference Wynne, Borg, Botma and Macgregor4 Here, we present an updated case series, with the aim of comparing our more recent findings with these historical data to highlight any changes in microbiology, sensitivity rates and antibiotic prescribing patterns for superficial lymph node abscesses. As the Royal Hospital for Children in Glasgow is the largest children's hospital in Scotland, with the busiest paediatric unit in the UK for emergency work (unpublished data from Civil Eyes Ltd, 2017), our results may be useful across the UK and beyond.
Materials and methods
This study reports a retrospective review of all patients admitted to the Royal Hospital for Children in Glasgow, between April 2018 and April 2021, with a superficial lymph node abscess of the neck requiring incision and drainage. Patients were primarily identified from the hospital's electronic operating theatre database, cross-referenced by manually searching an archive of the department's daily ward round handover sheets.
Patients were only included if they had culture and sensitivity testing performed on a sample obtained intra-operatively, were prescribed antibiotics, and were aged 16 years or younger.
Deep neck space abscesses (retropharyngeal, parapharyngeal and prevertebral abscesses) were excluded, as were peritonsillar, pre-auricular, mastoid and dental abscesses. Cases of infection in a pre-existing congenital cyst (such as a thyroglossal cyst or lymphatic malformation) were excluded. Patients with underlying malignancy or immunodeficiency were also excluded. Finally, patients with microbiological data from swabs taken without surgical intervention, and those with lymphadenopathy undergoing excisional biopsies to exclude malignancy, were excluded.
The patients’ electronic records were reviewed for age, sex, diagnosis, antibiotic therapy, length of stay and microbiological culture results. All microbiological data were based on local conventional protocols for culture and sensitivity testing, following guidelines published by the European Committee on Antimicrobial Susceptibility Testing.5 This includes the agar types inoculated, incubation periods, and thresholds for minimal inhibitory concentrations or zone diameters.
Comparisons were made with the findings of a previously published study from our own hospital based on cases from 1996 to 2000.Reference Wynne, Borg, Botma and Macgregor4
Results
Patient characteristics
Thirty-eight children underwent 39 incision and drainage procedures for a superficial lymph node abscess of the neck, with a mean of 13 cases per year. One patient presented with a recurrence of the abscess, requiring a repeat incision and drainage procedure. The children ranged in age from 3 months to 15 years (median of 2 years; mean of 3 years and 2 months), and 22 (58 per cent) were female. Thirty-one patients (82 per cent) underwent surgical management within 24 hours of admission.
Culture results
The most common micro-organism identified was methicillin-susceptible Staphylococcus aureus, in a total of 11 cases (28 per cent), followed by Streptococcus pyogenes (5 cases, 13 per cent). Four abscesses (10 per cent) had species from the Streptococcus anginosus group isolated (which includes S anginosus, Streptococcus intermedius and Streptococcus constellatus). Mixed anaerobic organisms were identified in three cases. The patient with the recurrent neck abscess had the same micro-organism isolated both times (methicillin-susceptible S aureus). All identified micro-organisms are summarised in Table 1. A graphical comparison with the historical series is depicted in Figure 1.
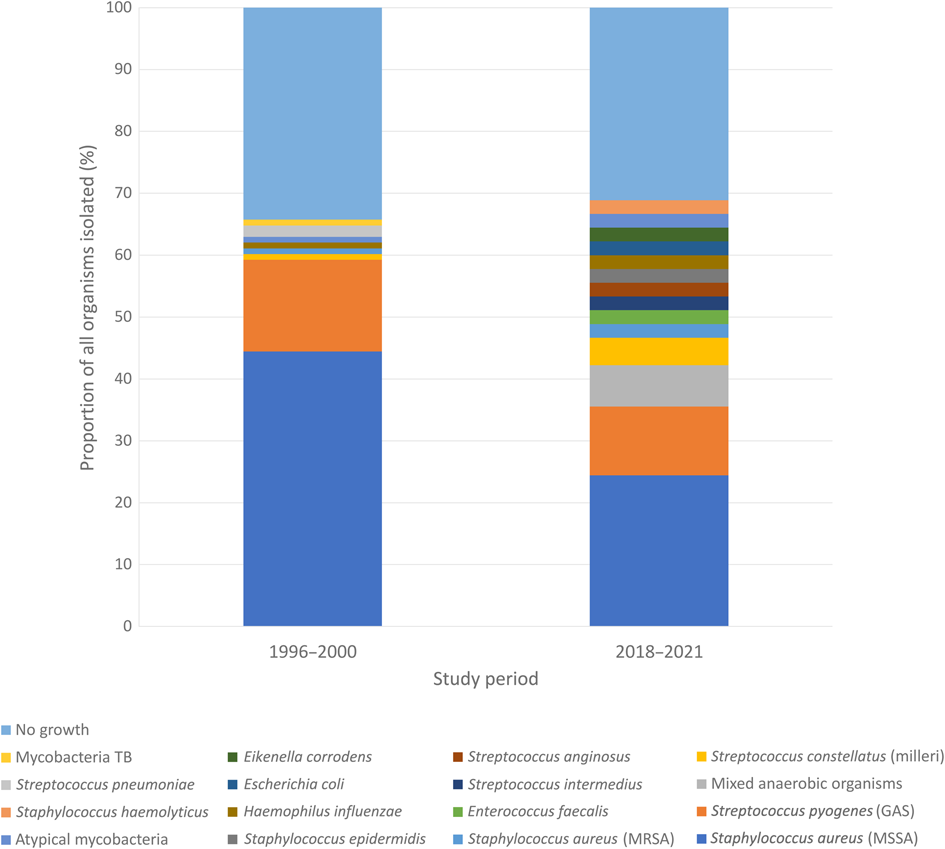
Fig. 1. Microbiology results for 2018–2021 and 1996–2000, expressed as a percentage of all microbes reported. TB = tuberculosis; MRSA = methicillin-resistant S aureus; GAS = group A streptococcus; MSSA = methicillin-susceptible S aureus
Table 1. Microbiology results for 2018–2021 and 1996–2000 study periods
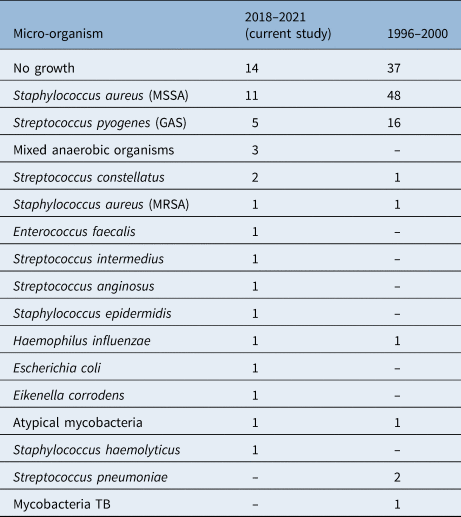
Data represent numbers of cases. MSSA = methicillin-susceptible S aureus; GAS = group A streptococcus; MRSA = methicillin-resistant S aureus; TB = tuberculosis
Antibiotic sensitivity and resistance
Sensitivity testing results for the commonest bacteria, S aureus and S pyogenes, are presented in Table 2. Of note, there was a single case of methicillin-resistant S aureus (MRSA), and another of S pyogenes resistant to both clarithromycin and clindamycin. Other resistances encountered among less common pathogens included ampicillin/amoxicillin-resistant Haemophilus influenzae, and Eikenella corrodens resistant to both metronidazole and clindamycin.
Table 2. Antibiotic sensitivities and resistances for Staphylococcus aureus and Streptococcus pyogenes *
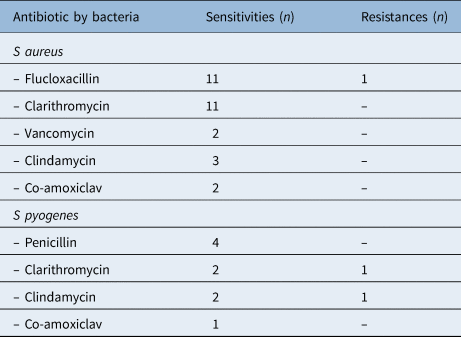
* For 2018–2021 study period
Empirical antibiotic therapy
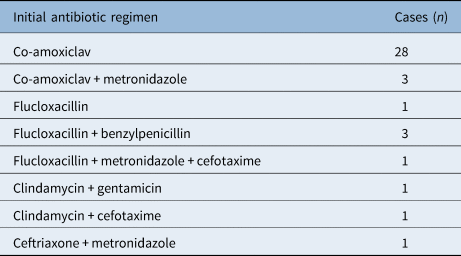
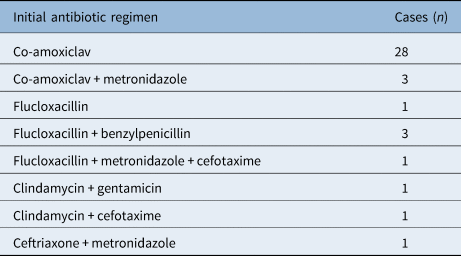
All initial antibiotic regimens are presented in Table 3, the most common being intravenous co-amoxiclav (28 cases, 72 per cent). One patient had their regimen changed before incision and drainage (co-amoxiclav switched to clindamycin and cefuroxime). Two patients had their antibiotics changed after a ‘no growth’ microbiology result because of a lack of clinical response: metronidazole was added to co-amoxiclav in one case, and co-amoxiclav was substituted with ceftriaxone in the other case.
Clinical outcomes
Length of hospital stay ranged from 1 to 5 days, with a mean of 2.8 days. At discharge from hospital, 36 patients (92 per cent) were prescribed ongoing oral antibiotic therapy. This consisted of co-amoxiclav in 34 children, with the remaining children receiving clindamycin (1 child) and clarithromycin (1 child). One child had a recurrence of their abscess, requiring re-admission and repeat incision and drainage. The child with the abscess due to non-tuberculous mycobacterial infection had ongoing discharge for approximately five months.
Discussion
Study strengths and weaknesses
Strengths of the study include the availability of historical data for comparison and thorough ascertainment by cross-referencing two sources of data. The study's main limitation is its retrospective design. Additionally, the inclusion criteria meant that only drained abscesses were analysed. Although medical records were all manually reviewed (meaning no relevant cases have been missed), some patients had their care continued at a different institution, which might have led to missing data on outcomes and complications. Furthermore, variability in clinician documentation of diagnosis, operative procedures and outcomes may have led to ascertainment bias. Finally, as most patients were administered antibiotics prior to drainage, the presence of certain pathogens may have been underestimated.
Patient characteristics
When compared to the historical data from 1996 to 2000, there was a drop in the mean number of abscesses drained, from around 24 to 13 per year.Reference Wynne, Borg, Botma and Macgregor4 This may be attributed to earlier identification of abscesses and prompt antibiotic management. Furthermore, lower thresholds for referrals by general practitioners might mean that only patients with abscesses caused by highly virulent micro-organisms proceed to undergo incision and drainage. Nevertheless, the role of the coronavirus disease 2019 pandemic, which significantly overlapped with the study's timeframe, cannot be negated. Social distancing undeniably blunted the transmission of common upper respiratory tract infections, which predispose to head and neck abscesses.Reference Nascimento, Baggio, Fascina and do Prado6
The relatively short mean length of hospital stay (2.8 days) compares favourably to historical data from our hospital (5 days) and to reports from other UK centres.Reference Wynne, Borg, Botma and Macgregor4,Reference Rustom, Sandoe and Makura7 This may be attributed to the exclusion of abscesses managed conservatively, which often require a lengthier period of hospitalisation.
Microbiology
We are pleased to see that almost all incision and drainage procedures resulted in a swab being sent to the microbiology department for culture testing. This in contrast to the situation in the original report from our hospital 20 years ago, when it was stated how it is ‘disappointing that incision and drainage procedures did not always result in the submission of a bacteriology swab for culture and sensitivity, the results of which could aid in the choice of antibiotic cover in difficult cases’.Reference Wynne, Borg, Botma and Macgregor4
A significant number of samples were reported as having ‘no growth’ in our study population (36 per cent of abscesses). This rate is similar to that in the 1996–2000 cohort, but slightly higher than reported elsewhere.Reference Rustom, Sandoe and Makura7,Reference Tom and Rice8 In some cases, this may be because of a failure to culture organisms that require special media, such as mycobacteria, but it is more likely to be because most patients received a dose of antibiotics from their general practitioner prior to admission.
Sensitivity reporting has evolved over the past two decades; therefore, direct comparison between our series and historical data are not always straightforward. For example, benzylpenicillin resistance is less often reported given its limited role in today's practice, and erythromycin has largely been replaced in clinical practice by clarithromycin.
The predominance of S aureus and S pyogenes in cultures from paediatric neck abscesses is common in published reports from the early 2000s, from the UK and elsewhere, including the historical series from our own hospital.Reference Wynne, Borg, Botma and Macgregor4,Reference Walker, Karnell, Ziebold and Kacmarynski9–Reference Fellner, Marom, Muallem-Kalmovich, Shlamkovitch, Eviatar and Lazarovitch11 Although methicillin-susceptible S aureus remains the most common cause of superficial neck abscesses in children, this study has shown a decline from a rate of 46 per cent to 28 per cent over the past 20 years. Similarly, there was a fall in the proportion of cases of S pyogenes, although this was less substantial. This was met by a shift towards a variety of rarer organisms.
Despite the shorter study period, 14 different pathogens were isolated between 2018 and 2021, whereas only 8 isolates were reported from 1996 to 2000. Bacteria solely identified in the more recent cohort include Escherichia coli, E corrodens, Enterococcus faecalis, S constellatus and S anginosus.
Among the less common micro-organisms, an increased prevalence of the S anginosus group (formerly known as Streptococcus milleri) is most noticeable, which includes S anginosus, S intermedius and S constellatus. Abscesses caused by the S anginosus group have reportedly increased over the last two decades, with recent studies highlighting their true pathogenic role.Reference Jiang, Li, Fu, Shan, Jiang and Shao12–Reference Siegman-Igra, Azmon and Schwartz14 This is likely to represent improved laboratory diagnostic techniques; in the past, S anginosus group micro-organisms were frequently mistaken for commensal contamination, especially in polymicrobial abscesses.Reference Asam and Spellerberg15 A study conducted at Texas Children's Hospital in 2019 showed an unexplained rise in S anginosus group infections among children with complicated sinusitis.Reference McNeil, Dunn, Kaplan and Vallejo16 A UK-based case series concluded that approximately 67 per cent of intracranial abscess and sinusitis cultures grew S anginosus group species.Reference Patel, Masterson, Deutsch, Scoffings and Fish17 Thus, although the specific role of the S anginosus group in neck abscesses has not yet been addressed in the literature, their prevalence in paediatric otolaryngological infections seems to be on the rise. This is of particular concern as S intermedius may be associated with haematogenous spread, and S anginosus poses a risk of Lemierre's syndrome (in addition to the more widely known Fusobacterium necrophorum).Reference Clarridge, Attorri, Musher, Hebert and Dunbar18,Reference Camacho-Cruz, Preciado, Beltrán, Fierro and Carrillo19
Streptococcus pneumoniae and Mycobacterium tuberculosis were the only two micro-organisms detected in the historical study but not in the current study. Over the past two decades, the UK has benefitted greatly from the widespread administration of the pneumococcal vaccine to children,Reference Yildirim, Shea and Pelton20 and, fortunately, tuberculosis remains rare.21
The rate of MRSA was relatively low in this series, with only one case identified. This is significantly lower than rates reported elsewhere, with some hospitals finding MRSA in up to 58 per cent of paediatric neck abscesses.Reference Brook22 Nevertheless, it is well-established that MRSA incidence remains low across Europe.Reference David and Daum23
• The yearly rate of drained superficial neck abscesses in children has fallen over the last two decades
• S aureus and S pyogenes remain the commonest micro-organisms, but their incidence has decreased significantly
• Relatively uncommon micro-organisms, particularly members of the S anginosus group, are now implicated in a greater proportion of neck abscesses
• Empirical co-amoxiclav monotherapy is recommended
• Second-line treatment options include clindamycin and cefotaxime
Implications for antibiotic therapy
There remains a lack of consensus regarding first-line antibiotic choices. Previous studies have proposed empirical use of flucloxacillin.Reference Rustom, Sandoe and Makura7 However, with the recent shift in microbiology, an anti-staphylococcal penicillin may not be always appropriate. As the majority of children given co-amoxiclav monotherapy did not require a change in their regimen (89 per cent), we recommend that it continues to be used as first-line treatment. Its wide coverage (including empirical anaerobic coverage) is advantageous with the increased prevalence of virulent microbes.
Nevertheless, strict policies on de-escalation are needed after microbiology results become available. A step-down approach would be reasonable in some instances to avoid the unnecessary administration of broad-spectrum antibiotics.
Overall, metronidazole was overused; it was administered to six patients, although mixed anaerobes were cultured in only three instances (8 per cent of abscesses – a significantly lower rate than suggested in the literature).Reference Rustom, Sandoe and Makura7,Reference Coticchia, Getnick, Yun and Arnold24 Metronidazole was not discontinued post-operatively in any of the remaining cases.
The occasional presence of relatively uncommon organisms in this context (including E faecalis, E coli and E corrodens), as well as virulence factors in common organisms, should be borne in mind if the child is not responding to standard empirical antibiotic therapy. Alternative antibiotics should be considered for use as second-line treatment. These may include agents such as vancomycin, clindamycin (which can suppress toxin production in some organisms), cefotaxime or other Gram-negative active agents, depending on culture results.
Conclusion
Our case series highlights a significant decline in the yearly number of drained abscesses. Similarly, the incidence of micro-organisms previously considered common is falling, including S aureus and S pyogenes. We now recommend co-amoxiclav monotherapy as a first-line treatment.
Data availability statement
Study data are available from the corresponding author upon reasonable request.
Competing interests
None declared