Introduction
Lobariaceae species are among the most conspicuous macrolichens (Galloway Reference Galloway1985, Reference Galloway2007; Brodo et al. Reference Brodo, Duran Sharnoff and Sharnoff2001; Wirth et al. Reference Wirth, Hauck and Schultz2013; Stenroos et al. Reference Stenroos, Velmala, Pykälä and Ahti2016). They are usually found in wet, temperate to tropical ecosystems. Currently, 466 species are accepted in 12 genera (Galloway & Elix Reference Galloway and Elix2013; Moncada et al. 2013 Reference Moncada, Lücking and Betancourt-Macuasea ; Galloway Reference Galloway2015; Jaklitsch et al. Reference Jaklitsch, Baral, Lücking and Lumbsch2016; Lücking et al. Reference Lücking, Hodkinson and Leavitt2017). Except for tropical species of Sticta (Schreb.) Ach., the family is richest in the Southern Hemisphere (Galloway et al. Reference Galloway, Stenroos and Ferraro1995; Galloway Reference Galloway2001) and has been particularly well studied in New Zealand with 66 species currently accepted (Galloway Reference Galloway2007). These correspond to ten genera under the current genus concept, two of them (Ricasolia De Not. and Yoshimuriella Moncada & Lücking) requiring confirmation (see below) and two, namely Dendriscosticta Moncada & Lücking and Lobariella Yoshim., being absent from the country (Moncada et al. Reference Moncada, Ranft, de Lange, Blanchon, Knight, Lumbsch and Lücking2016). Additional species have been reported from oceanic possessions of New Zealand (de Lange & Galloway Reference de Lange and Galloway2015).
Lichens have long been used as bioindicators and species in Lobariaceae are excellent indicators of forest health (Rose Reference Rose1974, Reference Rose1976, Reference Rose1992; Selva Reference Selva1994, Reference Selva1996; Zedda Reference Zedda2002). A prime example is Lobaria pulmonaria (L.) Hoffm., which has been extensively studied in this respect in Europe and North America (Søchting & Christensen Reference Søchting and Christensen1989; Gauslaa Reference Gauslaa1994; McCune Reference McCune2000; Campbell & Fredeen Reference Campbell and Fredeen2004; Kalwij et al. Reference Kalwij, Wagner and Scheidegger2005; Jüriado & Liira Reference Jüriado and Liira2009; Scheidegger Reference Scheidegger2009; Nascimbene et al. Reference Nascimbene, Brunialti, Ravera, Frati and Caniglia2010; Gustafsson et al. Reference Gustafsson, Fedrowitz and Hazell2013; Cansaran-Duman et al. Reference Cansaran-Duman, Altunkaynak, Aslan, Büyük and Aras2015; Giorgio et al. Reference Giorgio, Luisa and Sonia2015). Other Lobariaceae considered rare and threatened by land use change in the Northern Hemisphere, including Sticta fuliginosa (Dicks.) Ach., S. limbata (Sm.) Ach. and S. sylvatica (Huds.) Ach. (Hodkinson et al. Reference Hodkinson, Lendemer, McDonald and Harris2014; Magain & Sérusiaux Reference Magain and Sérusiaux2015), offer similar potential for bioindication of ecological continuity. Recent phylogenetic studies have shown that species concepts in these taxa require critical revision (Moncada et al. 2014 Reference Moncada, Lücking and Suáreza ; Magain & Sérusiaux Reference Magain and Sérusiaux2015), which will have an influence on their use as bioindicators.
In the tropics and the Southern Hemisphere, Lobariaceae have been used or mentioned as potential bioindicators of forest health based on studies in Cuba (Rosabal et al. Reference Rosabal, Burgaz and De la Masa2010), Colombia (Simijaca-Salcedo et al. Reference Simijaca-Salcedo, Vargas-Rojas and Morales-Puentes2014; Ramírez-Morán et al. Reference Ramírez-Morán, León-Gómez and Lücking2016), French Guiana (Normann et al. Reference Normann, Weigelt, Gehrig-Downie, Gradstein, Sipman, Obregon and Bendix2010), Chile (Galloway Reference Galloway1992) and Thailand (Wolseley et al. Reference Wolseley, Moncrieff and Aguirre-Hudson1994). Despite a wealth of literature dealing with Lobariaceae (Galloway Reference Galloway1985, Reference Galloway1988, Reference Galloway2007; de Lange et al. Reference de Lange, Galloway, Blanchon, Knight, Rolfe, Crowcroft and Hitchmough2012; de Lange & Galloway Reference de Lange and Galloway2015), New Zealand lags behind in using lichens (and especially Lobariaceae) for conservation monitoring (Galloway Reference Galloway2008; de Lange et al. Reference de Lange, Galloway, Blanchon, Knight, Rolfe, Crowcroft and Hitchmough2012). This is all the more remarkable considering that New Zealand’s native ecosystems, like many other island biotas, have been and continue to be severely affected by humans and invasive plants and animals (Atkinson Reference Atkinson1989; Holdaway Reference Holdaway1989; King Reference King1990; Anderson Reference Anderson2002; Worthy & Holdaway Reference Worthy and Holdaway2002; Tennyson & Martinson Reference Tennyson and Martinson2006; Prebble & Wilmshurst Reference Prebble and Wilmshurst2009; de Lange et al. Reference de Lange, Heenan, Norton, Rolfe and Sawyer2010; Brown et al. Reference Brown, Stephens, Peart and Fedder2015).
Hutcheson et al. (Reference Hutcheson, Walsh and Given1999) provided a summary of the use of bioindicators in New Zealand as a means of determining ecosystem health, pointing out the general lack of studies and near absence of work with lichens; observations confirmed by Galloway (Reference Galloway2008). De Lange et al. (Reference de Lange, Galloway, Blanchon, Knight, Rolfe, Crowcroft and Hitchmough2012) published the first ever threat assessment for the New Zealand lichen biota, including their lichenicolous fungi, noting that for lichens assessed as ‘threatened’ or ‘at risk’, ecosystem degradation and habitat loss were significant factors in causing their decline. The majority of New Zealand’s lichen biota listed by de Lange et al. (Reference de Lange, Galloway, Blanchon, Knight, Rolfe, Crowcroft and Hitchmough2012) were assessed as ‘data deficient’, one of the issues being the correct application of names, especially of presumably cosmopolitan or predominantly Northern Hemisphere lichens thought to occur in New Zealand, including those in Lobariaceae.
Only a small number of studies have so far addressed the problem of species recognition in lichen fungi in New Zealand with molecular techniques (Summerfield et al. Reference Summerfield, Galloway and Eaton-Rye2002; Thomas et al. Reference Thomas, Ryan, Farnden and Galloway2002; Parnmen et al. Reference Parnmen, Rangsiruji, Mongkolsuk, Boonpragob, Nutakki and Lumbsch2012, Reference Parnmen, Leavitt, Rangsiruji and Lumbsch2013; Buckley et al. Reference Buckley, Rafat, Ridden, Cruickshank, Ridgway and Paterson2014; Hayward et al. Reference Hayward, Blanchon and Lumbsch2014; Myles Reference Myles2014; Pino-Bodas et al. Reference Pino-Bodas, Burgaz, Martín, Ahti, Stenroos, Wedin and Lumbsch2015). Multi-locus approaches or studies using the ITS barcoding locus (Schoch et al. Reference Schoch, Seifert, Huhndorf, Robert, Spouge, Levesque and Chen2012) have shown that species delimitation in Lobariaceae is complex and that names globally applied to certain morphotypes, such as Pseudocyphellaria crocata (L.) Vain., Sticta dichotoma Bory and S. fuliginosa, represent numerous lineages (Magain et al. Reference Magain, Goffinet and Sérusiaux2012; Moncada et al. 2014 Reference Moncada, Lücking and Suáreza , Reference Moncada, Reidy and Lückingb ; Magain & Sérusiaux Reference Magain and Sérusiaux2015). As Lobariaceae are conspicuous lichens within the majority of New Zealand’s ecosystems and have tremendous potential for use as bioindicators, testing current species delimitations (Galloway Reference Galloway1985, Reference Galloway1988, Reference Galloway1997, Reference Galloway2007) using molecular data is critically needed, enabling accurate quantitative studies to establish monitoring protocols with these lichens.
Here we focus on the Sticta filix morphodeme, pedunculate species of Sticta that associate with green-algal primary photobionts but often form dendriscocauloid photomorphs with cyanobacterial secondary photobionts. This enigmatic Sticta-Dendriscocaulon morphodeme is restricted to the Southern Hemisphere, being replaced in the tropics and the Northern Hemisphere by other genera with morphologically similar cyanobionts, such as Dendriscosticta, Ricasolia and Yoshimuriella (Tønsberg & Goward Reference Tønsberg and Goward2001; Stenroos et al. Reference Stenroos, Stocker-Wörgötter, Yoshimura, Myllys, Thell and Hyvönen2003; Takahashi et al. Reference Takahashi, Wang, Tsubota and Deguchi2006; Moncada et al. 2013 Reference Moncada, Lücking and Betancourt-Macuasea ; Tønsberg et al. Reference Tønsberg, Blom, Goffinet, Holtan-Hartwig and Lindblom2016). Although Ricasolia (with R. adscripta (Nyl.) Nyl.) and Yoshimuriella (with Lobaria dictyophora (Müll. Arg.) D. J. Galloway) are possibly present in New Zealand (Moncada et al. Reference Moncada, Ranft, de Lange, Blanchon, Knight, Lumbsch and Lücking2016), they do not form dendriscocauloid cyanomorphs; L. dictyophora has been suggested to be included in the L. peltigera group (=Yoshimuriella; Moncada et al. 2013 Reference Moncada, Lücking and Betancourt-Macuasea ) by Galloway (Reference Galloway2007), but differs in its primary cyanobacterial photobiont (which is why it would not be expected to form a dendriscocauloid cyanomorph) and its chemistry, lacking gyrophoric acid (Galloway Reference Galloway1985, Reference Galloway2007).
In New Zealand, the S. filix morphodeme is found in temperate rainforests, mostly in shaded microhabitats growing near the base or on the lower part of large trees among bryophytes, small ferns and other small, shade-tolerant plants. Galloway (Reference Galloway1985, Reference Galloway1988, Reference Galloway1997, Reference Galloway2007) listed three species of this ‘guild’ for New Zealand: S. filix (Sw.) Nyl., S. lacera (Hook. f. & Taylor) Müll. Arg. and S. latifrons A. Rich. Of these, S. filix and S. latifrons form large, conspicuous lichens whereas S. lacera is comparatively small and easily overlooked or mistaken for Pseudocyphellaria multifida with which it often co-occurs. Sticta lacera is presumed to be endemic for New Zealand while the other two species have been reported from (south-)eastern Australia (Galloway Reference Galloway2001). To provide a solid base for the use of this guild and its photomorphs as bioindicators, we assessed its phylogenetic relationships and species delimitation using a combination of molecular (ITS barcoding locus) and morphological data.
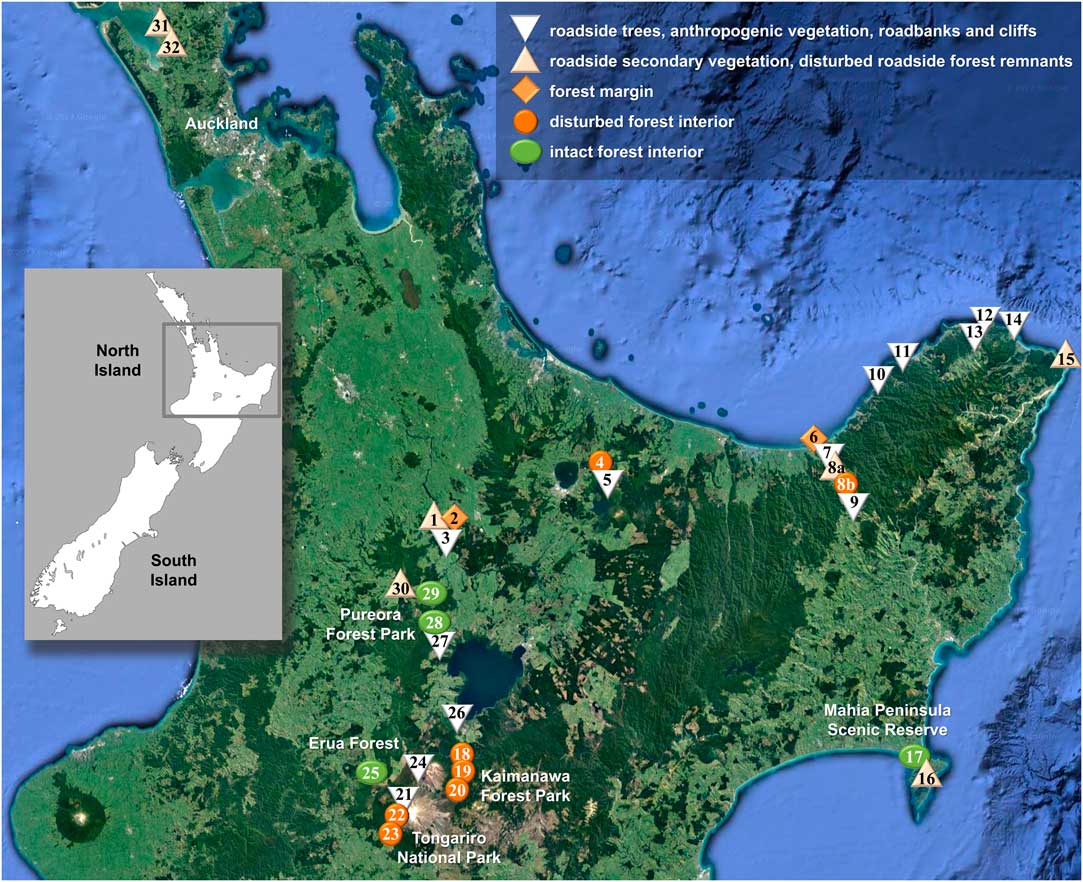
Fig. 1 Map showing location of sites visited for this study on New Zealand’s North Island plotted on a Google Earth satellite map depicting forest cover (https://www.google.com/earth; ©Nasa, TerraMetrics). Symbols correspond to conservation status categories as indicated in legend. The site numbers correspond to those in Table 1. In colour online.
Materials and Methods
Fieldwork was undertaken by BM, RL and PDL, accompanied in part by D. Blanchon, in February 2015 on New Zealand’s North Island, visiting a total of 33 sites (Table 1, Fig. 1). 1084 specimens were collected, 745 of which were Lobariaceae; all specimens were photographed in situ and then curated for voucher documentation with small pieces of medulla used for DNA extraction. Specimens were deposited in AK and UNITEC, with duplicates in B and F. At the Field Museum, dried Lobariaceae specimens were scanned by HR, both upper and lower side, yielding 1374 high resolution images (352 for Sticta), for assessment of morphometric characters such as lobe width, internode length, cyphellae diameter, as well as other morphological features. Additional images, particularly of dendriscocauloid morphs, were taken at the Botanic Garden and Botanical Museum (BGBM) with a SONY Cybershot G digital camera attached to a Zeiss Zoom 2000 dissecting microscope. Morphological and anatomical characters were further assessed using Leica MS5 and Motic K400 dissecting microscopes and Zeiss Axioscop 2 VistaVision VWR V036 compound microscopes. Material representing the Sticta filix morphodeme was initially identified with the five names provided by Galloway (Reference Galloway2007), namely the chloromorphs as S. filix, S. lacera and S. latifrons, and the cyanomorphs as Dendriscocaulon dendriothamnodes and D. dendroides.
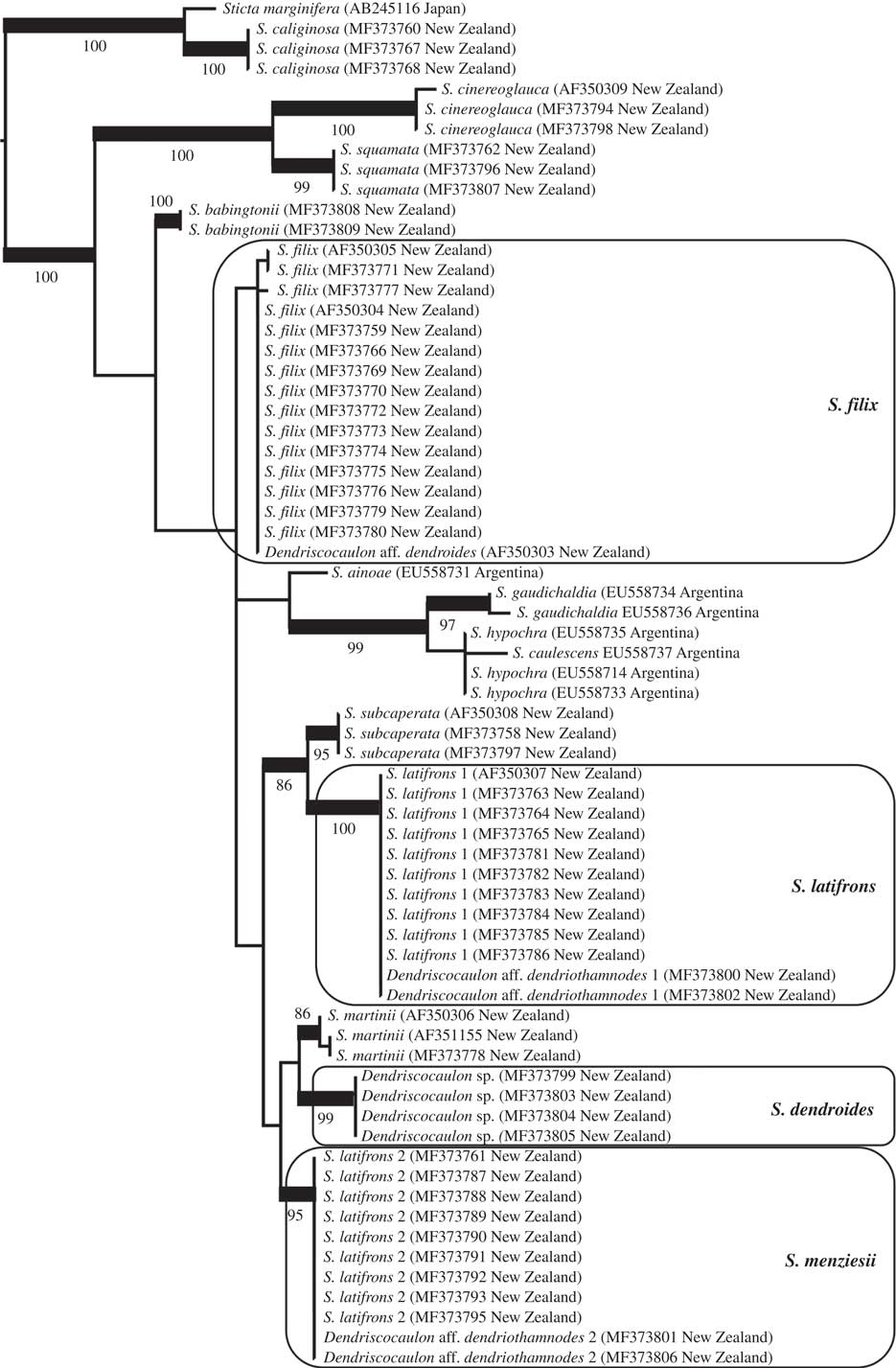
Fig. 2 Best-scoring maximum likelihood tree based on phylogenetic analysis of Sticta species using the ITS barcoding locus, focusing on the S. filix guild and other, mostly New Zealand taxa found basally in the Sticta phylogeny (see Moncada et al. 2014 Reference Moncada, Lücking and Suáreza : 218, fig. 3, for a broader context). Supported clades are thickened and bootstrap values indicated. The clades of interest are marked in boxes and final names applied to each clade are given.
Table 1 Sites visited for this study on New Zealand’s North Island, including coordinates (Lat., Long.), altitude, vegetation type and habitat, conservation status (Stat), and number of lichen specimens (Spec) collected at each site. The site numbers correspond to those in Fig. 1
DNA extraction and sequencing were performed by BM at the Pritzker Laboratory for Molecular Systematics and Evolution at the Field Museum. Sequences of the internal transcribed spacer (ITS) were targeted for all specimens of the Sticta filix morphodeme and 52 new sequences were generated for this study. DNA was extracted using the SIGMA REDExtract-N-Amp Plant PCR Kit (St. Louis, Missouri, USA). Dilutions of 10:1 up to 10:2 were used for PCR amplifications with the primer pairs ITS1F and ITS4 (Gardes & Bruns Reference Gardes and Bruns1993; White et al. Reference White, Bruns, Lee and Taylor1990). The 25 µl PCR reactions contained 2·5 µl buffer, 2·5 µl dNTP mix, 1 µl of each primer (10 µM), 5 µl BSA, 2 µl Taq, 2 µl genomic DNA extract and 9 µl distilled water. The thermal cycling parameters were set as follows: initial denaturation for 3 min at 95°C, followed by 30 cycles of 1 min each at 95°C, 52°C and 73°C with a final elongation for 7 min at 73°C. Amplification products were mounted on 1% agarose gels stained with ethidium bromide and, after cutting of the target bands, purified using the Qiagen QIAquick PCR Purification Kit or NucleoSpin DNA Purification Kit (Macherey-Nagel). Fragments were sequenced using the BigDye Terminator reaction kit (ABI PRISM, Applied Biosystems). Sequencing and PCR amplifications were performed using the same sets of primers. Cycle sequencing was executed with the following setting: 25 cycles of 95°C for 30 s, 48°C for 15 s and 60°C for 4 min. Sequenced products were precipitated with 10 µl of sterile dH2O, 2 µl of 3 M Napa and 50 µl of 95% EtOH, and subsequently loaded on an ABI 3100 (Applied Biosystems) automatic sequencer. Sequence fragments obtained were assembled with DNASTAR SeqMan 4.03, manually inspected and adjusted, and submitted to GenBank (Table 2).
Table 2 GenBank Accession numbers and voucher information for the ITS sequences used in this study. Newly generated sequences are in bold
The obtained sequences were aligned with 16 additional sequences of related Sticta species from GenBank (Table 2), using the S. caliginosa D. J. Galloway-S. marginifera Mont. clade as outgroup (Moncada et al. 2014 Reference Moncada, Lücking and Suáreza ). The alignment was assembled in BioEdit 7.2.5 (Hall Reference Hall1999) and the final alignment was made with MAFFT 7.244 (Katoh et al. Reference Katoh, Misawa, Kuma and Miyata2002, Reference Katoh, Asimenos and Toh2009) using the [--auto] option. The final alignment included 67 ingroup OTUs and was 626 bases long. Sequences obtained from specimens originally identified as S. lacera were shown to be conspecific with S. filix and the only available sequence in GenBank labelled with that name also clustered with S. filix. Hence, our dataset for this morphodeme presumably represented only two species, S. filix and S. latifrons, with their corresponding cyanomorphs whereas S. lacera might not be a good species but instead a depauperate ecomorph of S. filix. Phylogenetic analysis was performed using maximum likelihood in RAxML 8.2.8 (Stamatakis Reference Stamatakis2015) applying the GTR-Gamma model and 1000 bootstrap replicates. The resulting trees were visualized in FigTree 1.4.2 (Drummond & Rambaut Reference Drummond and Rambaut2007).
Based on structural and floristic characteristics (closed forest cover, tree composition, extension, proximity to roads or urban areas), the 33 sites were classified into five conservation status categories (Table 1, Fig. 2): strongly disturbed (category 1), moderately disturbed (2), intermediate (3), slightly disturbed (4) and intact (5). For each taxon delimited from the phylogenetic analysis, we computed the proportion of specimens among all collected lichen specimens per site and subsequently the average proportion per conservation category over all sites pertaining to a given category. None of the newly delimited taxa occurred in site classes 1–3. In order to test whether a given species was found more frequently in intact (site class 5) as opposed to slightly disturbed forest (site class 4), we compared the frequency values using a one-tailed Wilcoxon-Mann-Whitney U-test (Marx et al. Reference Marx, Backes, Meese, Lenhof and Keller2016; https://ccb-compute2.cs.uni-saarland.de/wtest). While this approach is not based on thorough quantitative sampling, it gives an estimate of the relative frequency of each taxon in relation to site conservation status that can be used as a hypothesis for more detailed studies.
Results
The Sticta filix morphodeme does not form a monophyletic clade; instead, the target samples are dispersed over several early diverging clades of the Sticta tree following Moncada et al. (2014 Reference Moncada, Lücking and Suáreza : 218, fig. 3). Within this assemblage interspecies relationships are, with a small number of exceptions, not supported (Fig. 2). Next to the outgroup, two basal clades are formed by S. cinereoglauca Hook. f. & Taylor, S. squamata D. J. Galloway and S. babingtonii D. J. Galloway, all from New Zealand and the last two for the first time provided with ITS sequence data. The 43 specimens of the target taxa are found within a larger, unsupported clade and form four clades, three of them well supported, instead of the two expected clades (filix and latifrons). Sticta filix is monophyletic but in this analysis the clade is not supported. A second clade is formed by South American taxa: S. ainoae D. J. Galloway & J. Pickering, S. gaudichaldia Delise, S. hypochra Vain. and S. caulescens De Not. A third, larger subclade contains the samples identified as S. latifrons and an additional clade composed only of a dendriscocauloid cyanomorph. Here, S. latifrons s. lat. falls into two distinct, distantly related clades, S. latifrons 1 and S. latifrons 2. The latter is more closely related (though unsupported) to the dendriscocauloid clade plus S. martinii D. J. Galloway, whereas S. latifrons 1 is supported sister to S. subcaperata (Nyl.) Nyl. Sticta latifrons 1 and S. latifrons 2 each have two dendriscocauloid cyanomorphs, whereas a fourth cyanomorph clustered with S. filix. The cyanomorph related to S. latifrons 2 and S. martinii does not represent any of the other known green species of Sticta from New Zealand, viz. S. babingtonii, S. caperata, S. cinereoglauca, S. livida Kremp., S. martinii, S. squamata and S. subcaperata (all sequenced except S. livida and none conspecific with the cyanomorph clade). A possible exception is S. lacera, which has not yet been sequenced but is a doubtful taxon (see above in Material and Methods) from which no cyanomorph has been reported (Galloway Reference Galloway1985, Reference Galloway1997, Reference Galloway2007).
Comparison with type material revealed that the Sticta latifrons 1 clade corresponds to S. latifrons s. str. whereas the S. latifrons 2 clade agrees with the type of S. menziesii, thus far subsumed under synonymy with S. latifrons (Fig. 2). Sticta menziesii is therefore reinstated below. High-resolution digital scanning of the sequenced samples and careful qualitative and quantitative analysis of features such as lobe configuration, pigmentation and cyphellae size revealed that the three phylogenetically recognized, green-algal morphs can be distinguished as follows:

Whereas the conspicuous, vein-like pattern of the underside and the strongly and finely dissected lobes readily distinguish Sticta filix from the other two species, we found that the best character to separate morphologically intermediate forms of S. menziesii and S. latifrons from each other is the disposition, size and shape of the cyphellae, a character that also separates S. filix from the other two species, further underlining the potential use of cyphellae morphology for species delimitation in Sticta (Moncada et al. 2013 Reference Moncada, Coca and Lückingb ).
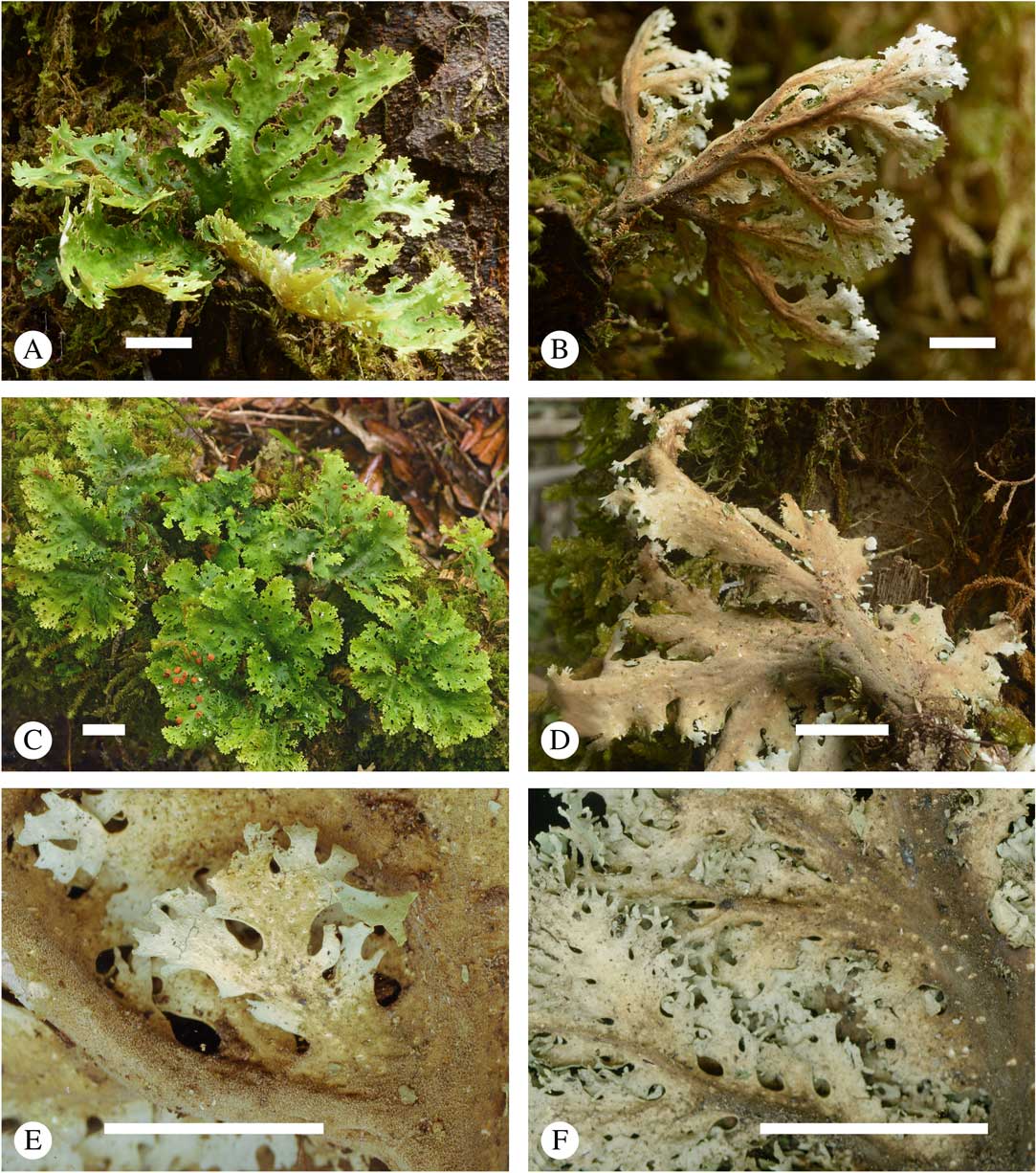
Fig. 3 Sticta filix, general habit and morphological details of the chloromorph. A & C, upper side; B & D, lower side; E & F, lower side enlarged showing cyphellae (A & B, Lücking et al. 39036; C & E, Lücking et al. 38159; D, Lücking et al. 38156; F, Lücking et al. 39016). Scales=10 mm. In colour online.

Fig. 4 Sticta latifrons s. str., general habit and morphological details of the chloromorph. A–D, upper side (in D dried); E, lower side; F, lower side enlarged showing cyphellae (A & F, Lücking et al. 38441; B, Lücking et al. 38446; C–E, Lücking et al. 38110). Scales=10 mm. In colour online.

Fig. 5 Sticta menziesii, general habit and morphological details of the chloromorph. A–C, upper side; D, lower side; E & F, lower side enlarged showing cyphellae (A, Lücking et al. 38029; B–D, Lücking et al. 38178; E, Lücking et al. 38009; F, Lücking et al. 38194). Scales=10 mm. In colour online.
Regarding the cyanomorphs and the two available names, Dendriscocaulon dendroides and D. dendriothamnodes, the type of the latter is from Australia and associated with Sticta stipitata C. Knight ex F. Wilson (Galloway Reference Galloway1983, Reference Galloway2007), a species of the S. filix morphodeme not occurring in New Zealand. Hence, D. dendriothamnodes becomes a synonym of S. stipitata and the name must be excluded from the New Zealand biota. A morphologically similar cyanomorph is associated with S. latifrons (James & Henssen Reference James and Henssen1976; Galloway Reference Galloway2007) which was confirmed here with molecular data; in spite of its similarity, this cyanomorph is not conspecific with the type of D. dendriothamnodes and its correct name is S. latifrons. A third, also similar cyanomorph was found associated with S. menziesii and consequently must bear that name. A problem then arises with the cyanomorph associated with S. filix and the one forming a separate clade without a known chloromorph (Fig. 2). We were unable to obtain the sequenced material of the Sticta filix cyanomorph for study; however, according to James (in Mark et al. Reference Mark, Scott, Sanderson and James1964), James & Henssen (Reference James and Henssen1976), Thomas et al. (Reference Thomas, Ryan, Farnden and Galloway2002) and Galloway (Reference Galloway2007), this cyanomorph corresponds to the morphology of D. dendroides but also to that of the additional cyanomorph forming a separate clade, sister to S. menziesii. Unfortunately, the type of D. dendroides is very depauperate and cannot be unambiguously assigned to either the S. filix cyanomorph or the cyanomorph forming a separate clade, so we epitypify D. dendroides with a specimen of the latter following recent recommendations (Ariyawansa et al. Reference Ariyawansa, Hawksworth, Hyde, Jones, Maharachchikumbura, Manamgoda, Thambugala, Udayanga, Camporesi and Daranagama2014), making the epithet dendroides available for that clade (as Sticta dendroides; see below) rather than describing a new species for it (Fig. 2). The possibility still exists that this cyanomorph is associated with S. lacera; if that turns out to be the case, then S. dendroides becomes a synonym of S. lacera without further nomenclatural disruptions. However, as noted under Material and Methods, because sequenced specimens originally identified as S. lacera, including one forming part of a previous study (AF350305; Thomas et al. Reference Thomas, Ryan, Farnden and Galloway2002), turned out to be conspecific with S. filix, the possibility must be considered that S. lacera is a depauperate form or ecomorph of S. filix.
As a consequence, the four dendriscocauloid cyanomorphs now known from New Zealand have the following names: S. dendroides, S. filix (=D. aff. dendroides), S. latifrons (=D. aff. dendriothamnodes 1) and S. menziesii (=D. aff. dendriothamnodes 2). Aside from the S. filix cyanomorph, which we did not obtain for study, the other three cyanomorphs present morphological and anatomical features that make their distinction readily possible:

Since Sticta menziesii was previously synonymized under S. latifrons (Galloway Reference Galloway1985, Reference Galloway1997, Reference Galloway2007), their cyanomorphs were also not separated and instead considered a single entity, Dendriscocaulon dendriothamnodes (Galloway Reference Galloway1983, Reference Galloway1985, Reference Galloway2007); however, this is the cyanomorph of an Australian species, S. stipitata (Galloway Reference Galloway2001, Reference Galloway2007). The macroscopic and microscopic differences found between the sequenced cyanomorphs further support the separation of S. menziesii from S. latifrons. Other than imagery, we were unable to investigate the type of Dendriscocaulon dendriothamnodes from Australia, so we are unable to state at present how this S. stiptata cyanomorph differs in morphological and anatomical features from the cyanomorphs of S. latifrons and S. menziesii.
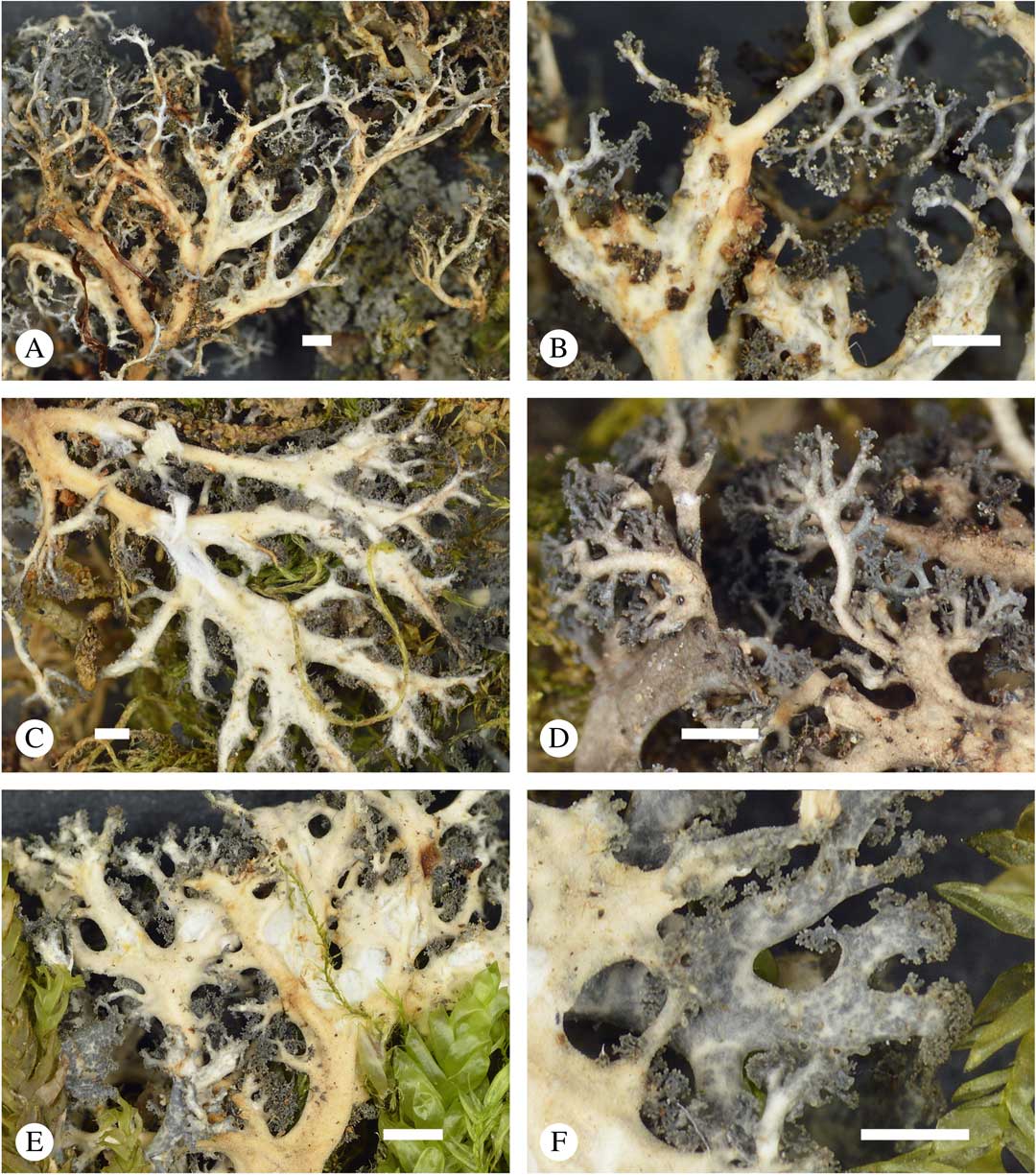
Fig. 6 Sticta dendroides, general habit and morphological details of the cyanomorph. A, C & E, lower side; B & D, lower side and tips enlarged; F, upper side and tips enlarged (A & B, Lücking et al. 38317; C, Lücking et al. 38053; D, Lücking et al. 38734; E & F, Lücking et al. 38039). Scales=1 mm. In colour online.

Fig. 7 Sticta menziesii, general habit and morphological details of the cyanomorph. A, thallus; B, thallus enlarged; C & D, tips enlarged; E, apical branches in microscope view; F, section through main stem showing cortical layers and hairs (A, B & F, Lücking et al. 38195; C–E, Lücking et al. 39004). Scales: A & B=1 mm; C & D=0·5 mm; E & F=100 µm. In colour online.

Fig. 8 Sticta latifrons, general habit and morphological details of the cyanomorph. A & C, thallus; B & D, thallus enlarged; E, apical branches in microscope view; F, section through main stem showing cortical layers and hairs (A, B & F, Lücking et al. 39011; C–E, Lücking et al. 38815). Scales: A=1 mm; B, C & D=0·5 mm; E & F=100 µm. In colour online.
Of the 33 sites visited during our fieldwork in 2015, 13 classify as strongly disturbed (1), seven as rather strongly disturbed (2), two as intermediate (3), seven as slightly disturbed (4), and four as intact (5). All four of the above species were found only at sites corresponding to categories 4 and 5, being absent from intermediate to strongly disturbed sites (categories 1–3). Notably, the four species exhibit two different patterns (Fig. 9): Sticta filix and S. latifrons were both found in slightly disturbed and intact sites but were relatively more common in slightly disturbed sites, whereas the previously unrecognized S. dendroides and S. menziesii were almost entirely restricted to intact sites. This difference is significant for both S. dendroides (one-tailed Mann-Whitney U-test, P=0·0121) and S. menziesii (Mann-Whitney U-test, P=0·0242).
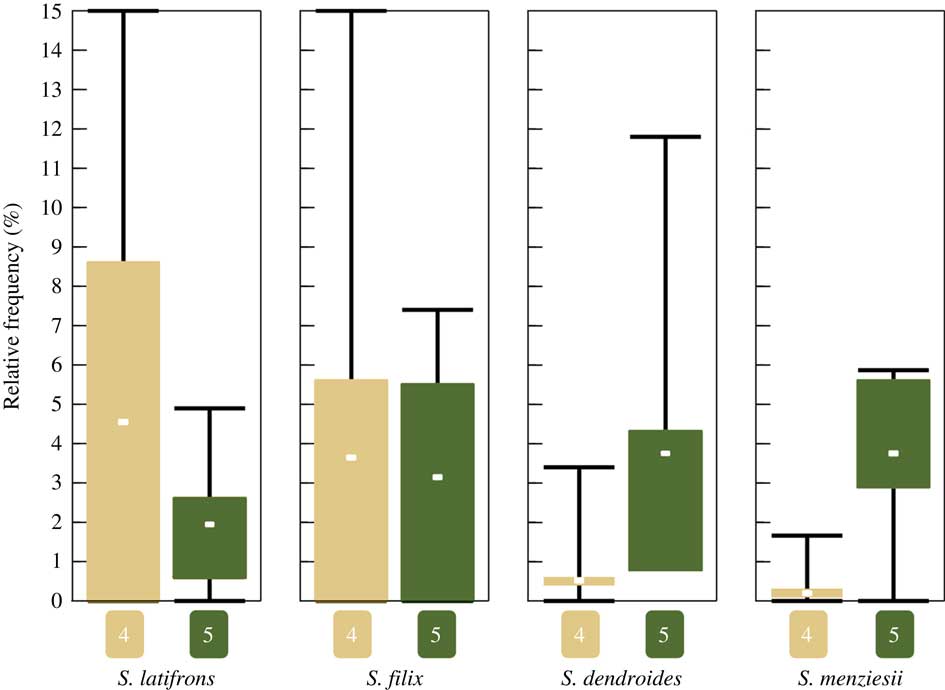
Fig. 9 Boxplot showing mean relative frequency of the four recognized Sticta species in intact (conservation status category 5) and slightly disturbed (category 4) forest sites (all four species are absent from sites corresponding to categories 1–3). Relative frequency is the number specimens of a species found at a site expressed as a percentage of the total number of specimens recorded at the same site. Whiskers indicate min/max, boxes indicate 25th and 75th percentiles; n=7 for slightly disturbed sites, n=4 for intact sites. In colour online.
Discussion
The results of our molecular phylogenetic study of the Sticta filix morphodeme in New Zealand, focusing on the two large species of this ‘guild’, S. filix and S. latifrons, and their cyanobacterial photomorphs, revealed the presence of a third large species which agrees morphologically with the type material of S. menziesii Hook. f. & Taylor, previously treated as a synonym of S. latifrons (Galloway Reference Galloway1985, Reference Galloway1997, Reference Galloway2007). Accordingly, we reinstate S. menziesii formally below. Ecologically, the species has a preference for dense, humid forests, growing on the lower trunks of trees and associated shrubs and saplings within the understorey and shrub layers, and being virtually absent from even only slightly disturbed sites. This sets it aside from S. latifrons s. str., which has broader habitat preferences and can be found on exposed trunks and canopy branches in lowland or riparian forest remnants, especially in places where cool, moisture-laden air ponds. Under such conditions, S. latifrons can be locally common even in somewhat degraded forest remnants. The reinstatement of Sticta menziesii not only clarifies an apparent morphological discrepancy and adds a further, potentially endemic, taxon to New Zealand’s lichen biota, but because of its observed ecological preferences it also recognizes a species with the capacity to be an excellent bioindicator of forest health in New Zealand.
Based on the results of our phylogenetic study, we also recognize four different dendriscocauloid photomorphs, one conspecific with each of Sticta filix, S. latifrons and S. menziesii, and a fourth one epitypified to represent the existing name Dendriscocaulon dendroides (Nyl.) R. Sant. ex H. Magn., below recombined in the genus Sticta as S. dendroides. The newly recognized taxon, S. dendroides, also discriminates between well-conserved and somewhat degraded forest. In our semi-quantitative analysis, S. dendroides is the only one of the four taxa exclusively represented by its cyanomorph, whereas both morphs were included for S. latifrons and S. menziesii and chloromorphs only for S. filix. In S. latifrons, the cyanomorphs behave in a similar way as the chloromorphs, being found in both well-conserved and somewhat degraded forest, whereas in S. menziesii the cyanomorphs were exclusively found in intact forests. Thus, the cyanomorphs also discriminate between conservation status and could theoretically be used as bioindicators in addition to the chloromorphs, although their morphological distinction might prove challenging in the field.
The present work underlines the importance of a polyphasic species delimitation approach, taking into consideration both macroscopic and microscopic phenotype characters and molecular data. The need for thorough taxonomic re-evaluation, even of taxa generally believed to be well understood, is particularly obvious when using lichens as bioindicators to assess ecosystem health. The two species S. dendroides and S. menziesii had not been properly recognized before and therefore their discrimination of intact versus disturbed forest had not been perceived either, as they appear to be better indicators of well-conserved, native forest than the two previously recognized taxa, S. filix and S. latifrons; the latter two not only seem to tolerate certain levels of disturbance but appear to thrive better under these circumstances.
New Zealand has a very rich assembly of Lobariaceae macrolichens, a family that has been identified as containing potentially excellent bioindicators of forest health (Rose Reference Rose1974, Reference Rose1976, Reference Rose1992; Galloway Reference Galloway1985, Reference Galloway1988, Reference Galloway1992, Reference Galloway2007, Reference Galloway2009; Søchting & Christensen Reference Søchting and Christensen1989; Gauslaa Reference Gauslaa1994; Selva Reference Selva1994, Reference Selva1996; Wolseley et al. Reference Wolseley, Moncrieff and Aguirre-Hudson1994; McCune Reference McCune2000; Zedda Reference Zedda2002; Campbell & Fredeen Reference Campbell and Fredeen2004; Kalwij et al. Reference Kalwij, Wagner and Scheidegger2005; Jüriado & Liira Reference Jüriado and Liira2009; Scheidegger Reference Scheidegger2009; Nascimbene et al. Reference Nascimbene, Brunialti, Ravera, Frati and Caniglia2010; Gustafsson et al. Reference Gustafsson, Fedrowitz and Hazell2013; Simijaca-Salcedo et al. Reference Simijaca-Salcedo, Vargas-Rojas and Morales-Puentes2014; Cansaran-Duman et al. Reference Cansaran-Duman, Altunkaynak, Aslan, Büyük and Aras2015; Giorgio et al. Reference Giorgio, Luisa and Sonia2015; Ramírez-Morán et al. Reference Ramírez-Morán, León-Gómez and Lücking2016). However, despite the fact that New Zealand is currently regarded as the best studied area when it comes to the taxonomy of Lobariaceae (Galloway Reference Galloway2009), our results show that species delimitations are far from settled, which has implications for our understanding of species-level ecology. The past treatment of Sticta menziesii as part of the natural variation exhibited by S. latifrons meant that its potential as a bioindicator of intact forest was not recognized. The preference of S. menziesii for intact forests could also make it a useful visual aid to monitor invasive browsing animals such as deer (including Cervus elaphus scoticus, C. nippon, C. unicolour, Dama dama and Odocoileus virginianus) and possum (Trichosurus vulpecula), all deliberately introduced and now widespread in New Zealand (Atkinson Reference Atkinson1989; King Reference King1990; Anderson Reference Anderson2002; Worthy & Holdaway Reference Worthy and Holdaway2002; Prebble & Wilmshurst Reference Prebble and Wilmshurst2009; Brown et al. Reference Brown, Stephens, Peart and Fedder2015). While these animals do not necessarily feed on this lichen, their impact is likely to affect its presence and abundance through secondary effects of changes in vegetation structure and microclimate.
The four Sticta species recognized here can be readily recognized in the field, although tools such as a hand lens are required for proper recognition, particularly of the cyanomorphs. This makes these lichens good candidates for use in rapid field assessments of the ecological integrity and vegetation health of forest ecosystems. Considering the rather limited use of lichens as bioindicators in New Zealand (Hutcheson et al. Reference Hutcheson, Walsh and Given1999; Galloway Reference Galloway2008; de Lange et al. Reference de Lange, Galloway, Blanchon, Knight, Rolfe, Crowcroft and Hitchmough2012), we recommend that their potential for use in the New Zealand-wide monitoring of indigenous vegetation health should be further explored through quantitative assessments, and to this end we designed a field guide to aid in the critical identification of the species of interest (see Supplementary Material Figure S1, available online).
Taxonomic Novelties
Sticta dendroides (Nyl.) Moncada, Lücking & de Lange comb. nov.
MycoBank No.: MB 821871
Leptogium dendroides Nyl., Flora 50: 438 (1867); Dendriscocaulon dendroides (Nyl.) R. Sant. ex H. Magn., Tillägg och Ändringar till Förteckning över Skandinaviens Växter 4, Lavar: 9 (1950); Dendriscocaulon filicinellum Nyl., Lichenes Novae Zelandiae (Paris): 10 (1888) [nom. illeg., ICN Art. 52.1]; type: New Zealand, Lyall s. n. (H-NYL 41030—lectotype!, Galloway Reference Galloway1985: 154); New Zealand, North Island, Erua Forest, Lücking et al. 38053 (F—epitype!, designated here; MBT 377561).
Diagnostic characters. Photobiont cyanobacterial (Nostoc). Thallus corticolous, often between bryophytes, microfruticulose, 3–5(–10) cm high, dendroid with distinct main stems and obliquely inserted lateral branches. Main branches flattened, dorsiventral, with the upper side bluish grey with cream-coloured maculae and the lower side cream-coloured. Stem and branch surface glabrous to sparsely pilose.
Notes. After its original description in the genus Leptogium, Nylander (Reference Nylander1888) realized that the species should belong in Dendriscocaulon but renamed it D. filicinellum, creating an illegitimate name. This dendriscocauloid cyanomorph was usually associated with the chloromorph Sticta filix (Galloway Reference Galloway1985, Reference Galloway2007), even in the protologue (Nylander Reference Nylander1867). However, in the type material there is no evidence for a direct connection with that particular species. Unfortunately, we could not locate the sequenced specimen of the cyanomorph that clusters with S. filix (Thomas et al. Reference Thomas, Ryan, Farnden and Galloway2002: AF350303), but based on the description given by Galloway (Reference Galloway1985, Reference Galloway2007) it is expected to be quite similar to the clade of the unique cyanomorph found here. Since the type of Leptogium dendroides is very depauperate, we decided to epitypify the name with a specimen from this clade. Thus, instead of making Leptogium dendroides a synonym of S. filix, this epitypification makes the name available for the separate clade and avoids the description of a new species.
Specimens examined. New Zealand: North Island: Hawke’s Bay, Mahia Peninsula, Kinikini Road, Mahia Peninsula Scenic Reserve, 56 km SSW of Gisborne, trail through reserve, 39°07'28''S, 177°52'27''E, 150–200 m, kohekohe (Dysoxylum spectabile), nikau (Rhopalostylis sapida) and tawa (Beilschmiedia tawa) forest transitioning into riparian podocarp forest dominated by kahikatea (Dacrycarpus dacrydioides), matai (Prumnopitys taxifolia) and totara (Podocarpus totara var. totara), with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), kotukutuku (Fuchsia excorticata), horoeka (Pseudopanax crassifolius) and kaikomako (Pennantia corymbosa), well-preserved forest, on Schefflera, 2015, R. Lücking, B. Moncada & P. de Lange 38734 (F); Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175°22'35''E, 800–900 m, podocarp tree land dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, on Weinmannia, 2015, R. Lücking, B. Moncada & P. de Lange 38039 (F); ibid., on Cyathea, 38053 (F); Manawatu-Manganui, Hauhangaroa Range, Pureora Forest Park, Waihora Lagoon, 39 km WNW of Taupo, trail from Waihora Lagoon car park to lagoon, 38°38'46''S, 175°39'37''E, 550–560 m, dense podocarp forest dominated by rimu (Dacrydium cupressinum), matai (Prumnopitys taxifolia), miro (P. ferruginea), kahikatea (Dacrycarpus dacrydioides) and totara (Podocarpus totara var. totara) with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata), kotukutuku (Fuchsia excorticata) and kaikomako (Pennantia corymbosa), well-preserved forest, on Melicytus lanceolatus, 2015, R. Lücking, B. Moncada & P. de Lange 39007a (F).
Sticta menziesii Hook. f. & Taylor
MycoBank No.: MB 406297
Sticta menziesii Hook. f. & Taylor in Hooker, The Botany of the Antarctic Voyage of H. M. Discovery Ships Erebus and Terror 1839–1843 1: 198 (1844); Sticta latifrons var. menziesii (Hook. f. & Taylor) Bab. in Hooker, The Botany of the Antarctic Voyage of H. M. Discovery Ships Erebus and Terror 1839–1843 2: 277 (1855); type: New Zealand, Dusky Bay, Menzies s. n. (BM—lectotype!, Galloway Reference Galloway1997: 143).
Diagnostic characters (chloromorph). Photobiont green (Dictyochloropsis). Thallus corticolous, macrofoliose, distinctly stalked, up to 10 cm high. Lobes flabellate to ligulate, with sinuose margins, (5–)10–30 mm broad; underside medium to dark brown, rarely pale brown; cyphellae of regular size, 0·3–1·0 mm diam., regularly rounded. For diagnostic characters of the cyanomorph, see key above.
Notes. This species is reinstated here from prior synonymy with Sticta latifrons based on the results from the molecular phylogenetic analysis and the morphological differences with the latter, outlined in the key above. The type specimen, shown in a photograph in Galloway (Reference Galloway1997: 144) and beautifully illustrated by Babington in Hooker (Reference Hooker1855: plate CXXII; Fig. 10), is a typical representative of this taxon, agreeing perfectly with well-developed specimens sequenced here.

Fig. 10 Illustration of the type material of Sticta menziesii by Babington in Hooker (Reference Hooker1855), clearly showing the typical features of the species. In colour online.
All other synonyms listed under Sticta latifrons in Galloway (Reference Galloway1985, Reference Galloway1997, Reference Galloway2007) represent S. latifrons s. str. This also applies to the names Sticta menziesii var. dissecta Kremp. and S. menziesii var. palmata Kremp., as well as S. menziesii var. ochroleuca (C. Bab.) Kremp.
Specimens examined (chloromorphs). New Zealand: North Island: Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175° 22'35''E, 800–900 m, podocarp forest dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38008 (AK, F), 38009 (F), 38029 (AK, F, UNITEC), 38048 (AK, F, UNITEC), 38194 (F), 38202 (F), 38941 (F); ibid., on Weinmannia, 38178 (AK, F); Manawatu-Manganui, Hauhangaroa Range, Pureora Forest Park, Waihora Lagoon, 39 km WNW of Taupo, trail from Waihora Lagoon car park to lagoon, 38°38'46''S, 175°39'37''E, 550–560 m, dense podocarp forest dominated by rimu (Dacrydium cupressinum), matai (Prumnopitys taxifolia), miro (P. ferruginea), kahikatea (Dacrycarpus dacrydioides) and totara (Podocarpus totara var. totara) with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata), kotukutuku (Fuchsia excorticata) and kaikomako (Pennantia corymbosa), well-preserved forest, on Dacrycarpus dacrydioides, 2015, R. Lücking, B. Moncada & P. de Lange 39010 (AK, F, UNITEC); ibid., on Prumnopitys ferruginea; 39001 (F); Waikato, Pureora Forest Park, Link Road Saddle, Link Track to Mount Pureora at Forestry Road, 42 km NW of Taupo, Link Road Pureora Track car park to base of Mount Pureora, 38°32'11''S, 175°38'44''E, 800–820 m, dense podocarp forest dominated by rimu (Dacrydium cupressinum), matai (Prumnopitys taxifolia), miro (P. ferruginea), kahikatea (Dacrycarpus dacrydioides) and totara (Podocarpus totara var. totara) with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata), kotukutuku (Fuchsia excorticata) and kaikomako (Pennantia corymbosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 39050 (AK, F, UNITEC); Waikato, Tongariro National Park, Tree Trunk Gorge Road, 38 km E of National Park village, roadside, 39°09'56''S, 175°48'11''E, 745 m, mountain beech (Fuscospora cliffortioides) forest with makahikatoa (Kunzea serotina), margin of well-preserved forest along road, 2015, R. Lücking, B. Moncada & P. de Lange 38782 (F).
Specimens examined (cyanomorphs). New Zealand: North Island: Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175° 22'35''E, 800–900 m, podocarp forest dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38195 (F); Manawatu-Manganui, Hauhangaroa Range, Pureora Forest Park, Waihora Lagoon, 39 km WNW of Taupo, trail from Waihora Lagoon car park to lagoon, 38°38'46''S, 175°39'37''E, 550–560 m, dense podocarp forest dominated by rimu (Dacrydium cupressinum), matai (Prumnopitys taxifolia), miro (P. ferruginea), kahikatea (Dacrycarpus dacrydioides) and totara (Podocarpus totara var. totara) with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata), kotukutuku (Fuchsia excorticata) and kaikomako (Pennantia corymbosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 39004 (F).
Specimens of Sticta filix examined. New Zealand: North Island: Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175°22'35''E, 800–900 m, podocarp forest dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38024a (F), 38028a (AK, F, UNITEC), 38090 (F), 38112 (F), 38149 (AK, F); ibid., on Coprosma grandifolia, 38094 (AK, F); ibid., on Pseudowintera, 38125 (AK, F); ibid., on Weinmannia, 38107 (AK, F, UNITEC), 38148 (AK, F); Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175°22'35''E, 800–900 m, podocarp forest dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38156 (F), 38159 (AK, F), 38190 (F); Manawatu-Manganui, Hauhangaroa Range, Pureora Forest Park, Waihora Lagoon, 39 km WNW of Taupo, trail from Waihora Lagoon car park to lagoon, 38°38'46''S, 175°39'37''E, 550–560 m, dense podocarp forest dominated by rimu (Dacrydium cupressinum), matai (Prumnopitys taxifolia), miro (P. ferruginea), kahikatea (Dacrycarpus dacrydioides) and totara (Podocarpus totara var. totara) with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata), kotukutuku (Fuchsia excorticata) and kaikomako (Pennantia corymbosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 39016 (F), 39034 (F), 39036 (F); ibid., on Dacrycarpus dacrydioides, 39009 (F); ibid., on Prumnopitys ferruginea, 39003 (AK, F, UNITEC); Waikato, Kaimanawa Forest Park adjacent to Tongariro National Park, Tree Trunk Gorge Road, 39 km E of National Park village, roadside, 39°10'31''S, 175°48'33''E, 725 m, mountain beech (Fuscospora cliffortioides), silver beech (Lophozonia menziesii) and red beech (Fuscospora fusca) forest, margin of well-preserved forest along road, 2015, R. Lücking, B. Moncada & P. de Lange 38844b (AK, F, UNITEC), 38850a (F), 38851 (AK, F, UNITEC); Waikato, Kaimanawa Forest Park, Rangipo Intake Road, 36 km ESE of National Park village, road head at hydroelectric dam of Tongariro River, 39°12'38''S, 175°46'48''E, 720–730 m, mountain beech (Fuscospora cliffortioides), silver beech (Lophozonia menziesii) and red beech (Fuscospora fusca) forest, margin of well-preserved forest along road, 2015, R. Lücking, B. Moncada & P. de Lange 38864 (AK, F, UNITEC).
Specimens of Sticta latifrons examined (chloromorph). New Zealand: North Island: Bay of Plenty, Lake Okataina Foreshore, Tauranganui Bay, 18 km ENE of Rotorua, parking lot facing lake near Lakes Lodge, 38°06'02''S, 176°25'49''E, 320 m, seral vegetation dominated by kamahi (Weinmannia racemosa) and ti kouka or cabbage tree (Cordyline australis), with pohutukawa (Metrosideros excelsa), exposed, planted trees along the beach near the lodge, with gully tree fern (Cyathea cunninghamii) and mamaku (C. medullaris), on Cordyline australis, 2016, R. Lücking, B. Moncada & P. de Lange 38357 (F); Bay of Plenty, Lake Okataina Road, 18 km NE of Rotorua, roadside along main road, 38°04'36''S, 176°24'51''E, 140 m, rimu (Dacrydium cupressinum) and tawa (Beilschmiedia tawa) forest, with a dense mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata) and tree fern (Dicksonia fibrosa and D. squarrosa) understorey, shady forest edge along road, 2016, R. Lücking, B. Moncada & P. de Lange 38303 (F), 38310 (AK, F), 38318 (AK, F, UNITEC), 38327 (AK, F); Bay of Plenty, Toatoa to Motu Road, 17 km ESE of Opotiki, roadside along main road, 38°03'30''S, 177°27'46''E, 85 m, rimu (Dacrydium cupressinum), tawa (Beilschmiedia tawa) and hard beech (Fuscospora truncata) forest, with a dense mahoe (Melicytus ramiflorus subsp. ramiflorus), pate (Schefflera digitata) and tree fern (Dicksonia fibrosa and D. squarrosa) understorey, shady forest edge along road and shady roadbank, 2015, R. Lücking, B. Moncada & P. de Lange 38441 (F), 38446 (AK, F, UNITEC), 38447 (F); Hawke’s Bay, Mahia Peninsula, Kinikini Road, Mahia Peninsula Scenic Reserve, 56 km SSW of Gisborne, trail through reserve, 39°07'28''S, 177°52'27''E, 150–200 m, kohekohe (Dysoxylum spectabile), nikau (Rhopalostylis sapida) and tawa (Beilschmiedia tawa) forest transitioning into riparian podocarp forest dominated by kahikatea (Dacrycarpus dacrydioides), matai (Prumnopitys taxifolia) and totara (Podocarpus totara var. totara), with an understorey of mahoe (Melicytus ramiflorus subsp. ramiflorus), kotukutuku (Fuchsia excorticata), horoeka (Pseudopanax crassifolius) and kaikomako (Pennantia corymbosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38657 (F), 38696c (AK, F, UNITEC), 38763 (F); ibid., on Beilschmiedia tarairi, 38767b (F); ibid., on Kunzea robusta, 38669 (AK, F); ibid., on Kunzea, 38773a (F), 38774 (F); ibid., on Melicytus ramiflorus, 38662 (AK, F); Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175°22'35''E, 800–900 m, podocarp treeland dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, on Carpodetus serratus, 2015, R. Lücking, B. Moncada & P. de Lange 38111 (F); Waikato, Tongariro National Park, Tree Trunk Gorge Road, 38 km E of National Park village, roadside, 39°09'56''S, 175°48'11''E, 745 m, mountain beech (Fuscospora cliffortioides) forest with makahikatoa (Kunzea serotina), margin of well-preserved forest along road, 2015, R. Lücking, B. Moncada & P. de Lange 38835 (F).
Specimens of Sticta latifrons examined (cyanomorph). New Zealand: North Island: Manawatu-Manganui, Erua Forest, Fishers Road, 1 km W to 5 km SW of National Park village, Tupapakukura Waterfall Track, 39°10'19''S, 175°22'35''E, 800–900 m, podocarp forest dominated by totara (Podocarpus totara var. totara) and miro (Prumnopitys ferruginea), with an understorey of kamahi (Weinmannia racemosa), well-preserved forest, 2015, R. Lücking, B. Moncada & P. de Lange 38019 (AK, F); Waikato, Tongariro National Park, Tree Trunk Gorge Road, 38 km E of National Park village, roadside, 39°09'56''S, 175°48'11''E, 745 m, mountain beech (Fuscospora cliffortioides) forest with makahikatoa (Kunzea serotina), margin of well-preserved forest along road, 2015, R. Lücking, B. Moncada & P. de Lange 38815 (AK, F).
Funding for field and laboratory work for this study was provided by a grant from the National Science Foundation (NSF) to The Field Museum: DEB-1354884 ‘Collaborative Research: Evolution, Diversification, and Conservation of a Megadiverse Flagship Lichen Genus’ (PI HTL, CoPI RL). The Field Museum’s Prince Fund supported the internship of HR as part of this research project. The Department of Conservation in Auckland, New Zealand, is warmly thanked for logistic support and for making possible the participation of PJDL in the fieldwork. Unitec Institute of Technology (Auckland) and Dan Blanchon kindly provided assistance for organizing a workshop on the use of Lobariaceae as bioindicators of environmental health. Dan Blanchon also participated in part of the fieldwork and Allison Knight provided additional material and helped trace voucher specimens from previous phylogenetic studies deposited in OTA. Dhahara Ranatunga of the Auckland Museum kindly helped with the logistics of depositing voucher specimens at AK and sending material to F. Konstanze Bensch assisted with MycoBank registration of the nomenclatural novelties and the GenBank Direct Submission staff promptly provided the GenBank Accession numbers.
Supplementary Material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0024282917000706














