Acute kidney injury (AKI) is a serious hospital-acquired disease caused by various clinical factors, and it is a common complication of local and systemic inflammation, with high mortality(Reference Hoste, Clermont and Kersten1,Reference Doi and Rabb2) . It occurs in 10–15 % of patients admitted to the hospital and sometimes more than 50 % of patients in intensive care units(Reference Al-Jaghbeer, Dealmeida and Bilderback3,Reference Hoste, Bagshaw and Bellomo4) . Evidence has shown that malnutrition, defined as any nutritional imbalance, is very common in patients with AKI, especially critically ill patients, and thiamine deficiency is a type of malnutrition(Reference Woolum, Abner and Kelly5,Reference Ostermann, Summers and Lei6) . The recommended intake of thiamine for adults is 1·1–1·2 mg/d(Reference Whitfield, Bourassa and Adamolekun7). However, the half-life of thiamine in the human body is short, and humans store less thiamine than do many other mammals. Therefore, if the dietary intake of thiamine is not maintained, thiamine deficiency can occur within a few weeks(Reference Whitfield, Bourassa and Adamolekun7). Critically ill patients with AKI may be at risk for thiamine deficiency due to pre-existing malnourishment, possible decreased nutritional intake and chronic comorbidities(Reference McClave, Taylor and Martindale8–Reference Cano, Fiaccadori and Tesinsky10). In addition, the high metabolic rate in critically ill patients can further accelerate the consumption of thiamine and lead to thiamine deficiency.
Thiamine is an essential coenzyme of several decarboxylases required for the Krebs cycle, glucose metabolism, the pentose phosphate pathway and the generation of ATP and NADPH(Reference Cruickshank, Telfer and Shenkin11,Reference Collie, Greaves and Jones12) . Thiamine pyrophosphate is also a key coenzyme in the pyruvate dehydrogenase complex, which is the rate-limiting component of the citric acid cycle(Reference Bukhari, Moradi and Gollapudi13). Thiamine deficiency is associated with a variety of clinical manifestations, including heart failure, hypotension, lactic acidosis, peripheral neuropathy and encephalopathy(Reference Collie, Greaves and Jones12,Reference Manzanares and Hardy14,Reference Costa, Gut and de Souza Dorna15) . Several studies have shown that thiamine supplementation may be beneficial for critically ill patients. Donnino et al. found that thiamine supplementation significantly decreased serum lactate levels in critically ill patients with thiamine deficiency(Reference Donnino, Andersen and Chase16), in addition, secondary analysis found that patients with septic shock randomised to receive thiamine had lower serum creatinine levels and a lower rate of progression to renal replacement therapy (RRT) than those randomised to receive the placebo(Reference Moskowitz, Andersen and Cocchi17). However, there is currently no evidence to support supplementation with specific nutrient solutions in critically ill patients with AKI or specialised supplementation with vitamins and trace elements(Reference Levey and James18). In addition, the role of early thiamine supplementation in critically ill patients with AKI has not been studied. The aim of this study was to evaluate the association of early thiamine use with clinical outcomes in critically ill patients with different AKI stages.
Materials and methods
Database introduction
Medical Information Mart for Intensive Care III (MIMIC III) is a publicly and freely available ICU database containing patient data from 2001 to 2012(Reference Shen, Zhang and Shen19). Database use was authorised by the Institutional Review Board of the Massachusetts Institute of Technology. To protect patient privacy, the information of all patients was anonymised. One author (XL) had access to this database (certification number 35970146) and was responsible for data extraction.
Ethical approval
The establishment of the MIMIC III database was approved by the Massachusetts Institute of Technology (No. 0403000206) and Beth Israel Deaconess Medical Center (2001-P-001699/14). Our study used the anonymous data of this database and hence the need for informed consent was waived. The study complied with the ethical standards stipulated in the declaration of Helsinki in 1964 and its subsequent amendments.
Inclusion and exclusion criteria
Patients were included if they met criteria for AKI according to the Kidney Disease: Improving Global Outcomes 2012 criteria, which were as follows(Reference Andrassy20): (1) an increase in Scr to more than 1·5-fold the baseline value within the prior 7 d; (2) a ≥ 0·3 mg/dl increase in Scr within the past 48 h or (3) UO < 0·5 ml/kg per h for 6 h or more. The minimum Scr value within 7 d prior to ICU admission was used as the baseline Scr level(Reference Huber, Ozrazgat-Baslanti and Thottakkara21,Reference Siew, Ikizler and Matheny22) . The first Scr value measured after ICU admission was used as the baseline Scr when the pre-ICU Scr was not available(Reference Angeli, Ginès and Wong23). The number of patients without a creatinine level before ICU admission was 3464 (23·0 %). AKI stages were defined as the maximum AKI stage within 48 h after ICU admission as determined by both Scr and the volume of UO during the first 48 h after ICU admission. Patients were excluded if they met one of the following characteristics: (1) younger than 18 years; (2) death or discharge within 48 h after ICU admission or (3) less than two Scr tests. For patients who were admitted to the ICU more than once, only the first stay was included.
Data extraction
Data extracted from the MIMIC III database included age, sex, weight, emergency status, maximal AKI stage within 48 h after ICU admission, comorbidities, sequential organ failure assessment score, Simplified Acute Physiology Score II (SAPS II), biochemical indices, thiamine use, use of vasopressors, estimated glomerular filtration rate (eGFR), mechanical ventilation and mean arterial pressure. The lab measurement data, mean arterial pressure, RRT, enteral nutrition, use of vasopressors and mechanical ventilation were extracted within the first 24 h after patient ICU admission. Comorbidities, including diabetes, heart failure, chronic lung disease, chronic liver disease, fluid and electrolyte disorders, hypertension and cardiac arrhythmias, were defined as per the Implementation of the International Statistical Classification of Disease and Related Health Problems, 10th Revision coding systems(Reference Quan, Sundararajan and Halfon24). Sepsis was defined as life-threatening organ dysfunction resulting from a patient’s dysfunctional response to infection. In this study, patients with recorded or suspected infection plus an acute sequential organ failure assessment score elevation of ≥ 2 points were considered to have sepsis. Acute-on-chronic (A-on-C) renal injury was defined by a history of chronic kidney diseases (CKD) in patients with AKI(Reference Zhao, Xu and Ying25). CKD was defined as an abnormality in kidney structure or function that persisted for more than 3 months. This included 1 or more of the following: (1) an eGFR <60 ml/min/1·73 m2; (2) albuminuria (i.e. a urine albumin level ≥ 30 mg per 24 h or a urine albumin:creatinine ratio ≥ 30 mg/g); (3) abnormalities in urine sediment, histology or imaging suggestive of kidney damage; (4) renal tubular disorders or (5) a history of kidney transplantation(26). In the present study, thiamine use was defined as intravenous administration of ≥ 100 mg of thiamine within 48 h after ICU admission. The data from patients discharged alive were censored at the time of hospital discharge.
Endpoints
The primary endpoint of this study was in-hospital mortality. Recovery of renal function, 90-d mortality and lengths of stay in the ICU and hospital were considered secondary outcomes. Recovery of kidney function was defined as discharge from the ICU with a level <1·5 times the baseline value and normal UO (> 0·5 ml/kg per h for 24 h).
Management of missing data
Missing data variables are common in the MIMIC III database. In this study, the missing values for all variables accounted for <5 % (see additional file in online Supplementary Table S1). The missing values were replaced by mean or median values. The components that were missing >20 %, such as serum lactate, albumin, aspartate aminotransferase, C-reactive protein and alanine aminotransferase, were removed from this analysis.
Statistical analysis
Continuous variables are expressed as medians (interquartile ranges) or means ± sd in the present study and compared using Student’s t test and the Mann–Whitney U test. Categorical variables are expressed as numbers and percentages. The χ 2 test or Fisher’s exact test was used as appropriate.
In the present study, propensity score matching (PSM) was performed to minimise the imbalance between patients with and without early thiamine use. A 1:1 nearest neighbour matching algorithm was applied using a caliper width of 0·05. We selected the following variables to generate the propensity score: age, sex, weight, emergency status, maximal AKI stage within 48 h after ICU admission, CKD, diabetes, heart failure, chronic lung disease, chronic liver disease, fluid and electrolyte disorders, hypertension, sepsis, cardiac arrhythmias, sequential organ failure assessment score on ICU admission, SAPS II on ICU admission, Scr, white blood cell count, haemoglobin (Hb) level, platelet level, glucose (Glu) level, eGFR, mechanical ventilation, vasopressors use and mean arterial pressure. The kernel density plot of P was used to check the degree of PSM in the present study. Finally, 734 matched pairs were generated and subjected to further analyses.
The Kaplan–Meier method and log rank tests were used to compare survival distributions among patients with and without thiamine use. A logistic regression model and linear regression model were used to estimate the associations between early thiamine administration and clinical outcomes with adjustments for confounding variables. Stepwise regression was used to eliminate the collinearity between independent variables. Stratified analyses were performed to explore whether the association between thiamine administration and in-hospital mortality differed across various subgroups classified by different AKI stages, heart failure, sepsis, chronic lung disease, chronic liver disease and acute-on-chronic renal injury.
Two-tailed tests were performed, and P < 0·05 was considered statistically significant. The statistical analyses were performed using Stata 14·0 (Stata Corp.).
Results
Baseline characteristics and propensity score matching
A total of 23 649 critically ill patients with AKI within 48 h after ICU admission were included in the MIMIC III database. After exclusion according to the exclusion criteria, 15 066 patients were eligible for inclusion in the present study; 735 patients were administered thiamine within the first 48 h after ICU admission and 14 331 patients did not early receive thiamine (Fig. 1). The baseline characteristics and comparisons between the early thiamine use group and non early thiamine use group are presented in Table 1. There were no differences between the two groups in weight, maximal AKI stage within 48 h after ICU admission, sequential organ failure assessment score, Scr or white blood cell count. However, there were significant differences in age, sex, emergency status and comorbidities between the two groups. In addition, the early thiamine use group had a higher Hb level, eGFR, mean arterial pressure and mechanical ventilation rate and a lower SAPS II, platelet level, Glu level and vasopressors use rate than the nonearly thiamine use group (Table 1).

Fig. 1. Flow chart of patient selection from the MIMIC III database.
Table 1. The baseline characteristics of patients with early and non early thiamine use before and after PSM

AKI: acute kidney injury, CKD: chronic kidney diseases, COPD: chronic obstructive pulmonary disease, ARDS: acute respiratory distress syndrome, IQR: interquartile range, SOFA: sequential organ failure assessment, SAPS II: Simplified Acute Physiology Score II, eGFR: estimated glomerular filtration rate, MAP: mean arterial pressure, ICU: intensive care unit. Scr: serum creatinine, WBC: white blood cell, Hb: hemoglobin, PLT: platelet, Glu: glucose.
Values are shown as means ± standard deviations unless otherwise indicated.
* The maximum values during the first day after ICU admission were recorded.
† The status during the first day after ICU admission were recorded.
‡ The mean values during the first day after ICU admission were recorded.
To eliminate this bias, we used PSM to establish two additional 1:1 matched cohorts. For PSM, 734 patients who received thiamine in the early stage were matched to 734 patients who did not in the early stage. The quality of the matched samples was assessed by graphing the propensity scores of the two groups and comparing the sd of the means (Fig. 2). After matching, there were no significant differences in the baseline data of twenty-four covariates between the two groups (Table 1).

Fig. 2. Kernel density plots of the propensity scores before and after PSM.
Association between early thiamine use and clinical outcomes before and after propensity score matching
Kaplan–Meier survival curves were used to depict the survival distributions of patients with and without early thiamine administration within 28 d of ICU admission. The survival of patients in the early thiamine use group was better before and after PSM than the survival of patients in the non early thiamine use group (Fig. 3).

Fig. 3. Kaplan–Meier survival curves of the study population.
Logistic regression was used to estimate the association of thiamine use on mortality outcomes, recovery of renal function and RRT use. We found that early thiamine use was associated with reduced in-hospital mortality (OR 0·61; 95 % CI 0·48, 0·77; P < 0·001) and 90-d mortality (OR 0·52; 95 % CI 0·42, 0·64; P < 0·001) after adjustment for possible confounding factors associated with mortality (Table 2). After the adjustment, significant differences were observed between the early thiamine use group and the nonearly thiamine use group in the rate of renal function recovery (OR 1·28; 95 % CI 1·18, 1·37; P < 0·001) (Table 2), but not in that of RRT use (OR 0·86; 95 % CI 0·63, 1·15; P = 0·305) (online Supplementary Table S2). Linear regression was used to evaluate the association between early thiamine use and the length of stay. After the adjustment, significant differences were observed between the two groups in the lengths of stay in the ICU (β 0·41; 95 % CI 0·27, 0·57; P = 0·005) and hospital (β 0·30; 95 % CI 0·11, 0·50; P = 0·007) (Table 2).
Table 2. Associations between early thiamine use and clinical outcomes in critically ill patients with acute kidney injury (AKI) before and after propensity score matching (PSM) (Numbers and percentages; odd ratios and 95 % confidence intervals)
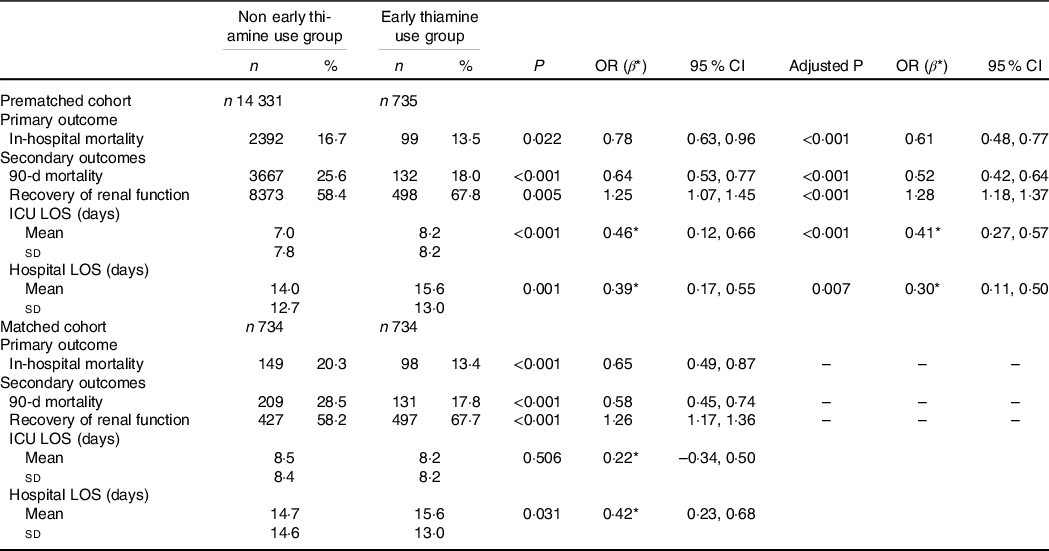
ICU, intensive care unit; LOS, lengths of stay; AKI, acute kidney injury; CKD, chronic kidney diseases; SOFA, sequential organ failure assessment; MAP, mean arterial pressure; PLT, platelet; eGFR, estimated glomerular filtration rate.
Logistic regression was performed to estimate the association of thiamine use on mortality outcomes and recovery of renal function, adjusted by age, sex, weight, emergency status, AKI stage, CKD, diabetes, heart failure, chronic liver disease, fluid and electrolyte disorders, hypertension, sepsis, SOFA score on ICU admission, Hb, PLT, eGFR and MAP. Recovery from AKI was defined as discharge from the ICU with a Scr level less than 1·5 times the baseline value and normal UO (> 0·5 ml/kg per h). Linear regression was used to evaluate the association between thiamine use and length of stay, adjusted by age, sex, weight, emergency status, AKI stage, CKD, diabetes, heart failure, chronic liver disease, fluid and electrolyte disorders, hypertension, sepsis, SOFA score on ICU admission, Hb, PLT, eGFR and MAP.
Before PSM, P represents the P value of univariate analysis, and the adjusted P represents the P value after multivariate analysis. After PSM, P represents the P value of univariate analysis.
* β replaces a regression coefficient.
Similarly, among the 734 propensity-matched pairs, hospital mortality (OR 0·65; 95 % CI 0·49, 0·87; P < 0·001) and 90-d mortality (OR 0·58; 95 % CI 0·45, 0·74; P < 0·001) were significantly reduced in the early thiamine use group. Early thiamine use was also associated with improved the chance of renal function recovery (OR 1·26; 95 % CI 1·17, 1·36; P < 0·001), but was not associated with the application of RRT (OR 0·88; 95 % CI 0·63, 1·25; P = 0·483) (online Supplementary Table S2). Additionally, early thiamine use was associated with increased lengths of stay in hospital (β 0·42; 95 % CI 0·23, 0·68; P = 0·031) (Table 2).
Subgroup analysis
The subgroup analyses based on maximal AKI stage within 48 h after ICU admission, heart failure, sepsis, chronic lung disease, chronic liver disease and A-on-C renal injury are presented in Fig. 4. Considering the Kidney Disease: Improving Global Outcomes criteria, early thiamine administration was associated with lower in-hospital mortality in patients with stages 1 to 2 AKI but not in those with stage 3 AKI (Fig. 4). When the analysis was restricted to patients with chronic lung disease or chronic liver disease, early thiamine use was not associated with reduced in-hospital mortality. There were no significant differences in the subgroups of heart failure, sepsis and A-on-C renal injury (Fig. 4).

Fig. 4. Subgroup analyses of the association between early thiamine use and in-hospital mortality.
Discussion
In this retrospective analysis, our results show for the first time that early thiamine use is associated with reduced in-hospital mortality and 90-d mortality in critically ill patients with AKI within 48 h after ICU admission. This result remained robust in the PSM analysis after adjusting for covariates. The findings of the subgroup analysis suggest that early thiamine use was possible beneficial role in patients with stages 1 to 2 AKI according to the Kidney Disease: Improving Global Outcomes criteria.
Several possible mechanisms by which thiamine could exert protective effects against mortality in critically ill patients with AKI have been proposed. Thiamine is a cofactor of pyruvate dehydrogenase, which plays a key role in aerobic metabolism(Reference Manzanares and Hardy14,Reference Frank, Leeper and Luisi27) . In the absence of thiamine, pyruvic acid cannot enter the Krebs cycle, resulting in a shift in metabolism to the anaerobic pathway. Pyruvate is converted to lactic acid instead of acetyl coenzyme A, resulting in elevated serum lactate levels, cell apoptosis and organ damage (including renal injury)(Reference Costa, Gut and de Souza Dorna15,Reference Zarbock, Gomez and Kellum28,Reference Donnino, Miller and Goyal29) . Thiamine supplementation may improve mitochondrial function, increase cellular energy production and prevent apoptosis-related cell death in renal tubular cells, which may contribute to the reduced incidence of AKI(Reference Honore, Jacobs and De Waele30–Reference Amrein, Oudemans-van Straaten and Berger32). In addition, thiamine plays a key role in nerve tissue repair and nerve signal modulation(Reference Manzetti, Zhang and van der Spoel33). Thiamine also produces anti-inflammatory effects by inhibiting oxidative stress and activating NF-κB(Reference Umezawa34). In view of the above, thiamine has attracted extensive attention as a drug for ‘metabolic resuscitation’ in recent years.
Thiamine deficiency is relatively more common in critically ill patients with AKI due to their high metabolic status and inadequate dietary intake. However, due to the lack of rapid tests for the serum thiamine concentration, the thiamine status of patients cannot be accurately determined in a timely manner, and thiamine deficiency is often ignored. At present, evidence-based medicine guidelines do not support routine thiamine supplementation in all critically ill patients. Thus, clinicians must use other indirect cues, such as clinical manifestations (i.e. cardiovascular and gastrointestinal beriberi), diet and related laboratory tests (i.e. serum lactic acid), to identify the most at-risk patients who need to receive thiamine supplementation. Moreover, additioanl randomised controlled trials are needed to confirm whether thiamine is necessarily beneficial for all critically ill patients with AKI or may be beneficial only for those with thiamine deficiency. In the present study, we found that the thiamine group seemed to have a longer lengths of stay in the ICU and hospital, and this partly may be caused by the differences in the survival rate between the two groups, that is, some patients who died early in the ICU would have a short ICU stay. It needs to be confirmed by further randomised controlled trials.
In the subgroup analysis, we found that early thiamine use did not associate with the outcome of the patients with sepsis. In recent years, the use of thiamine as a new adjuvant treatment for sepsis has gradually increased in clinical practice. More recently, a number of studies have explored whether there is any relationship between thiamine administration and the outcome in septic shock patients with AKI. Preventive interventions have shown controversial results. Woolum et al. analysed 369 patients with septic shock and reported that thiamine was associated with lower short-term mortality(Reference Woolum, Abner and Kelly5). In a randomised, double-blind controlled trial involving seventy patients, patients with septic shock who received thiamine had lower serum creatinine levels and a lower rate of progression to RRT than patients who received a placebo(Reference Moskowitz, Andersen and Cocchi17). In contrast, a recent multicentre observational study of 18 780 patients showed that thiamine was not associated with decreased mortality in patients with septic shock(Reference Miyamoto, Aso and Iwagami35). In a randomised clinical trial of vitamin supplementation, Fujii et al. demonstrated that the use of vitamin C together with thiamine did not significantly improve the survival duration in 216 patients with sepsis shock(Reference Fujii, Luethi and Young36). Therefore, one question is whether thiamine has renoprotective properties. Many researchers have speculated that in addition to renal hypoperfusion in sepsis-related AKI, some other mechanisms, such as nephron apoptosis and vascular endothelial cell damage, may play roles. Thiamine supplementation may prevent apoptosis-related cell death in renal tubular cells. More evidence is needed for further verification. A phase II randomised trial (NCT03550794) is underway to test the impact of thiamine in general septic subjects with renal injury.
In the present study, we found for the first time that the prognostic impact of early thiamine use seemed to be weak in patients with stage 3 AKI, while it was relatively strong in patients with stages 1 to 2 AKI. However, the specific mechanism is still unclear, and we need further research, especially prospective studies, to confirm the relationship between thiamine and clinical outcomes.
A-on-C renal injury refers to AKI in the context of previous CKD, and its clinical incidence in AKI patients ranges from 13 % to 35 %(Reference Ali, Khan and Simpson37). In the unmatched cohort, the incidence of A-on-C renal injury was 9·2 %. Many studies have shown that there is a significant difference in the prognosis of AKI patients with and without CKD(Reference Ali, Khan and Simpson37,Reference Wu, Huang and Lai38) . In this study, we found that early thiamine use was not associated with improved short-term survival in patients with A-on-C renal injury.
This study has several potential limitations. First, due to its retrospective design, we were unable to obtain the blood thiamine concentration of critically ill patients with AKI within 48 h after ICU admission; therefore, we do not know the rate of thiamine deficiency in these patients and whether thiamine supplementation was the direct cause of the reduction in mortality. Second, in our study, there was no further analysis of the relationship between thiamine dosage and mortality reduction in critically ill patients with AKI within 48 h after ICU admission. In addition, it is unknown whether patients will be treated with thiamine in conjunction with other trace elements in clinical practice. Third, there are many factors that may confound the clinical prescription of thiamine, such as the ICU admission reason, disease severity, nutritional status, liver function status and clinician preference. This study may be affected by selection bias. In this study, we did not include AKI patients with ICU for less than 48 h, which may lead to differences in early administration of thiamine and further lead to selection bias. In addition, AKI stage was performed within 48 h after admission in ICU, but the maximum AKI stage in the first 48 h was not necessarily consistent with the maximum AKI stage in ICU, which may be biased. Due to lack of data on whether patients began to use RRT before admission, RRT was not used as a criterion for the diagnosis and stage of AKI, which may be biased. Despite PSM, residual confounding cannot be completely excluded. Therefore, prospective studies are needed to further validate the conclusions of the present study. Fourth, the subgroup analysis in different AKI stages may be biased by the absence of a true baseline creatinine level in a considerable number of patients. Finally, this was a single centre study, and our conclusions need to be further verified by multicentre trials.
Conclusions
Early thiamine use was associated with improved short-term survival in critically ill patients with AKI within 48 h after ICU admission. Thiamine was possible beneficial in patients with stages 1 to 2 AKI according to the Kidney Disease: Improving Global Outcomes criteria.
Acknowledgements
Not application.
This work was supported by grants from the National Natural Science Foundation of China (81770679, 81470973 and 81970582) and the National Natural Science Foundation of Shandong Province (ZR2020QH060). No funding bodies had any role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Study design: X. L., H. L., C. Y., Y. X. and J. P. Data collection: X. L. Data analysis: X. L., H. L., N. N., Y. F., H. Z. and Q. D. Data interpretation: X. L., H. L., L. Z., B. Z., W. J., X. F., L. C., C. Y. and H. Y. Drafting of the manuscript: X. L. and H. L. Revision of manuscript content: X. L. and H. L. Approved the final version of the manuscript: X. L., H. L., N. N., X. L., X. F., C. J., Y. X. and J. P. All the authors have been critically reviewing and approved themanuscript.
The authors have declared that no competing interests exist
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114521003111









