Introduction
The cestode parasite Taenia solium is responsible for three clearly defined pathological entities: human taeniasis and neurocysticercosis (NC), and porcine cysticercosis. Humans intestinally infected with the adult Taenia worm, are the source of eggs that develop into the larval metacestodes when ingested by pigs (porcine cysticercosis) or man. Porcine cysticercosis is responsible for economic losses by small-scale pig producers. Human cysticercosis is of particular concern as, in addition to establishment of the metacestode in muscle and other organs such as the heart, it is localized in the brain, causing NC (Garcia et al., Reference Garcia, Nash and Del Brutto2014). Indeed, T. solium is the most common parasite infection of the brain, frequently prevalent in poor rural communities without access to medical facilities, and resulting in severe, debilitating, often fatal symptoms (FAO/WHO, 2014).
Taenia solium has a worldwide distribution, with serious public health and agricultural impact in many poor countries of Latin America, Africa and Asia, but also in developed countries, in the latter, mainly due to immigration from endemic countries (Carabin et al., Reference Carabin, Wrinkler and Dorny2017). Current estimates indicate a prevalence of greater than 50 million people for NC, resulting in an impact of 2.8 million disability adjusted life years (DALYs) (Havelaar et al., Reference Havelaar, Kirk and Torgerson2015). Significantly, NC is responsible for 10% of acute case admissions to neurological wards in countries where it is endemic (FAO/WHO, 2014). In Latin America alone, an estimated 75 million people are living in areas of T. solium endemicity. Of these, there are between 0.45 and 1.35 million people with symptoms of epilepsy attributed to NC (Bern et al., Reference Bern, Garcia and Evans1999; Coyle et al., Reference Coyle, Mahanty and Zunt2012). These endemic areas typically share the obvious risk factors: poverty, free-foraging pigs, an absence of meat inspection and poor or absent sanitation. Indeed, the presence of T. solium in a community is unequivocal evidence of deficient socio-economic conditions. In addition to these environmental risk factors, a major barrier to the control of NC is the common lack of information and awareness of the dangers of T. solium within the rural population (Willingham & Engels, Reference Willingham and Engels2006).
In Venezuela, cysticercosis has been reported in humans and pigs through serological evidence (Ferrer et al., Reference Ferrer, Cortez and Perez2002, Reference Ferrer, Cabrera and Rojas2003; Guzman et al., Reference Guzman, Guilarte del and Urdaneta2004; Cortez et al., Reference Cortez, Boggio and Guerra2010; Toquero et al., Reference Toquero, Morocoima and Ferrer2017; Rojas et al., Reference Rojas, Patiño and Pérez2019). In addition, there is coprological evidence for taeniasis (Rojas et al., Reference Rojas, Aguilar and Alviarez2008; Parkhouse et al., Reference Parkhouse, Rojas and Aguilar2020). In this report, we present serological, parasitological and socio-epidemiological evidence consistent with endemic transmission of taeniasis/cysticercosis (T/C) in three rural Venezuelan communities: Valle del Rio and Potrero Largo in the state of Cojedes and Palmarito in Portuguesa. Finally, as we have employed the detection of anti-metacestode antibodies and HP10 secreted metacestode glycoprotein to detect cysticercosis, these results do not formally discriminate between somatic cysts and NC.
Materials and methods
General design
The survey of human and porcine cysticercosis was prompted by anecdotal references suggesting possible endemic T/C by T. solium in the villages of Potrero Largo and Palmarito and a proven case of adult NC in Valle del Rio, reported by Dr CM Aguilar. Human and porcine cysticercosis was serologically identified through enzyme-linked immunosorbent assay (ELISA) by the detection of anti-metacestode antibodies and the HP10 secreted metacestode glycoprotein. In addition, relevant risk factors were established through an epidemiological survey and a questionnaire.
Study area and population
Valle del Río is located in Cojedes state at 09°49′49.7″N, 68°29′31.4′W, 588 m above sea level, with 166 inhabitants; Potrero Largo is 4 km away from Valle del Rio and is located at 09°49′17.8″N, 68°29′53.6″W, 152 m above sea level, with 300 inhabitants; Palmarito (Planahom area) is located in Portuguesa state at 09°44′26.1″N, 69°18′22.3″W, 1050 m above sea level with a population of 114 inhabitants. Mean temperatures range from 25 to 27°C for Valle del Rio and Potrero Largo, and from 12 to 28°C in Palmarito. Annual rainfalls (mm) are 91–107 in Valle del Rio and Potrero Largo, and 700–1000 in Palmarito.
Field procedures
The field procedures have been previously described (Rojas et al., Reference Rojas, Patiño and Pérez2019). Briefly, community visits were conducted between 2006 and 2008 (Valle del Rio and Potrero Largo) and in 2015 (Palmarito), by a team consisting of a medical doctor, four bioanalysts and five medical students. Prior to the experimental work, a meeting with the community was organized in order to explain the study objective and to invite their voluntary participation. Home visits were then made in order to provide responses to a structured questionnaire designed to record socio-sanitary information and risk factors for cysticercosis (housing conditions, water and sanitary facilities, public health services, number of pigs per household, recognition of metacestode-infected pigs and observation of proglottids in human faeces). As part of the interview, the inhabitants were shown samples of T. solium proglottids and photographs of infected pig meat. Individuals who had lived more than six months in the village were classified as residents. The main occupation of the members of the communities is agriculture and animal farming (chickens, pigs, goat and cows). Domestic pigs were the commonest animals, and the majority were allowed to roam and forage freely in the streets between the houses and their backyards.
Sample collection and storage
Faecal samples: three stool samples were collected on alternative days in disposable small plastic boxes from all consenting individuals. The villagers were carefully instructed on adequate hygiene to avoid contamination of themselves or the environmental area.
Serum samples: approximately 5 ml of human venous blood was drawn by venepuncture from all consenting villagers aged five years or older. Samples were centrifuged the same day, aliquoted in 1.5-ml vials and frozen until use. Porcine blood was collected by sampling the jugular vein by trained veterinarians or animal health assistants.
The total numbers of participants were 144, 248 and 100 for faecal samples and 139, 213 and 81 for serum samples in Valle del Río, Potrero Largo and Palmarito, respectively.
Parasitological diagnosis
Direct (Lugol and saline solution) and concentration methods (Kato and Willis–Molloy) were used to detect parasite and Taenia spp. eggs in the faecal samples (Botero & Restrepo, Reference Botero and Restrepo2012). Taenia solium adults were identified by morphological examination of expelled proglottids.
Antiparasitic treatment
Individuals positive for intestinal parasites were treated with anthelmintic or anti-protozoan medications. The patients found to be positive for Taenia spp. eggs in the coprological analysis were treated within one week of positive diagnosis under medical surveillance, according to the WHO/FAO/OIE (2005) protocol. After fasting for 10 h, a single dose of praziquantel (Cisticide® Merck, FCD Laboratorios, Cd. De México, Mexico)) (10 mg/kg) was given to each patient. One hour later, the patients were purged (Fleet phospho-soda oral) and were then kept on a light diet (juices and soups) for 12 h. Faeces were collected for 6 h and examined for helminth eggs and adult Taenia proglottids and scolices. Faecal samples from the positive carrier patients were examined at monthly intervals for four months in order to confirm successful treatment.
Detection of anti-metacestode antibodies and the secreted metacestode glycoprotein HP10 in human and porcine sera
The presence of anti-metacestode antibodies was measured using T. solium metacestode vesicular fluid as the antigen target in the ELISA protocol, according to Larralde et al. (Reference Larralde, Laclette and Owen1986), with minor modifications, as previously described (Cortez et al., Reference Cortez, Boggio and Guerra2010). The presence of the HP10 secreted metacestode glycoprotein was measured using an antigen-trapping ELISA as published (Harrison et al., Reference Harrison, Joshua and Wright1989), with minor modifications (Cortez et al., Reference Cortez, Boggio and Guerra2010).
ELISA cut-off values for the anti-metacestode antibodies and the HP10 secreted metacestode glycoprotein were calculated from the mean plus three standard deviations (for the antibody ELISA) and three or four standard deviations (for the HP10 antigen ELISA of humans or pigs, respectively) of the optical density readings obtained from analysis of 23 negative human control sera. Thus, 0.140 and 0.160 were the cut-off values for the human antibody ELISA and the HP10 ELISA, respectively. For analysis of porcine sera evaluation, 30 negative porcine control sera were assayed, to give cut-off values of 0.200 and 0.390 for the antibody ELISA and the HP10 ELISA, respectively (Rojas et al., Reference Rojas, Patiño and Pérez2019).
Statistical analysis
All the data were entered into an Excel (Microsoft Office Excel 2007 (PC Actual, Madrid, Spain)) spreadsheet and analyses were conducted in Stata 8.0 (http://www.stata.com).
Results
Socio-sanitary conditions and T/C risk factors in the three rural communities studied
Personal, socio-sanitary conditions and T/C risk factors were determined by house-to-house visits through responses to a structured questionnaire (summarized in table 1). All three communities have a basic rural school, but a primary health service was only available in two of the communities, being absent in Potrero Largo. In the absence of paved streets, the houses are connected by earthen pathways. There is neither public transport nor organized disposal of domestic waste, and environmental sanitation is scarce. Thus, latrines were only present in 2/35, 27/55 and 3/23 of the households in Valle del Rio, Potrero Largo and Palmarito, respectively, and the majority of the population routinely defecated in the open. Piped water was intermittently available in Valle del Rio and Potrero Largo, but the only source of water in Palmarito was a stream. Finally, the illiteracy level among the inhabitants was 25.7% in Valle del Rio, 10.1% in Potrero Largo and 39% in Palmarito.
Table 1. Socio-sanitary conditions of households in the three studied communities.
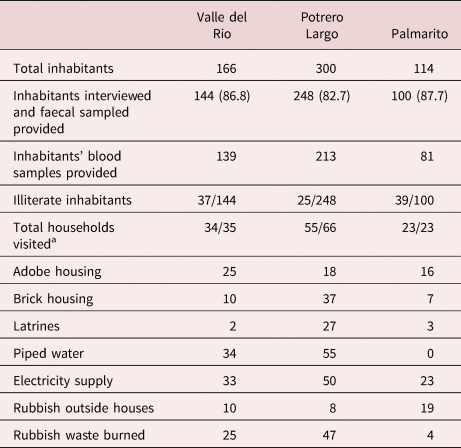
a Number of households visited/total number of households.
Risk factors for T/C in the communities
The risk factors for the three communities studied are presented in table 2. The number of householders raising pigs was nine, 32 and 13 in Valle del Rio, Potrero Largo and Palmarito, respectively, at the time of the study. The pigs were free-roaming or kept in backyard areas close to the houses. Only two families (in Potrero Largo) had an appropriate pigpen.
Table 2. Taeniasis/cysticercosis risk factors in the three rural communities.
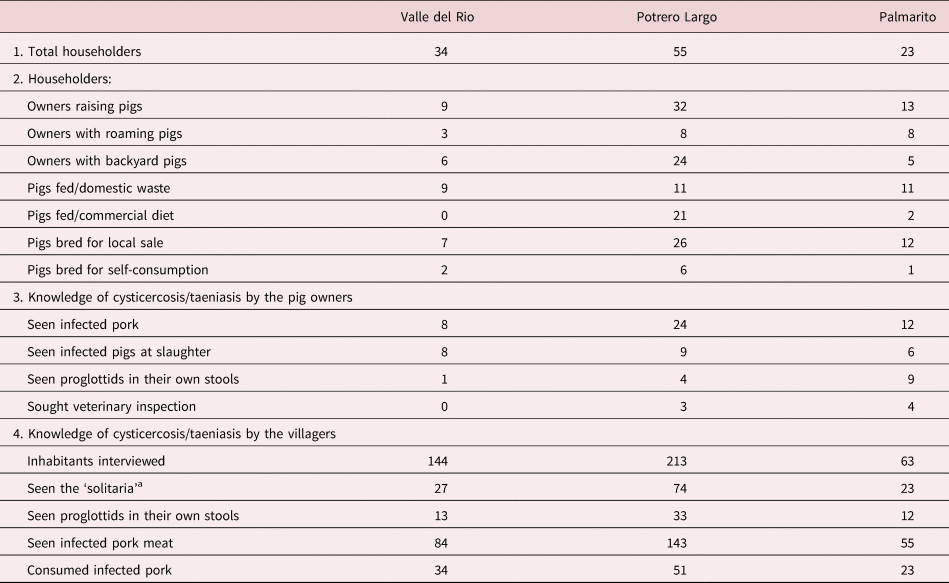
a Local name for adult of Taenia sp.
Particularly relevant, some inhabitants admitted to having consumed infected pig meat (locally known as ‘frutica’) and others to having observed expulsed proglottids in their own stools. In spite of these observations, all of those interviewed were ignorant of the parasite life cycle and attendant risk factors. An important observation was that in all of the three communities all the pig owners sacrificed the animals themselves in the yard of their houses and without any sanitary or veterinary surveillance.
Intestinal parasite infections in the three rural communities
The coprological examination of faecal specimens presented in table 3 indicates a very high level of soil-transmitted parasites in the three communities: 86.1%, 81.1% and 87.0% total parasitized individuals in Valle del Rio, Potrero Largo and Palmarito, respectively, many being infected with Trichuris trichiura and Ancylostoma sp. (table 4). All the parasitized patients were given anti-parasite treatment.
Table 3. Parasite-positive faecal samples from individuals in the three rural communities studied.
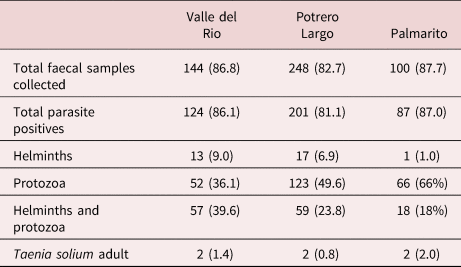
Faeces were collected, processed and analysed as described in the Materials and Methods section. Total faecal samples collected = the number of individuals that gave faeces, with the percent of the total population of each village in brackets. Total parasite positives: the number of positive samples for a given parasite infection, with the percent of faeces analysed in brackets. Protozoa include: Giardia intestinalis, Entamoeba histolytica/E. dispar, E. coli, Iodamoeba bütschlii, Endolimax nana, Blastocystis sp., Isospora belli. Helminths include: Ascaris lumbricoides, Trichuris trichiura, Ancylostoma sp., Enterobious vermicularis, Hymenolepis nana, Taenia solium.
Table 4. Protozoa and helminth species identified in faecal samples from individuals in the three rural communities studied.
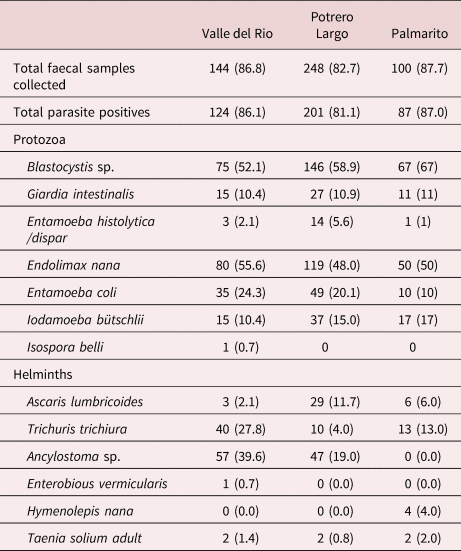
One important finding was the observation of Taenia spp. eggs in the faeces of two individuals in Valle del Rio, two individuals in Potrero Largo and two individuals in Palmarito. These six patients were treated with praziquantel and a purge. Their faeces were examined; the expelled parasite material (proglottids and scolices) was analysed, resulting in the formal identification of T. solium in all six patients. The other parasites present in the faeces from infected individuals are specified in tables 3 and 4.
Detection of anti-metacestode antibodies and HP10 secreted metacestode glycoprotein in human and porcine sera
As can be seen, the positive serological evidence clearly indicated endemic human and porcine (table 5) cysticercosis in all of the three studied communities. Thus, the HP10 secreted metacestode glycoprotein was detected in 36.0%, 12.2% and 29.6% human sera from Valle del Rio, Potrero Largo and Palmarito, respectively, and anti-metacestode antibodies were detected in 46.0%, 19.2% and 27.2% of the samples of human sera from Valle del Rio, Potrero Largo and Palmarito, respectively.
Table 5. Anti-metacestode antibody and HP10 glycoprotein levels in human and porcine sera in three rural communities.
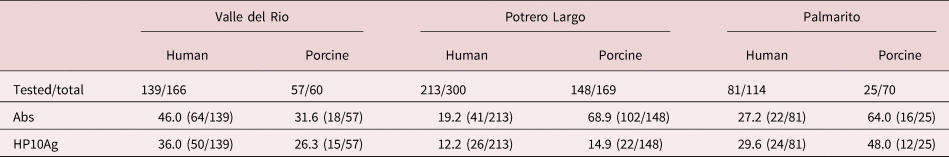
Similarly, in the pig serum samples, we observed anti-metacestode antibodies in 31.6%, 68.9% and 64.0% of the sera and HP10 secreted metacestode glycoprotein in 26.3%, 14.9% and 48.0% of the pig sera in Valle del Rio, Potrero Largo and Palmarito, respectively.
Discussion
The study of the three communities was prompted by anecdotal references of possible T. solium transmission and a proven case of NC in Valle del Rio.
We first determined the presence of the typical risk factors for T/C in the three communities. All communities were characterized by their obvious high level of poverty, with poorly structured villages and houses, a large absence of latrines, scarce public services, an absence of waste disposal services, irregular or absent water supply and significant illiteracy. Given these conditions, the observed high levels of soil-transmitted parasites (helminths and protozoa) were not a surprise: 86.1%, 81.1% and 87.0% in Valle del Rio, Potrero Largo and Palmarito, respectively. Similar risk factors have been reported for other rural Venezuelan communities (Toquero et al., Reference Toquero, Morocoima and Ferrer2017; Rojas et al., Reference Rojas, Patiño and Pérez2019) and in other countries with endemic cysticercosis (Ng-Nguyen et al., Reference Ng-Nguyen, Stevenson and Breen2018; Openshaw et al., Reference Openshaw, Medina and Felt2018). Indeed, the socio-economic conditions in these communities constitute a perfect panorama for the establishment of diverse infectious diseases, collectively called Neglected Tropical Diseases, and sustained by a vicious cycle of poverty, disease and underdevelopment (Ault, Reference Ault2007; WHO, 2010).
Specifically, and in relation to T/C, we observed routine human defecation in the open, and free-roaming pigs killed locally without veterinary inspection and for local consumption. These conditions not only favour the perpetuation of the T. solium transmission cycle, but also provide an informal route for entry of infected pig meat into urban areas (Bern et al., Reference Bern, Garcia and Evans1999). Perhaps our most depressing observation was that some people had seen Taenia sp. proglottids (in their faeces) and/or metacestodes in pork, but never associated these observations with the disease.
An important aspect of our study was the identification of two T. solium adult worm carriers in each of the three communities. Their subsequent treatment is not only expected to diminish local T. solium transmission, but may also provide an objective lesson to the inhabitants through highlighting the presence and danger of T. solium carriers in their communities.
In addition to the presence of adult worm carriers and the classical T/C risk factors, high levels of anti-T. solium metacestode antibodies and HP10 secreted metacestode glycoprotein were observed in both humans and pigs. While it is true that these serological procedures do have limitations (Carabin et al., Reference Carabin, Wrinkler and Dorny2017; Cortez et al., Reference Cortez, Rojas and Parkhouse2018; Parkhouse et al., Reference Parkhouse, Carpio and Campoverde2018), they are the only currently feasible practical tools available for the economically identification of cysticercosis in rural communities of low-income countries. Imaging technology, unfortunately, is not a practical solution for the diagnosis of poor rural populations because of its non-availability and high cost. The detection of porcine cysticercosis has been suggested as a possible alternative to serology for the identification of endemic human cysticercosis. Pigs are infected early in life and are usually slaughtered at, or before, one year of age (Bern et al., Reference Bern, Garcia and Evans1999). Thus, porcine sero-surveys may be the fastest, least expensive way to determine the level of ongoing transmission of cysticercosis.
Transmission of cysticercosis has also been reported for other rural Venezuelan communities. Thus, Ferrer et al. (Reference Ferrer, Cortez and Perez2002, Reference Ferrer, Cabrera and Rojas2003), using the same approach, found anti-metacestode antibodies in an Amerindians population (Amazonas state) and in rural communities (Lara and Carabobo states). A significant seroprevalence has also been reported in an indigenous population of Zulia state (Freites et al., Reference Freites, García and Díaz-Suárez2015) and in two rural communities of Anzoátegui state (Toquero et al., Reference Toquero, Morocoima and Ferrer2017).
In conclusion, we have observed the classical risk factors for the transmission of cysticercosis and taeniasis in all three of the communities studied. In addition, there was clear evidence for the transmission of cysticercosis through the identification and treatment of carriers of the adult T. solium, and through serological evidence for both cases of human and porcine cysticercosis. This study constitutes the first step towards eradication of the diseases in these communities.
Financial support
This work was supported by Ministerio PPP de Educación Superior Venezuela, OPSU Alma Mater programme (GR 2003–2007), the Consejo de Desarrollo Científico y Humanístico, Universidad de Carabobo (projects CDCH-UC 001-2004 MMC and 1143-2005 GR), Ministerio de Ciencia y Tecnología, Venezuela (project LOCTI-1.52 GR) and FUNDABIOMED, Universidad de Carabobo.
Conflicts of interest
None.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Signed informed consent was obtained for all adult participants, and parents or legal guardian of minors. Also, the authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. Animals were handled following good animal practice, as defined by OIE'S Terrestrial Animal Health Code for use in research and education (https://www.oie.int/fileadmin/Home/eng/Health_standards/tahc/current/chapitre_aw_research_education.pdf) and the guidelines provided by the National Centre for Replacement, Refinement and Reduction of Animals in Research.







