Introduction
Biologically active enzymes derived from invertebrate-associated gut symbionts represent a promising and underexplored resource. These enzymes play a crucial role in the efficient utilization of diverse nutrient resources by invertebrates (Jing et al., Reference Jing, Qi and Wang2020). Consequently, animal husbandry has shown considerable interest in harnessing these invertebrate-derived bioactive enzymes (Kannan et al., Reference Kannan, Mubarakali, Thiyonila, Krishnan, Padmanaban and Shantkriti2019).
Arazyme, an alkaline metalloprotease, is secreted by Serratia proteamaculans, a Gram-negative aerobic bacterium belonging to the genus Serratia. It is a symbiotic bacterium isolated from the intestinal ecosystem of Nephila clavata (Fig. 1), a species of arachnid known as ‘棒络新妇’ in Chinese and ‘무당거미’ in Korean (Bersanetti et al., Reference Bersanetti, Park, Bae, Son, Shin, Hirata, Juliano, Carmona and Juliano2005; Kwak et al., Reference Kwak, Lee, Shin, Maeng, Park, Oh, Son, Bae and Park2007). Arazyme plays a pivotal role in facilitating the digestion process in N. clavata. With a size of 51.5 kDa, arazyme has been approved as a food additive (registration No. 2007-SA-0007) by the Ministry of Food and Drug Safety in the Republic of Korea. Notably, it exhibits remarkable enzymatic activity over a wide range of pH and temperature and displays resistance to detergents (Bersanetti et al., Reference Bersanetti, Park, Bae, Son, Shin, Hirata, Juliano, Carmona and Juliano2005).

Figure 1. Image of N. clavata (source: https://commons.wikimedia.org/wiki/File:Nephila_clavata%28Female,Japan,2017.10.08%29.jpg).
As a novel invertebrate-derived bioactive enzyme, arazyme holds broad application prospects in animal husbandry. This paper provided a review of the current knowledge and understanding of arazyme, aiming to offer valuable references for its potential applications.
Characteristics of arazyme
Arazyme, an enzyme with zinc-containing metalloprotease activity, relies on the presence of zinc for its function (Kwak et al., Reference Kwak, Lee, Shin, Maeng, Park, Oh, Son, Bae and Park2007). Its deduced amino acid sequence exhibits significant homology to serralysins found in other enteric bacteria (Kwak et al., Reference Kwak, Lee, Shin, Maeng, Park, Oh, Son, Bae and Park2007). Arazyme acts as a proteolytic enzyme, capable of hydrolysing substances such as albumin, casein, elastin, haemoglobin, keratin and collagen (Piao et al., Reference Piao, Shen, Sun, Piao and Zhang2009). Notably, its proteolytic activity remains unaffected by inhibitors of aspartate, cysteine and serine proteases, as well as pepsin, trypsin and chymotrypsin. However, its activity is strongly inhibited by compounds like 1,10-phenanthroline and ethylenediaminetetraacetic acid (Bersanetti et al., Reference Bersanetti, Park, Bae, Son, Shin, Hirata, Juliano, Carmona and Juliano2005; Kwak et al., Reference Kwak, Lee, Shin, Maeng, Park, Oh, Son, Bae and Park2007). Furthermore, arazyme demonstrates optimal enzymatic activity at pH levels of 6.0 or higher and can function efficiently at temperatures up to 50°C (Bersanetti et al., Reference Bersanetti, Park, Bae, Son, Shin, Hirata, Juliano, Carmona and Juliano2005).
Immunoregulatory property
Arazyme has been extensively studied for its immunoregulatory properties. Pereira et al. (Reference Pereira, Melo, de Melo, Mourão-Sá, Silva, Berzaghi, Herbozo, Coelho-dos-Reis, Scutti, Origassa, Pereira, Juliano, Juliano, Carmona, Câmara, Tsuji, Travassos and Rodrigues2016) demonstrated that in vivo injection of both active and heat-inactivated arazyme exhibited significant effects in reducing tumour development, including primary and metastatic tumours. These effects were attributed to the activation of Toll-like receptor 4, leading to increased levels of interferon gamma and elevated counts of activated CD8+ T lymphocytes. In a separate study, Pereira et al. (Reference Pereira, Ferreira-Guimaraes, Paschoalin, Scutti, Melo, Silva, Melo, Silva, Tiago, Matsuo, Juliano, Juliano, Carmona, Travassos and Rodrigues2014) found that intraperitoneal administration of arazyme exerted a dose-dependent cytostatic effect on human and murine tumour cells by reducing the expression of CD44 molecules on the tumour cell surface, thereby interfering with cell adhesion. Additionally, arazyme induced the production of protease-specific immunoglobulin G, which exhibited cross-reactivity with tumour matrix metalloproteinase-8. Ghadaksaz et al. (Reference Ghadaksaz, Imani Fooladi, Mahmoodzadeh, Nejad and Amin2022) developed a fusion protein, arazyme-linker-TGFαL3, with high affinity for the epidermal growth factor receptor. In silico immune simulation demonstrated that this chimaera protein has the potential to prevent cancer development by inducing an immune response and inhibiting cell proliferation. Similarly, Rahmani et al. (Reference Rahmani, Imani, Ajoudanifar and Soleimani2023) designed a fusion protein, arazyme-linker-herceptin-HER2, which showed promising results as a candidate for breast cancer treatment. In silico immune simulation demonstrated that the predicted B-cell and T-cell epitopes of the fusion protein were capable of eliciting an immune response.
Overall, the mechanism of arazyme's immunoregulatory properties involves Toll-like receptor 4 activation, increased interferon gamma levels, activation of CD8+ T lymphocytes, reduction of CD44 molecules and production of specific immunoglobulins. These studies collectively highlight the immunomodulatory potential of arazyme and its ability to impact immune responses, offering promising prospects for its application in immunotherapy.
Anti-inflammatory property
Bersanetti et al. (Reference Bersanetti, Park, Bae, Son, Shin, Hirata, Juliano, Carmona and Juliano2005) observed that arazyme exhibited high hydrolytic activity on substance P, which is secreted by inflammatory cells, as well as peptides related to bradykinin, during in vitro co-culture. Additionally, Kim et al. (Reference Kim, Lee, Baek, Kim, Kim, Jeong, Shin, Park and Lee2015) conducted studies demonstrating that oral treatment of arazyme attenuated the development of atopic dermatitis-like lesions in mice induced by 2,4-dinitrochlorobenzene. This attenuation was achieved through the reduction of epidermal thickening, infiltration of inflammatory cells into the dermis and suppression of interleukin 4, interleukin 13 and immunoglobulin E levels. In another study by Kim et al. (Reference Kim, Kim, Shin, Son, Park and Lee2013), THP-1 human monocytic and EoL-1 human eosinophilic cells were treated in vitro with the Dermatophagoides pteronyssinus extract and administered with arazyme. The researchers observed that arazyme alleviated the severity of atopic dermatitis by regulating the expression of thymus and activation-regulated chemokine and skin barrier proteins in keratinocytes. They found that arazyme inhibited the production of monocyte chemoattractant protein 1, interleukin 6 and interleukin 8 in THP-1 and EoL-1 cells. Furthermore, arazyme suppressed the secretion of interleukin 6 and interleukin 8 in HMC-1 cells, reduced thymus and activation-regulated chemokine, monocyte chemoattractant protein 1, interleukin 6 and interleukin 8 levels in HaCaT cells, and upregulated the production of filaggrin, involucrin and loricrin in HaCaT cells. Additionally, Kim et al. (Reference Kim, Yang, Shin, Son, Park and Lee2014) observed that arazyme administration inhibited lipopolysaccharide-induced apoptosis in human umbilical vein endothelial cells in vitro. This inhibition was attributed to the suppression of monocyte chemoattractant protein 1 and interleukin 6 secretion as well as the downregulation of vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 expression, and reduction in reactive oxygen species production. Kim and Lee (Reference Kim and Lee2014) investigated the inhibitory effects of arazyme on neutrophil apoptosis in allergic diseases, specifically allergic rhinitis and asthma in vitro. They discovered that arazyme effectively inhibits neutrophil apoptosis through the PI3K/Akt/ERK/NF-κB pathway and the caspase 9/3 pathway. In the context of animal health, providing arazyme-containing diet has shown potential in reducing the occurrence of subclinical mastitis in dairy cows (Liu et al., Reference Liu, Cheng, Lu and Jin2007). This reduction is manifested by a decrease in somatic cell count in milk.
In summary, the mechanism underlying arazyme's anti-inflammatory property involves multiple potential pathways. Arazyme may interact with protease-activated receptors and exhibit high hydrolytic activity on specific peptides. Additionally, it regulates the production of pro-inflammatory cytokines and chemokines and protects against apoptosis and oxidative stress. Although more research is needed to fully understand the precise molecular mechanisms, these findings collectively suggest that arazyme acts as an anti-inflammatory agent through various mechanisms, making it a promising candidate for inflammation regulation.
Anti-bacterial property
In a study conducted by Kim et al. (Reference Kim, Lee, Bae, Shin, Ku, Park and Jeong2021), it was found that arazyme at a concentration of 250 mg/ml exhibited remarkable anti-bacterial properties in vitro. The researchers observed that arazyme inhibited the growth of oral opportunistic pathogens such as Candida albicans, Enterococcus faecalis, Staphylococcus epidermidis and Streptococcus mutans. Notably, arazyme demonstrated over 80% inhibition against C. albicans, which is a major contributor to denture stomatitis. These findings highlight the potential of arazyme as an effective agent for controlling harmful bacterial growth and preventing oral infections associated with denture use.
However, it is important to note that while this study demonstrated the anti-bacterial properties of arazyme, further research is necessary to elucidate the underlying mechanisms by which arazyme exerts its effects on bacterial growth. Future experiments can help unravel the specific molecular interactions and pathways involved in the anti-bacterial activity of arazyme, providing a deeper understanding of its potential applications in controlling bacterial infections.
Organ protection property
In the study conducted by Park et al. (Reference Park, Jeong, Park, Son, Shin, Do, Yang, Yuan, Hong, Goo, Lee, Ki, Ishigami and Jeong2008), the effects of oral arazyme on acute hepatic injury induced by CCl4 in mice were investigated. The findings revealed that arazyme plays a protective role by upregulating the expression of antioxidative proteins, inhibiting the TGF-β/Smad3 signalling pathway through downregulation of Smad3 and p-Smad3 expression levels, and preventing the decrease of SMP30 expression. These results suggest that arazyme has the potential to protect hepatocytes from chemokine-induced hepatic damage. In a separate study by Li et al. (Reference Li, Yoo, Park, Lim, Shin, Kim, Park and Jeong2019), the hepatoprotective effects of oral arazyme in high-fat diet-induced non-alcoholic fatty liver disease-like mice were examined. The administration of arazyme demonstrated protective effects against hepatic steatosis and hepatitis, and inhibited the progression of non-alcoholic fatty liver disease. Arazyme exerted its hepatoprotective effects by inhibiting hepatic fatty acid and triglyceride synthesis, suppressing SREBP-1-mediated lipid accumulation and macrophage-mediated inflammation, and reducing palmitic acid-induced lipogenesis in HepG2 hepatocytes. Moreover, arazyme reduced macrophage recruitment by inhibiting the expression of inflammatory cytokines in the liver. Li et al. (Reference Li, Park, Bak, Lim, Shin, Park and Jeong2016) further investigated the hepatoprotective effects of oral arazyme in high-fat diet-induced non-alcoholic fatty liver disease-like mice. The supplementation of arazyme in the diet exhibited hepatoprotective effects by decreasing hepatic lipid accumulation and improving insulin resistance. Arazyme supplementation resulted in reduced plasma levels of alanine aminotransferase, thyroglobulin, non-esterified fatty acids, glucose and haemoglobin A1C. Additionally, arazyme improved hepatic steatosis and fibrosis, decreased hepatic triglyceride and total cholesterol contents, improved glucose tolerance and reduced pancreatic insulin contents and islet size. In cell studies, arazyme increased AKT phosphorylation in palmitic acid-induced HepG2 cells, improved insulin secretion and synthesis in pancreatic MIN6 β-cells and reduced hepatic lipid accumulation while reversing insulin resistance.
Overall, oral arazyme administration is able to upregulate antioxidative proteins, inhibit the TGF-β/Smad3 signalling pathway, prevent the decrease of SMP30 expression, inhibit hepatic fatty acid and triglyceride synthesis, enhance AKT phosphorylation and improve insulin resistance. These mechanisms collectively contribute to the hepatoprotective effects of arazyme, ultimately improving liver function in various liver injury and non-alcoholic fatty liver disease models.
Application of arazyme in feedstuff industry
The feed industry can extract several benefits from the use of arazyme. Firstly, arazyme, as a proteolytic enzyme, has the ability to hydrolyse proteins in feedstuff materials (Piao et al., Reference Piao, Shen, Sun, Piao and Zhang2009). This enzymatic hydrolysis results in increased levels of digestible small molecular substances, particularly amino acids and peptides, present in the ingredient. Zi et al. (Reference Zi, Kim, Shin, Lee, Yeom and Choi2011) conducted an enzymatic hydrolysis study on soybean meal using a combination of 0.5% phytase and 0.02% arazyme, which resulted in increased levels of free cysteine, aspartic acid, threonine, methionine and leucine in the hydrolysed soybean meal. Kim (Reference Kim2009) reported that when arazyme was applied to hydrolyse corn gluten meal, it led to elevated levels of free leucine. Similarly, hydrolysis of soybean meal with arazyme resulted in an increased level of free phenylalanine, valine, leucine and isoleucine (Kim, Reference Kim2009). Furthermore, the hydrolysis of distiller's dried grains with solubles using arazyme increased the contents of free cysteine, valine and isoleucine (Kim, Reference Kim2009). Fish meal hydrolysed with arazyme also exhibited increased level of free cysteine (Kim, Reference Kim2009). Piao et al. (Reference Piao, Shen, Sun, Piao and Zhang2009) note that when arazyme was applied to hydrolyse meat meal, fish meal, soybean meal, cottonseed meal, rapeseed meal, corn flour and corn gluten meal, there were notable increases in the contents of both essential and non-essential free amino acids, particularly lysine, threonine and tryptophan. Additionally, Liang and Piao (Reference Liang and Piao2010) found that arazyme hydrolysis of black rice bran resulted in increased free peptide content. By enhancing the free amino acid profiles of feedstuff materials, arazyme can improve the nutritional value of animal diets.
Additionally, the utilization of arazyme can help optimize feed formulations by providing a cost-effective alternative to expensive protein sources, such as plasma protein powder. Guan and Sun (Reference Guan and Sun2010) demonstrated that replacing 4% plasma protein powder in the diet with 0.1% arazyme and 3% fish meal effectively reduced feed costs without compromising growth performance.
Overall, the use of arazyme in the feed industry offers benefits such as improved feedstuff's free amino acid profiles and cost savings in feed formulation. These advantages contribute to the production of high-quality and cost-effective animal feeds, ultimately benefiting both feed manufacturers and livestock producers.
Application of arazyme in animal husbandry
In this paper, we conducted a review of scientific studies investigating the effects of dietary arazyme supplementation in various animal species, including poultry (Table 1), swine (Table 2), ruminant (Table 3) and aquatic animals (Table 4). The results of these studies consistently demonstrate the positive effect of incorporating arazyme into the diet of farming animals, leading to improvements in their growth and production performance.
Table 1. Application of arazyme in poultry husbandry

NH3, ammonia; BWG, body weight gain.
Table 2. Application of arazyme in swine husbandry
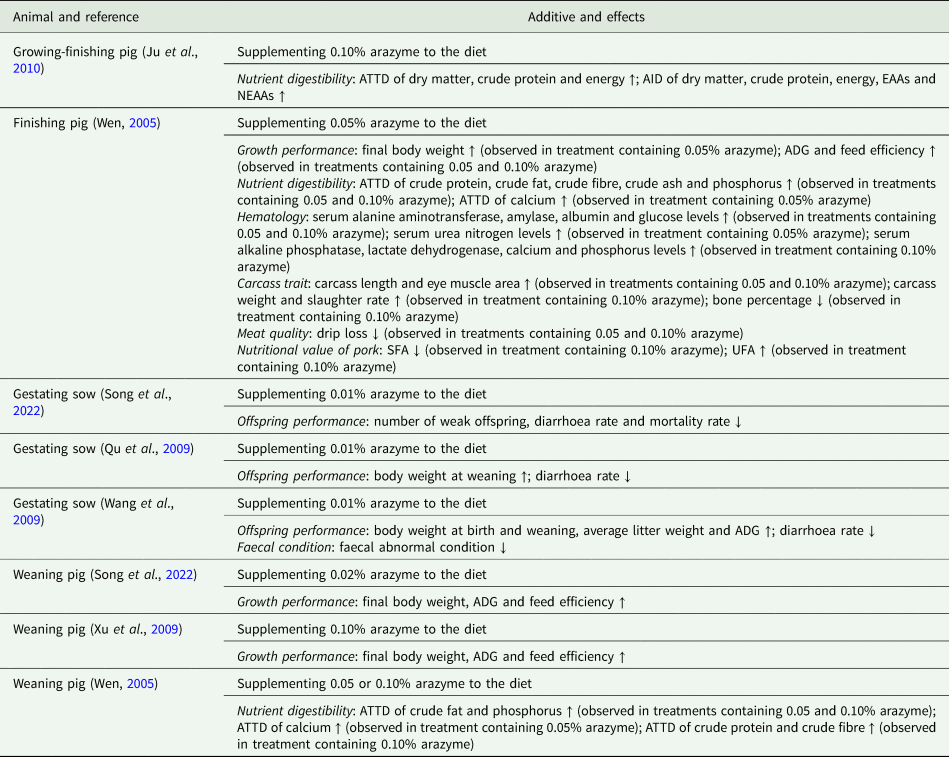
ADG, average daily gain; EAA, essential amino acid; NEAA, non-essential amino acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; ATTD, apparent total tract digestibility; AID, apparent ileal digestibility.
Table 3. Application of arazyme in ruminant husbandry

ADG, average daily gain.
Table 4. Application of arazyme in aquaculture
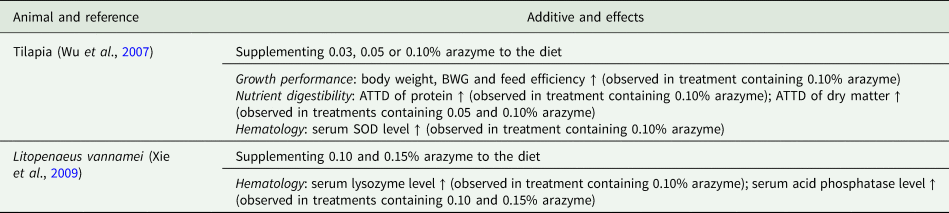
BWG, body weight gain; ATTD, apparent total tract digestibility; SOD, superoxide dismutase.
Specifically, in poultry husbandry, the supplementation of arazyme to a diet has been shown to improve performance and reduce excreta noxious gas emissions in both broilers and layers (Kim, Reference Kim2009; Kim et al., Reference Kim, Kim, Kim, Lee, Lee, Lee, You, Ahn, Kim, Park, Son, Shin and Kang2009). Similarly, in swine husbandry, arazyme supplementation has been found to enhance the efficiency of nutrient utilization, leading to improved growth performance, carcass traits, meat quality, nutritional value of pork and offspring quality (Wen, Reference Wen2005; Qu et al., Reference Qu, Li and Guan2009; Wang et al., Reference Wang, Li, Lin and Chen2009; Xu et al., Reference Xu, Gong, Zhang, Duan and Fan2009; Ju et al., Reference Ju, Shen and Zhang2010; Song et al., Reference Song, Li, Liu, Qi, Dou and Zheng2022). In ruminant husbandry, the inclusion of arazyme has shown promising results in improving rumen fermentation, ultimately translating into improved milk quality, milk production and growth performance (Zhang and Sun, Reference Zhang and Sun2009; Wang, Reference Wang2019). Furthermore, in the context of aquaculture, the addition of arazyme to fish and shrimp diets has demonstrated beneficial effects on nutrient digestibility, antioxidant capacity and growth performance (Wu et al., Reference Wu, Xie, Wang, Yu, Hu and Niu2007; Xie et al., Reference Xie, Yu, Wang, Wu, Hu and Guan2009).
The consistent positive outcomes observed across these studies highlight the potential of arazyme as a valuable tool in animal nutrition. The ability of arazyme to enhance growth and production performance in various species underscores its importance as a supplement in animal feed formulations. Further research and exploration of optimal dosage levels and other delivery methods are warranted to fully harness the benefits of arazyme in animal husbandry.
Conclusion
In conclusion, arazyme, a biologically active enzyme derived from invertebrate-associated gut symbionts, holds significant potential for various applications. It exhibits remarkable enzymatic activity over a wide range of pH and temperature, making it suitable for use under different conditions. Arazyme has been extensively studied for its immunoregulatory, anti-inflammatory, anti-bacterial and organ protection properties. In the feedstuff industry, arazyme has been proven effective in hydrolysing proteins in feed materials, leading to enhanced free amino acid profiles and peptide contents. This offers opportunities to improve the nutritional value of feed formulations and reduce feed costs. In animal husbandry, arazyme has consistently shown positive effects on growth and production performance in poultry, swine, ruminants and aquatic animals. It improves nutrient utilization, intestinal health and meat quality, while reducing noxious gas emission. These findings emphasize the potential of arazyme as a valuable tool in animal nutrition.
Overall, the comprehensive review of arazyme presented in this paper provides valuable references for its potential applications. Further research is warranted to explore optimal dosage levels, delivery methods and underlying mechanisms of action.
Data
No new data were generated or analysed in support of this research.
Author contributions
D. X. Dang: writing – original draft, writing – review and editing. W. S. Meng, S. H. Li: data collection and organization. T. Wang and D. Li: methodology, supervision, writing – review and editing.
Acknowledgments
The authors are deeply indebted to Xin Yan Fan and Shan Fang, without whose valuable assistance in collecting the large number of articles to be reviewed this paper would not have been possible.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interest
None.
Ethical standard
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.








