Creatine (Cr) is endogenously produced (approximately 1 g/d) as well as ingested from diet (approximately 1–5 g/d), mostly via meat intake. Approximately 95 % of total Cr content is localised in skeletal muscle, while smaller amounts can also be found in other excitatory tissues, such as the brain( Reference Wyss and Kaddurah-Daouk 1 ).
It has been generally accepted that vegetarians have lower tissue Cr content when compared with omnivores. This assumption particularly holds true with regard to skeletal muscle( Reference Harris, Soderlund and Hultman 2 ). Furthermore, a large body of evidence suggests that vegetarians respond better to Cr supplementation in terms of muscle Cr accretion( Reference Harris, Soderlund and Hultman 2 ). However, the extent to which dietary Cr may affect Cr content in other tissues is yet unknown.
In the brain, Cr is thought to exert an important energetic role. This notion has been supported by a large body of evidence, including (1) the presence of Cr kinase isoforms in the central nervous system( Reference Kaldis, Hemmer and Zanolla 3 ), (2) the relationship between brain Cr depletion and mental retardation, autism, speech delay and brain atrophy( Reference Salomons, van Dooren and Verhoeven 4 ) and (3) the reversal of these symptoms after oral Cr intake( Reference Salomons, van Dooren and Verhoeven 4 , Reference Stockler, Hanefeld and Frahm 5 ). Recently, it has been demonstrated that orally ingested Cr is able to penetrate the blood–brain barrier, though with some limitations, and improve brain energy metabolism in human subjects( Reference Dechent, Pouwels and Wilken 6 , Reference Lyoo, Kong and Sung 7 ). As a result, it has been suggested that Cr supplementation may alleviate mental fatigue induced by stressor stimulus, such as sleep deprivation combined with vigorous physical activity( Reference McMorris, Harris and Howard 8 ). Additionally, a recent study has demonstrated that in vegetarians, but not in omnivores, Cr supplementation improves memory (as assessed by the word recall test)( Reference Benton and Donohoe 9 ). The latter finding allows speculating that vegetarians may have suboptimal levels of brain Cr, similar to those observed in skeletal muscle.
The aim of the present study was to gather knowledge on the influence of diet on brain Cr content by comparing vegetarians (who consume virtually no Cr from diet) with omnivores.
Materials and methods
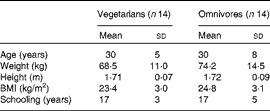
In the present cross-sectional study, fourteen healthy vegetarians who had been on a vegetarian diet for 9 (sd 10) years (six women and eight men) were compared with fourteen age- and BMI-matched omnivorous individuals (five women and nine men). No statistical differences between vegetarians and omnivores were detected for any of the demographic variables (Table 1).
Table 1 Characteristics of the participants (Mean values and standard deviations)
The participants were recruited by placing advertisement posters around the researchers' workplaces as well as in social networking websites. The individuals who volunteered for participation were self-identified as lacto-ovo-vegetarians (n 10), ovo-vegetarians (n 2), vegans (n 2) or omnivores (n 14). To ensure an accurate self-classification, all the subjects were provided, beforehand, with a comprehensive explanation of the definitions regarding vegetarianism and its subclassifications, according to well-accepted criteria( Reference Weinsier 10 ). Afterwards, a systematic dietary intake analysis was carried out by means of three 24 h food recalls undertaken on separate days (two weekdays and one weekend day) using a visual aid photo album of real foods, which did ensure that the vegetarians' diet was free of meat. Furthermore, dietary Cr content was estimated based on specific food composition tables( Reference Harris, Lowe and Warnes 11 , Reference Hulsemann, Manz and Wember 12 ).
Brain Cr content in the posterior cingulate cortex (Fig. 1) was assessed by non-invasive proton magnetic resonance spectroscopy (1H-MRS) using a single-voxel stimulated echo acquisition mode (STEAM) technique (voxel size 3 × 3 × 3 cm3) with emission time/repetition time (TE/TR) = 10/1839 ms and a whole-body 3·0T MRI scanner (Achieva Intera, Philips). Cr was examined in the posterior cingulate cortex since it is part of the limbic system and is related to emotion formation as well as cognitive function (e.g. processing, learning and memory)( 13 ). Metabolite quantification was carried out using LCModel software (s-provenches.com/pages/lcmodel.shtml)( Reference Provencher 14 ), and data are expressed as total Cr content, Cr:N-acetylaspartate ratio and Cr:choline ratio. Data were normally distributed as evidenced by the Shapiro–Wilk test. Differences between groups were examined by two-tailed unpaired t tests, and significance level was previously set at P< 0·05. Data are expressed as means and standard deviations, except when otherwise stated.

Fig. 1 T1-weighted magnetic resonance (MR) images showing the volume of interest selected for proton MR spectroscopy in the posterior cingulate cortex (total volume = 3 × 3 × 3 cm3).
The present study was approved by the local Ethics Committee (University of Sao Paulo, School of Medicine, Sao Paulo, Brazil), and all the subjects signed written informed consent. All the procedures followed were in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Results
As expected, vegetarians had lower dietary Cr intake than omnivores (0·03 (sd 0·01) v. 1·34 (sd 0·62) g/d, respectively; P= 0·0005). However, vegetarians and omnivores had comparable brain total Cr content (5·999 (sd 0·811) v. 5·917 (sd 0·665) IU, respectively; P= 0·77; Fig. 2), Cr:choline ratio (4·455 (sd 1·336) v. 4·470 (sd 0·476) IU, respectively; P= 0·97) and Cr:N-acetylaspartate ratio (0·770 (sd 0·047) v. 0·760 (sd 0·060) IU, respectively; P= 0·64).

Fig. 2 (a) Dietary creatine (Cr) intake in vegetarians and omnivores. Values are means, with standard deviations represented by vertical bars. * Mean value was significantly different from that of groups (P= 0·0005). (b) Brain total Cr content in vegetarians and omnivores (individual data, mean and 95 % CI). No significant difference was observed between the groups (P= 0·77). Data expressed as Cr:N-acetylaspartate ratio and Cr:choline ratio are summarised in the Results section.
Discussion
The main finding of the present study is that dietary Cr intake does not have any influence on brain Cr content in apparently healthy subjects, as opposed to what is observed in skeletal muscle.
It has been shown previously that oral Cr intake can have beneficial effects on cognitive function in vegetarians rather than in omnivorous individuals( Reference Benton and Donohoe 9 ), suggesting that the former may show some deficit in brain Cr content. However, the present study refutes this hypothesis, reinforcing previous experimental data suggesting that brain Cr content relies primarily on local endogenous synthesis rather than on Cr dietary intake( Reference Braissant, Henry and Loup 15 ). In fact, the key enzymes involved in the two-step Cr synthesis, i.e. arginine:glycine amidinotransferase and S-adenosyl-l-methionine:N-guanidinoacetate methyltransferase, are present in astrocytes, neurons and oligodendrocytes, giving the all the main cell types of the brain the potential to synthesise Cr( Reference Braissant, Henry and Loup 15 , Reference Andres, Ducray and Schlattner 16 ). In contrast, brain permeability to Cr is very limited, probably due to the absence of Cr transporter 1 expression in the astrocytes involved in the blood–brain barrier. Together, these observations have led some authors to consider that the brain could be a ‘near-independent compartment for the synthesis and use of Cr’( Reference Braissant, Henry and Loup 15 ). The present study supports this notion by demonstrating that dietary Cr has no effect on brain Cr content.
In apparent contrast to our findings, there is compelling evidence that Cr supplementation can increase brain Cr content and, hence, overcome cognitive impairments (e.g. mental retardation, autism and speech delay) in children with Cr deficiency syndromes( Reference Stockler, Hanefeld and Frahm 5 ). Additionally, a few( Reference McMorris, Harris and Howard 8 , Reference Watanabe, Kato and Kato 17 ) but not all( Reference Rawson, Lieberman and Walsh 18 ) studies have revealed a positive effect of Cr supplementation on cognition in individuals exposed to highly stressing conditions (e.g. sleep deprivation and exhausting exercise). In light of these data, it is possible to speculate that dietary Cr may be potentially relevant to brain metabolism in ‘extreme’ conditions where inherent (e.g. X-linked Cr transporter defect) or environmental (e.g. acute mental stressors) factors lead to a Cr depletion that cannot be compensated by endogenous Cr production. Based on the present findings, one can assume that vegetarianism is not a condition that predisposes to brain Cr depletion in the posterior cingulate cortex. However, further studies are needed to determine whether this is the case for other regions of the brain. Additionally, novel investigations must explore the potential differential response to high-dose Cr intake (i.e. Cr supplementation) in vegetarians and omnivores.
In conclusion, dietary Cr intake seemed not to influence brain Cr content in healthy adults, suggesting that in normal conditions, brain is dependent on its own Cr synthesis.
Acknowledgements
The authors thank the participants of the present study.
The authors are thankful to Fundação de Amparo à Pesquisa (FAPESP) do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for providing financial support. FAPESP and CNPq had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: M. Y. S., B. G. and G. G. A. wrote the manuscript; V. d. S. P., H. R. and M. C. O. reviewed and revised the manuscript; M. Y. S. and B. G. were responsible for the study concept and design; M. Y. S., V. d. S. P. and M. C. O. were responsible for data acquisition; M. Y. S., M. C. O. and B. G. analysed and interpreted the data; H. R., G. G. A. and B. G. contributed to the study through their statistical expertise.
None of the authors has any conflicts of interest to declare.





