Introduction
Worldwide, stroke is the second leading cause of death and a major cause of disability.Reference Katan and Luft1 Since the National Institute of Neurological Disorders and Stroke (NINDS) Intravenous alteplase to Intravenous tissue-type plasminogen activator (IVtPA) trial in 1995, a paradigm shift was initiated in the management of ischemic stroke.Reference Campbell, Meretoja, Donnan and Davis2,3 Timely administration of the drug effected better 90-day outcomes in carefully selected acute ischemic stroke patients.Reference Emberson, Lees and Lyden4 However, not all potentially eligible patients benefited from this form of treatment.Reference Lahr, Luijckx, Vroomen, van der Zee and Buskens5 Globally, only about 2%–10% of stroke patients received IVtPA.Reference Lahr, Luijckx, Vroomen, van der Zee and Buskens5,Reference Karlinski, Kobayashi, Mikulik, Sanak, Wahlgren and Czlonkowska6 Many patients failed to receive IVtPA on account of rigid guidelines that accompany the administration of the drug.Reference Fugate and Rabinstein7 Because of the criteria set by the landmark trial of the third European Cooperative Acute Stroke Study (ECASS III), patients with previous cerebral infarction (PCI) and diabetes mellitus (DM) were excluded in the 3–4.5 h extended time window.Reference Cronin, Sheth and Zhao8 The exclusion is based on the poorer outcomes observed in patients with DM and PCI compared with those patients who have neither comorbidities.Reference Karlinski, Kobayashi, Mikulik, Sanak, Wahlgren and Czlonkowska6,Reference Rubiera, Ribo and Santamarina9-Reference Cappellari, Moretto and Micheletti16 Recently published systematic review on early recurrent stroke and IVtPA, however, has shown similar findings in terms of functional outcome and symptomatic intracerebral hemorrhage (sICH) to that of published data on thrombolyzed patients without a recent stroke.Reference Ignacio, Diestro, Espiritu, Spears and San Jose17,Reference Sarmiento, Diestro, Espiritu and San Jose18 Independently, secondary analyses of trial data and observational studies have identified hyperglycemia or DM as a risk factor for sICH after thrombolytic therapy, as well as in untreated stroke.Reference Ehrlich, Liang and Xu19 Poorer response to IVtPA in these patients is considered to be a result of increased plasminogen activator inhibitor-1 activity, resistance to antithrombotic agents, and higher prevalence of atherosclerosis.Reference Kruyt, Biessels, DeVries and Roos11,Reference Nordt, Klassen, Schneider and Sobel20,Reference Pandolfi, Cetrullo and Polishuck21 Moreover, hyperglycemia has repeatedly shown a deleterious effect of exacerbating ischemic brain injury, accelerating the molecular processes leading to cell death, and resulting finally in larger infarct volumes and poorer outcomes, including higher mortality, poorer neurological and functional outcomes, longer hospital stay, higher readmission rates, and stroke recurrence.Reference Rubiera, Ribo and Santamarina9,Reference Lau, Lew, Borschmann, Thijs and Ekinci22 This can be explained from a pathophysiologic perspective, wherein excess perfusion after recanalization of local vascular occlusion as well as various mechanisms and factors involved in ischemic/reperfusion cascade leads to the damage of the blood–brain barrier and dysfunction of the vascular basal lamina, which may cause intracerebral hemorrhage (ICH).Reference Wu, Wu, Chen, Li and Ji23 Therefore, the exclusion criteria in previous studies and clinical practice may infer that the damage of blood–brain barrier and vascular basal lamina caused by previous infarction have not yet fully recovered; hence, intravenous thrombolysis with IVtPA may increase the risk for hemorrhage in these areas.Reference Wu, Wu, Chen, Li and Ji23
Regardless, contraindications to IVtPA vary among guidelines such as the American Heart Association (AHA) guidelinesReference Powers, Rabinstein and Ackerson24 and the European Stroke Initiative recommendations.13 The latest AHA/American Stroke Association (ASA) guidelines state that IVtPA in patients with a combined history of DM and PCI presenting in the 3–4.5 h time window, IVtPA may be as effective as treatment in the 0- to 3 h window.Reference Powers, Rabinstein and Ackerson24 Despite increasing data supporting the benefit of IVtPA in these patients, the European restriction of IVtPA in patients with previous stroke and DM is still enforced.13,25
This review aims to determine the safety and efficacy of IVtPA among diabetic patients with a history of stroke presenting in the 3–4.5 h time window using meta-analysis of relevant studies in terms of the following outcome measures: mortality, symptomatic intracranial hemorrhage (sICH), and functional outcomes using modified Rankin scale (mRS).
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) Consensus Statements guidelines were followed in this review.Reference Liberati, Altman and Tetzlaff26,Reference Stroup, Berlin and Morton27
Criteria for Inclusion of Studies
Types of Studies. We considered studies that employed randomized controlled trials and prospective or retrospective cohort studies. Studies that were designed with no comparator group were excluded. Only studies that reported primary data, expressed in the English language and available in full-text, were considered.
Types of Participants. Studies whose participants were selected according to the NINDS trial criteria in the administration of IVtPA were considered in this review.3 We included in the analyses the following patients who presented with a new acute ischemic stroke within 3–4.5 h time window and underwent IVtPA: (1) diabetic patients with PCI (DM+/PCI+) and (2) patients with no diabetes and PCI (No DM+/PCI+). PCI is defined as having a history of previous ischemic stroke in the last 3 months. The No DM+/PCI+ group includes patients with neither DM nor PCI, patients with DM only, and patients with PCI only.
Types of Interventions. We included studies which employed the standard 0.9 mg/kg dose of IVtPA within 3–4.5 h time window of stroke onset.
Types of Outcome Measures. The primary outcomes considered in this review are the safety endpoints of IVtPA measured in terms of mortality and sICH as defined by the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria.Reference Rubiera, Ribo and Santamarina9 The secondary endpoint considered in this review is the efficacy outcome of IVtPA on functional outcomes as measured by mRS at 3 months. A good functional outcome was variably defined as mRS of 0–2 indicating functional independence, while all others were considered poor outcome.
Search Methods for Identification of Studies
Literature search was done up to July 19, 2019, in the following databases: Medline via PubMed, ClinicalTrials.gov website, Cochrane Central Register for Controlled Trials (CENTRAL) by the Cochrane Library, and Scopus. The following general search and corresponding MeSH terms were used: “diabetes mellitus,” “previous stroke,” and “intravenous thrombolysis.” Alternate keywords such as “DM,” “previous cerebral infarction,” and “IV r-TPA” were also used. The detailed search strategies are listed in Appendix A.
Selection of Studies
Titles and abstracts obtained in the systematic search were evaluated using predefined screening criteria by two authors (ATP, AIE). After deduplication, full-text of articles which passed the screening criteria were obtained and evaluated by two authors (JDD, AIE). Studies which fulfilled the eligibility criteria were included in this review. Two authors (ATP, AIE) collected relevant data from the included studies.
Methodological Quality Assessment
The methodological quality of the studies included was conducted by two authors (ATP, JDBD). The risk of bias was assessed using the Newcastle–Ottawa Scale.Reference Wells, Shea and Connell28
Data Collection and Analysis
Odds ratios (ORs) with 95% confidence interval (CI) were used to compare measures of treatment effect. The data were analyzed using Stata 15.1 software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Dichotomous outcomes were pooled using the Mantel–Haenszel method. Random-effects model was used in the meta-analyses.Reference Higgins and Green29 Statistical significance was considered if the 95% CI of the OR between the intervention and the control arms did not include the number one.
Statistical heterogeneity was detected using the chi-squared test with a p-value < 0.10 to indicate statistically significant heterogeneity.Reference Higgins, Thompson, Deeks and Altman30 The I 2 statistic was employed to determine degree of heterogeneity, with an I 2 > 50% denoting substantial heterogeneity.Reference Higgins, Thompson, Deeks and Altman30
Results
Systematic Literature Review
Using the search terms, we identified a total of 13 records with only one duplicate removed (Figure 1). After applying the eligibility criteria, four papers were included in this review.Reference Fuentes, Martínez-Sánchez and Alonso de Leciñana31,Reference Mishra, Ahmed and Davalos32

Figure 1: PRISMA flow diagram for study selection.
Characteristics of Studies and Patients
The studies’ and patients’ characteristics are summarized in Appendix B and Table 1, respectively. All of the studies were registry based. Of the 44,572 patients in the combined registries, 2841 of them had both DM and PCI (DM+/PCI+), while 41,731 had DM only, PCI only, and neither DM nor PCI (No DM+/PCI+ group). Most of the patients were males (n = 24,305; 54.5%). The great majority of the patients were in the No DM+/PCI+ group (n = 41,731; 93.6%). DM+/PCI+ patients were older than the No DM+/PCI+ group. Baseline NIHSS scores were similar in both groups except for the study by Filipov which showed higher NIHSS on admission for the DM+/PCI+ group. Higher admission blood glucose was recorded in DM+/PCI+ patients. Furthermore, hypertension and atrial fibrillation are more common in the DM+/PCI+ group with note of higher number of antiplatelet use.
Table 1: Patient demographic and clinical characteristics

NR means not reported. *Only the mean is reported by Fuentes for the systolic blood pressure on admission.
Methodological Quality Assessment
The results were summarized in a risk of bias table (see Appendix C).
Outcomes
Unadjusted rates of sICH were not increased significantly among DM+/PCI+ patients who were given IVtPA (OR, 1.09; 95% CI, 0.88, 1.36) (see Figure 2).Reference Ehrlich, Liang and Xu19,25,Reference Fuentes, Martínez-Sánchez and Alonso de Leciñana31 However, mortality appears to be increased in the DM+/PCI+ group (OR, 1.81; 95% CI, 1.60, 2.06) (see Figure 3).25,Reference Fuentes, Martínez-Sánchez and Alonso de Leciñana31,Reference Mishra, Ahmed and Davalos32 In terms of functional outcome, IVtPA in DM+/PCI+ patients was more likely to be functionally independent at 3 months (mRS of 0–2) compared with the No DM+/PCI+ group (OR, 0.76; 95% CI, 0.61, 0.94) (Figure 4).Reference Fuentes, Martínez-Sánchez and Alonso de Leciñana31,Reference Mishra, Ahmed and Davalos32
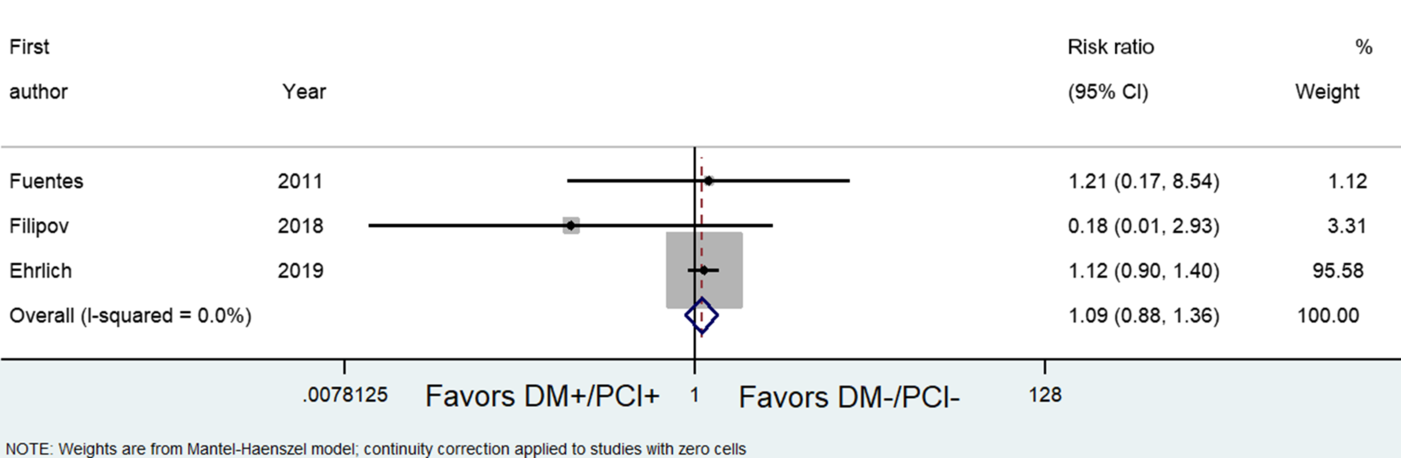
Figure 2: Forest plot showing odds ratios comparing the outcomes of diabetic patients with prior cerebral infarction (DM+/PCI+) versus patients with no diabetes and with no previous cerebral infarction (No DM+/PCI+) in terms of the risk of symptomatic intracranial hemorrhage.

Figure 3: Forest plot showing odds ratios comparing the outcomes of diabetic patients with prior cerebral infarction (DM+/PCI+) versus patients with no diabetes and with no previous cerebral infarction (No DM+/PCI+) in terms of the risk of mortality.

Figure 4: Forest plot showing odds ratios comparing the outcomes of diabetic patients with prior cerebral infarction (DM+/PCI+) versus patients with no diabetes and with no previous cerebral infarction (No DM+/PCI+) in terms of the risk of functional dependence.
Discussion
Most of the contraindications to IVtPA were derived from major stroke trials as expert consensus in the field for the NINDS trial.Reference Fugate and Rabinstein7 The basis for the exclusion of DM, PCI, or both in the administration of IVtPA was due to the findings that associate these comorbidities to a higher risk of hemorrhagic transformation after IVtPA administration.13 Compared to patients who were given IVtPA according to SITS-MOST criteria, a previous report suggested that patients with DM and PCI showed significantly less neurological improvement, more sICH, and higher mortality at discharge.Reference Rubiera, Ribo and Santamarina9
Contrary to these reports, using the 4 registries analyzed in this review, thrombolysis within the 3–4.5 h period post-ictus in DM+/PCI+ patients had no significant difference in terms of sICH compared to the No DM+/PCI+ group. DM+/PCI+ patients treated with IVtPA are more likely to be ambulatory at 3 months compared to the No DM+/PCI+ patients. However, higher mortality was observed after giving IVtPA in the DM+/PCI+ group compared to No DM+/PCI+ group. These results imply that despite having a higher likelihood of dying DM+/PCI+ patients who actually survive are less disabled than the patients in the No DM+/PCI+ group. However, we also note that the meta-analysis done for 3-month functional outcomes showed significant statistical heterogeneity at 51%. In addition, the study of Filipov was not included in the meta-analysis for functional outcomes but was included in the mortality meta-analysis.25 We recall that this study had significantly higher baseline NIHSS (7 vs 5) in the DM+/PCI+ group. Our pooled outcomes may be significantly different if we had functional outcome data from this study.
Several possible confounding factors may have contributed to the higher mortality in the DM+/PCI+ group, namely, age, presence of significant comorbidities at baseline, and raised blood glucose on admission. As previously mentioned, patients in the DM+/PCI+ group were older than their counterpart, and it has been shown in studies that age can significantly affect outcome resulting in higher ratio of unfavorable results.Reference Karlinski, Kobayashi, Mikulik, Sanak, Wahlgren and Czlonkowska6,Reference Fuentes, Martínez-Sánchez and Alonso de Leciñana31,Reference Sagnier, Galli and Poli33 Moreover, patients in the DM+/PCI+ group were more medically complex than their counterparts with higher number of significant comorbidities at baseline which may have complicated their condition further. Moreover, it has been known from the SITS-ISTR report that admission hyperglycemia is an independent predictor of poor outcome after stroke in IVtPA-treated patients.25,Reference Desilles, Meseguer and Labreuche34,Reference Miedema, Luijckx, Brouns, De Keyser and Uyttenboogaart35 Noncausal explanation posits that hyperglycemia may be merely an epiphenomenon indicative of an acute response to stress apart from a previous undiagnosed DM.Reference Miedema, Luijckx, Brouns, De Keyser and Uyttenboogaart35-Reference Ahmed37
Despite several studies supporting findings that DM+/PCI+ patients can be successfully treated with IVtPA with favorable outcomes,Reference Karlinski, Kobayashi, Mikulik, Sanak, Wahlgren and Czlonkowska6,Reference Guillan, Alonso-Canovas and Garcia-Caldentey38-Reference Kvistad, Logallo, Thomassen, Waje-Andreassen, Brøgger and Naess40 we recommend assessment of risk–benefit ratio and careful consideration of the characteristics of patients with DM and PCI for thrombolysis in acute ischemic stroke based on the results of the pooled analyses in this review. Although IVtPA may significantly improve functional disability and appear not to increase sICH events, this intervention should not be routinely given to all patients with DM and PCI due to possible increased mortality from yet undetermined, established factors. Thus, based on the data provided in this current study, limited evidence suggests that an individualized management approach in this set of population is encouraged considering all the confounding factors that could affect patient outcomes.
This study has some inherent limitations. The results were derived from looking into databases with previously collected data; hence, validity of the results was dependent on accurate documentation of clinical data and outcomes of patients in these registries. Some relevant follow-up data were missing including data in sICH in the study by Mishra.Reference Mishra, Ahmed and Davalos32 Finally, some results were difficult to combine due to differences in the reported outcome measures, e.g., variability in categorization of good and poor functional outcome, differences regarding timing of assessment of outcomes post-thrombolysis.
In line with this, performance of longitudinal, prospective, controlled studies may be imperative to provide a more conclusive evidence regarding the effectiveness and safety of IVtPA in patients with DM and PCI in acute ischemic stroke. In order to drive the performance of a sound and ethical clinical trial involving this subgroup of patients, provisions to demonstrate clinical equipoise, e.g., in terms of the characteristics of the participants that must be involved that could potentially benefit from this intervention, should be established. Based on the baseline characteristics of patients included in this review, younger patients with no other significant comorbidities are speculated to benefit from this intervention with no probable significant increase in sICH, and more importantly, in mortality. Another option to provide evidentiary support on the use of IVtPA in this population would be to conduct studies which were prospectively collected in countries wherein the presence of DM and PCI is not among the exclusion criteria to thrombolyze.Reference Mishra, Ahmed and Davalos32 Finally, interaction of different confounding factors that could possibly affect the outcome, such as stroke severity at onset as well as other medical conditions, may be analyzed in the future studies to evaluate predictors of outcomes in these patients.
Conclusion
In conclusion, limited evidence suggests that for patients who had an acute ischemic stroke within the 3–4.5 h period, diabetic patients with PCI showed no significant difference in terms of sICH compared to patients with no diabetes and PCI. Significantly more thrombolyzed diabetic patients with PCI were functionally independent at 3 months but also had higher mortality compared with patients with No DM and PCI. The decision to thrombolyze this subpopulation should be individualized with careful considerations on advanced age and significant comorbidities as to hypertension and atrial fibrillation. Further prospective, controlled studies may be necessary to substantiate the effectiveness and safety of this intervention in this subgroup population.
Acknowledgments
We acknowledge the support and encouragement received from our family and loved ones and from all of those who give us inspiration.
Conflict of Interest
None.
Statement of Authorship
ATP: Conceptualization, data curation, formal analysis, writing-original draft, writing-review, and editing. JDBD: Conceptualization, data curation, formal analysis, writing-original draft, writing-review, and editing. AIE: Conceptualization, data curation, formal analysis, writing-original draft, writing-review, and editing. RJCS: Conceptualization, data curation, formal analysis, writing-original draft, and writing-review. AAD, AE-M, and JS: Analysis and interpretation of data, drafting, or revising the article. MACB and MCZSJ: Conceptualization, formal analysis, writing-original draft.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.63.







