Coccidiosis, an intestinal disease caused by several species of Eimeria protozoa, is an economically important disease for commercial poultry production(Reference Lillehoj and Lillehoj1). Widespread use of antibiotic-based growth promoters has improved the efficiency of worldwide poultry production. However, due to the emergence of drug-resistant pathogens and the European Union's ban on the use of antibiotics as growth promoters in feeds, interest has shifted toward the development of alternative strategies, such as dietary supplementation with phytogenics, to control avian coccidiosis(Reference Casewell, Friis and Marco2). Phytogenics are a group of natural growth promoters derived from herbs, spices or other plants. In this regard, many medicinal foods and herbal products are highly effective in enhancing host defence against microbial infections, reducing tumorigenesis and decreasing oxidative stress(Reference Lee, Park and Park3–Reference Lee, Lillehoj and Hong6). Previous studies in our laboratory have demonstrated that chickens fed a diet supplemented with phytogenics and subsequently challenged with Eimeria parasites showed reduced gut lesions, enhanced body-weight gain and decreased excreta oocyst output compared with birds fed a control diet(Reference Lee, Lillehoj and Park7, Reference Lee, Lillehoj and Cho8). Furthermore, altered expression of immune-related genes in chickens was observed after the feeding of phytogenics, supporting their well-known medicinal effects(Reference Jamroz, Wertelecki and Houszka9, Reference Burt, Fledderman and Haagsman10). Therefore, it has been proposed that phytogenics augment host immunity against infectious agents through their ability to alter gene expression(Reference Lee, Lillehoj and Park7, Reference Lee, Lillehoj and Cho8).
Cinnamaldehyde (CINN) is a constituent of cinnamon (Cinnamomum cassia Presl (Lauraceae)) that is widely used as a flavoring compound and has been traditionally used to treat human diseases, including dyspepsia, gastritis and inflammatory diseases. CINN has been reported to possess antioxidant, antimicrobial and larvicidal activities(Reference Lin, Wu and Chang11–Reference Kim, Park and Park13), as well as to modulate T cell differentiation(Reference Koh, Yoon and Kwon14). CINN has been found to be active against human liver, lung and leukaemia cancer cells in anticancer studies(Reference Moon and Pack15–Reference Wu and Ng17), is the most potent antiproliferative constituent of C. cassia (Reference Ng and Wu18), and its antitumour effects have also been described using a murine A375 model of human melanoma(Reference Cabello, Bair and Lamore19).
At the physiological level, CINN protects the intestinal microvilli, which are responsible for the absorption of nutrients(Reference Rhodes20–Reference Tschirch22). Dietary feeding of CINN along with carvacrol and capsaicin, or capsicum, improved feed conversion, but did not improve body-weight gain compared with that of control chickens(Reference Jamroz, Wiliczkiewicz and Wertelecki23–Reference Hernández, Madrid and García25). While the mechanisms that are responsible for these phenomena are unknown, it has been suggested that they may involve morphological modification of gastrointestinal mucosal cells(Reference Jamroz, Wertelecki and Houszka9) and/or altered expression of metabolism-related genes(Reference Kim, Lillehoj and Lee26). Chickens fed a diet supplemented with CINN also displayed reduced intestinal colonisation by Escherichia coli, Clostridium perfringens and fungi, and increased colonisation by Lactobacillus spp., compared with controls(Reference Jamroz, Wiliczkiewicz and Wertelecki23). Our previous microarray study showed that feeding of CINN to chickens altered the expression of sixty-two genes (thirty-one up-regulated, thirty-one down-regulated) in intestinal intra-epithelial lymphocytes(Reference Kim, Lillehoj and Lee26). Therefore, the present investigation was performed to evaluate the effects of CINN on in vitro parameters of immunity and to assess its ability to reduce infection against avian coccidiosis in vivo.
Methods
Spleen lymphocyte proliferation
All experiments were approved by the Agricultural Research Service Institutional Animal Care and Use Committee. Specific pathogen-free Ross/Ross broiler chickens, aged 3 weeks (Longenecker's Hatchery, Elizabethtown, PA, USA), were euthanised by cervical dislocation. Spleens were then removed and placed in Petri dishes with 10 ml of Hanks' balanced salt solution supplemented with penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma, St Louis, MO, USA). Cell suspensions were prepared by gently flushing through a cell strainer and lymphocytes were purified by density gradient centrifugation through Histopaque-1077 (Sigma)(Reference Lee, Lillehoj and Cho4). The cells were adjusted to 1·0 × 107 cells/ml in Roswell Park Memorial Institute (RPMI) 1640 medium without phenol red (Sigma) supplemented with 10 % fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 μg/ml), and 100 μl/well were added to ninety-six-well flat-bottomed plates containing CINN at 100 μl/well (400, 100, 50 or 25 ng/ml) from Pancosma S.A. (Geneva, Switzerland), concanavalin A (500 ng/ml; Sigma) as a positive control, or medium alone as a negative control. The cells were incubated at 41°C in a humidified incubator (Forma, Marietta, OH, USA) with 5 % CO2 for 48 h and cell numbers were measured using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8; Dojindo Molecular Technologies, Gaithersburg, MD, USA) at 450 nm using a microplate spectrophotometer (BioRad, Hercules, CA, USA).
Nitric oxide production by macrophages
HD11 chicken macrophages were cultured at 1·0 × 106 cells/ml (100 μl/well) in ninety-six-well plates with CINN at 100 μl/well (1·2, 2·5 or 5·0 μg/ml), recombinant interferon (IFN)-γ(Reference Lillehoj and Choi27) (1·0 μg/ml) as a positive control, or medium alone as a negative control at 41°C and 5 % CO2 for 24 h. Cell culture supernatant fractions (100 μl) were mixed with 100 μl of Griess reagent (Sigma), incubated for 15 min at room temperature, optical densities at 540 nm were measured using a microplate spectrophotometer, and nitrite concentrations were determined using a standard curve generated with known concentrations of sodium nitrite.
Tumour cell cytotoxicity
Retrovirus-transformed chicken RP9 B cells(Reference Lee, Lillehoj and Cho4) were cultured at 1·0 × 106 cells/ml (100 μl/well) in ninety-six-well plates with CINN at 100 μl/well (0·3, 0·6, 1·2 or 2·5 μg/ml), recombinant chicken NK-lysin (1·0 μg/ml) as a positive control, or medium alone as a negative control at 41°C and 5 % CO2 for 48 h, and cell numbers using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8) were measured at 450 nm using a microplate spectrophotometer.
Parasite cytotoxicity
Freshly sporulated E. tenella oocysts were disrupted with 0·5 mm glass beads for 5–7 s and the freed sporocysts were incubated in PBS containing 0·014 m-taurodeoxycholic acid and 0·25 % trypsin to release sporozoites at 41°C for 45 min. Sporozoites were filtered, washed three times with RPMI 1640 at 2100 rpm at 4°C for 10 min, incubated at 1 × 106/ml with 100 or 10 μg CINN per ml at 41°C for 24 h, and viability was measured by trypan blue exclusion.
Quantification of intestinal cytokine mRNA levels
Chickens, aged 1 d (four birds per group), were housed at 29°C in Petersime brooder units and were fed ad libitum with a control diet (US Department of Agriculture Feed Mill, Beltsville, MD, USA) or the control diet supplemented with CINN at 14·4 mg/kg. This concentration of CINN was chosen based upon pilot studies of cytokine mRNA levels. The control diet contained 24·2 % crude protein, 4·7 % fat, 2·4 % fibre, 1·3 % linoleic acid, 1 % Ca, 0·4 % available P, 0·8 % K, 1·5 % arginine, 1·2 % lysine and 0·8 % methionine + cystine. At 14 d post-hatch, the birds were euthanised by cervical dislocation. Their intestinal tissues were removed, cut longitudinally and washed three times with ice-cold Hanks' balanced salt solution containing penicillin (100 U/ml) and streptomycin (100 μg/ml) (Sigma). The mucosal layer was carefully removed using a surgical scalpel and total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). Then 5 μg of total RNA were treated with 1·0 U of DNase I and 1·0 μl of 10X reaction buffer (Sigma), incubated at room temperature for 15 min, 1·0 μl of stop solution was added to inactivate DNase I, and the mixture was heated at 70°C for 10 min. RNA was reverse-transcribed using the StrataScript first-strand synthesis system (Stratagene, La Jolla, CA, USA) according to the manufacturer's recommendations. Quantitative RT-PCR oligonucleotide primers for chicken IL-1β, IL-6, IL-15 and IFN-γ and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) internal control are listed in Table 1. Amplification and detection were carried out using equivalent amounts of total RNA using the Mx3000P system and Brilliant SYBR Green qPCR master mix (Stratagene). Standard curves were generated using log10 diluted standard RNA and the levels of individual transcripts were normalised to those of GAPDH by the QGene program(Reference Muller, Janovjak and Miserez28). Each sample was analysed in triplicate. To normalise individual replicates, the logarithmic-scaled threshold cycle (Ct) values were transformed to linear units of normalised expression before calculating means and sem for the references and individual targets, followed by the determination of mean normalised expression using the QGene program(Reference Muller, Janovjak and Miserez28).
Table 1 Oligonucleotide primers used for quantitative RT-PCR of chicken cytokines

GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN, interferon.
Experimental animals, diets and Eimeria infection
The immunomodulatory effect of CINN against avian coccidiosis was evaluated in chickens infected with E. tenella, E. acervulina or E. maxima. Briefly, chickens aged 1 d (twenty per group) were fed with a control diet or a diet supplemented with CINN at 14·4 or 125 mg/kg (the concentrations in diets). These concentrations of CINN were chosen based upon pilot studies of the immunomodulatory effect of CINN on Eimeria-infected birds. At 14 d post-hatch, the birds were transferred to cages (two birds per cage) for excreta collection and were either uninfected or orally infected with 2·0 × 104 sporulated oocysts of E. tenella, E. maxima or E. acervulina as described(Reference Lee, Lillehoj and Cho29). Body-weight gains were calculated between 0 and 9 d post-infection. For determination of excreta oocyst shedding, birds were placed in cages (two birds per cage, twelve per group) and the excreta samples were collected daily between 5 and 9 d post-infection and then pooled. Oocyst numbers per bird over 4 d were calculated as described(Reference Lee, Lillehoj and Jang30) using a McMaster chamber according to the formula: total oocysts/bird = (oocyst count × dilution factor × (excreta sample volume/counting chamber volume))/2.
Serum antibody levels
Blood was obtained by cardiac puncture (four birds per group) following euthanasia at 9 d post-infection and sera were collected by centrifugation. Diluted sera (1:100, 100 μl/well) were added to ninety-six-well microtitre plates precoated with 10 μg per well of EtMIC2, a purified recombinant microneme protein from E. tenella, as described(Reference Lee, Lillehoj and Park31), incubated with agitation at room temperature for 1 h, and washed with PBS containing 0·05 % Tween 20. Bound antibody was reacted with peroxidase-conjugated rabbit anti-chicken IgG (Sigma) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma), and optical density at 450 nm was determined using a microplate spectrophotometer.
Statistical analyses
Each sample was analysed in triplicate or quadruplicate. Statistical analyses were performed using SPSS software (SPSS 15.0 for Windows; SPSS, Inc., Chicago, IL, USA), and all data were expressed as mean values with their standard errors. Comparisons of the mean values were performed by one-way ANOVA, followed by Student's t test or Duncan's multiple-range test, and differences were considered statistically significant at P < 0·05.
Results
Effects of cinnamaldehyde on in vitro and in vivo parameters of immunity
Dietary CINN increased splenocyte proliferation at all concentrations tested compared with the medium control (P < 0·001) (Fig. 1(A)). Cell proliferation with CINN at 400 ng/ml was comparable with that of the concanavalin A-stimulated positive control. NO levels in the cell culture media of CINN-treated HD11 macrophages were greater than those of cells treated with medium alone (P < 0·001) (Fig. 1(B)). CINN had no observable toxic effects on spleen cells or macrophages at any of the concentrations tested. Treatment of RP9 tumour cells with CINN at 0·6, 1·2 or 2·5 μg/ml reduced cell viability compared with the medium control (P < 0·001) (Fig. 1(C)). CINN decreased E. tenella sporozoite viability at 10 μg/ml (P < 0·05) and 100 μg/ml (P < 0·001) compared with the medium control (Fig. 1(D)). Finally, the levels of transcripts encoding the pro-inflammatory cytokines IL-1β and IL-6, as well as the Th1-type cytokines IL-15 and IFN-γ, were increased in the intestine of chickens fed CINN at 14·4 mg/kg by 12, 2·0, 10 and 47-fold, respectively, compared with each of the non-supplemented control groups (P < 0·001) (Fig. 2).

Fig. 1 Effects of cinnamaldehyde (CINN) treatments on in vitro parameters of immunity. (A) Spleen cells were treated with the indicated concentrations of CINN, concanavalin A (Con A) (500 ng/ml) or medium (control; Cont) for 48 h and viable cell numbers were measured using 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8). (B) HD11 macrophages were treated with the indicated concentrations of CINN, recombinant chicken interferon (IFN)-γ (1·0 μg/ml) or medium (Cont) for 24 h and NO levels were measured using Griess reagent. (C) RP9 tumour cells were treated with the indicated concentrations of CINN, chicken NK-lysin (NKL; 1·0 μg/ml) or medium (Cont) for 48 h and viable cell numbers were measured using WST-8. (D) Eimeria tenella sporozoites were treated with the indicated concentrations of CINN or medium (Cont) for 24 h and viability was assessed by trypan blue exclusion. OD, optical density at 450 or 540 nm. Values are means (n 4), with standard errors represented by vertical bars. Mean value was significantly different from that of the medium-treated (Cont) group: * P < 0·05, *** P < 0·001 (Student's t test).
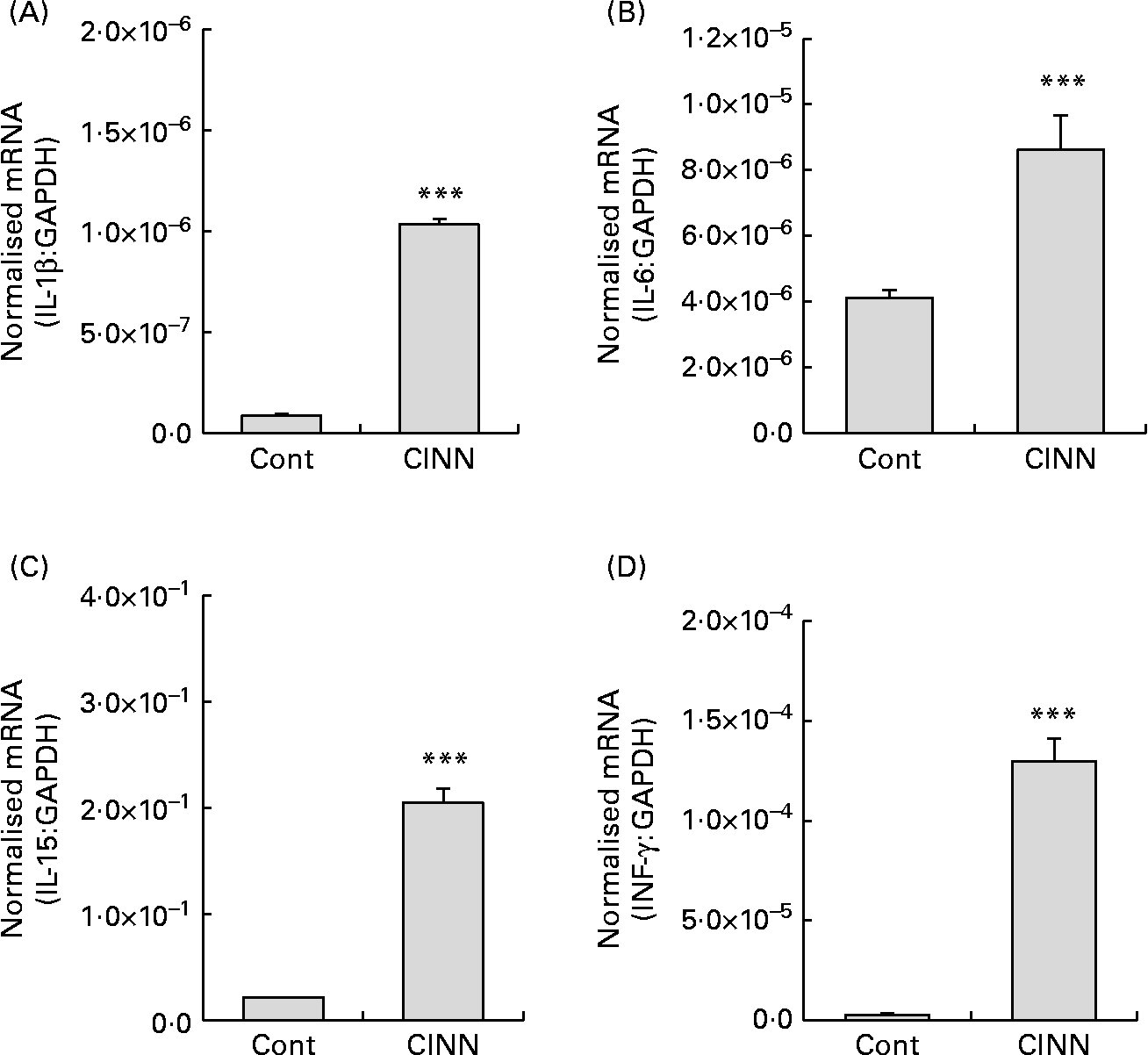
Fig. 2 Effects of a cinnamaldehyde (CINN)-supplemented diet on intestinal cytokine transcript levels. Chickens were fed a non-supplemented diet (control; Cont) or a diet supplemented with CINN at 14·4 mg/kg. At 14 d post-hatch, intestinal tissue was removed and the levels of transcripts for IL-1β (A), IL-6 (B), IL-15 (C) and interferon (IFN)-γ (D) were quantified by real-time RT-PCR. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Values are means (n 12), with standard errors represented by vertical bars. *** Mean value was significantly different from that of the group fed the non-supplemented diet (Cont) (P < 0·001; Student's t test).
Effect of cinnamaldehyde on in vivo protection against avian coccidiosis
Chickens that were fed CINN at 125 mg/kg and infected with E. acervulina, or were fed CINN at 14·4 mg/kg and infected with E. maxima, had significantly (P < 0·05) increased body-weight gains between 0 and 9 d post-infection compared with infected birds given a non-supplemented diet (Fig. 3(A) and (B)). By contrast, feeding of CINN at 14·4 mg/kg had no effect on body-weight gain of E. tenella-infected animals (Fig. 3(C)).

Fig. 3 Effect of cinnamaldehyde (CINN)-supplemented diets on body-weight gain following Eimeria infection. Chickens were fed a non-supplemented diet (control; Cont) or diets supplemented with CINN at 125 mg/kg (A) or 14·4 mg/kg (B, C). At 14 d post-hatch, chickens were uninfected or orally infected with 2·0 × 104 sporulated oocysts of Eimeria acervulina (A), E. maxima (B) or E. tenella (C) and body-weight gains were measured between 0 and 9 d post-infection. Values are means (n 20), with standard errors represented by vertical bars. a,b,c Mean values with unlike letters were significantly different (P < 0·05; Duncan's multiple-range test). The improvement in body-weight gain of birds fed the CINN-supplemented diet compared with those fed the non-supplemented diet following infection with E. acervulina was 16·5 % (A). The improvement in body-weight gain of birds fed the CINN-supplemented diet compared with those fed the non-supplemented diet following infection with E. maxima was 41·6 % (B).
Excreta oocyst number was reduced by 41 % in E. acervulina-infected chickens fed with CINN at 125 mg/kg compared with infected animals given the non-supplemented diet (P < 0·01) (Fig. 4). By contrast, excreta oocyst numbers of E. maxima- or E. tenella-infected chickens given non-supplemented or CINN-supplemented (14·4 mg/kg) diets were equal. Finally, the levels of serum antibodies reactive with the recombinant EtMIC2 protein were increased by 98 % in E. tenella-infected chickens fed the CINN-supplemented diet (P < 0·001), but not in the birds given the CINN diet and infected with E. acervulina or E. maxima, when compared with animals on the control diet (Fig. 5).

Fig. 4 Effect of cinnamaldehyde (CINN)-supplemented diets on excreta oocyst shedding following Eimeria infection. Chickens were fed a non-supplemented diet (control; Cont) or diets supplemented with CINN at 125 or 14·4 mg/kg. At 14 d post-hatch, chickens were orally infected with 2·0 × 104 sporulated oocysts of Eimeria acervulina (125 mg CINN/kg), E. maxima (14·4 mg CINN/kg) or E. tenella (14·4 mg CINN/kg) and excreta oocyst numbers were measured between 5 and 9 d post-infection. Values are means (n 12), with standard errors represented by vertical bars. ** Mean value was significantly different from that of the group fed the non-supplemented diet (Cont) (P < 0·01; Student's t test).

Fig. 5 Effect of cinnamaldehyde (CINN)-supplemented diets on EtMIC2 (a purified recombinant microneme protein from Eimeria tenella) serum antibody levels following Eimeria infection. Chickens were fed a non-supplemented diet (control; Cont) or diets supplemented with CINN at 125 or 14·4 mg/kg. At 14 d post-hatch, chickens were orally infected with 2·0 × 104 sporulated oocysts of E. acervulina (125 mg CINN/kg), E. maxima (14·4 mg CINN/kg) or E. tenella (14·4 mg CINN/kg) and EtMIC2 serum antibody levels were measured at 9 d post-infection. OD, optical density at 450 nm. Values are means (n 4), with standard errors represented by vertical bars. *** Mean value was significantly different from that of the group fed the non-supplemented diet (Cont) (P < 0·001; Student's t test).
Discussion
The present study demonstrated that CINN enhanced in vitro and in vivo parameters of immunity and reduced experimental Eimeria infection in vivo. It should be noted, however, that these effects were Eimeria species specific and were not detected across all observations. Treatment of chicken spleen cells or HD11 macrophages with CINN increased proliferation and NO production, respectively, and treatment of chicken RP9 tumour cells or E. tenella sporozoites with CINN decreased cell viability. For the in vivo studies, feeding of CINN increased the levels of intestinal mRNA encoding IL-1β, IL-6, IL-15 and IFN-γ, reduced E. acervulina- and E. maxima-induced body-weight loss, reduced E. acervulina oocyst shedding, and increased the E. tenella-stimulated EtMIC2 antibody response compared with feeding of the control diet.
Previous studies have demonstrated the beneficial effects of plant extracts in chicken diets for reducing the number of pathogenic gut bacteria without increasing digestibility of nutrients (crude protein, fibre and amino acids), and reducing body-weight loss due to Eimeria infection(Reference Jamroz, Wiliczkiewicz and Wertelecki23, Reference Idris, Bounous and Goodwin32). The results of the present study revealed that CINN-fed birds showed increased body-weight gain after E. acervulina or E. maxima infection and decreased oocyst shedding following E. acervulina infection compared with controls. The significant effect of CINN on body-weight gain and oocyst reduction in the E. acervulina-infected animals compared with the E. maxima- and E. tenella-infected groups may have been due to the relatively higher concentration of the phytogenic in the diet fed to E. acervulina birds (125 v. 14·4 mg/kg). However, when compared with the previous concentrations of dietary supplement used in coccidiosis control (ranging from 200 to 1000 mg/kg)(Reference Lee, Lillehoj and Cho8, Reference Lee, Lillehoj and Cho29, Reference Lee, Lillehoj and Park31), the concentration used in the present study (125 mg/kg) was relatively low. In addition, given that the challenge dose of Eimeria parasites used in the present investigation (2·0 × 104 oocysts per bird) is likely to be considerably higher than the exposure level in commercial production flocks, it remains to be determined whether the lower CINN supplementation also may provide protection against coccidiosis in poultry raised under normal field conditions. It is interesting to note, however, that chickens provided with the higher dose of CINN and infected with E. acervulina nevertheless failed to generate antibodies that cross-reacted with EtMIC2.
T and B lymphocytes, macrophages, monocytes and natural killer cells mediate innate and acquired immune defences. Macrophages play an important role in host defence against infectious agents and tumours, in part, through the production of effector molecules, such as NO, and IFN-γ-stimulated NO production by chicken macrophages has been reported(Reference Okamura, Lillehoj and Raybourne33). Previous studies have demonstrated that the effects of plant extracts on host defence against microbial pathogens and tumours directly correlated with increased cell-mediated immunity(Reference Lee, Lillehoj and Cho4, Reference Lee, Lillehoj and Chun5, Reference Lee, Lillehoj and Cho8). The present results demonstrating a stimulatory effect of CINN on in vitro NO production in chicken macrophages may be related to the result of the in vivo study in which CINN increased IFN-γ expression in the intestine. Moreover, the present data correlate well with previous reports that documented the bioactive properties of medicinal foods and herbs on macrophage activation(Reference Lee, Lillehoj and Hong6, Reference Sakagami, Aoki and Simpson34, Reference Suzuki, Takatsuki and Maeda35). On the other hand, other studies have reported that CINN suppressed NO production by lipopolysaccharide-activated mouse macrophages(Reference Kim, Lee and Lee36).
Protective immunity to Eimeria infection is accompanied by the production of a collection of cytokines, chemokines and other protein mediators of local inflammatory responses(Reference Lillehoj and Lillehoj1). For example, IL-1β is a pro-inflammatory cytokine that is produced by macrophages, monocytes, and dendritic cells that play a central role in the regulation of immune and inflammatory responses. In mammals, IL-1β increases the expression of cell adhesion molecules on endothelial cells to enable the transmigration of blood leucocytes to extravascular sites of infection(Reference Dinarello37). In chickens, IL-1β given simultaneously with a DNA vaccine following oral Eimeria infection exerted an adjuvant effect by reducing excreted oocyst shedding(Reference Waldmann and Tagaya38). IL-6 is produced by T cells and macrophages and acts as both a pro-inflammatory and an anti-inflammatory cytokine, depending upon the context of its expression, whereas IL-15 is primarily secreted by mononuclear phagocytes and enhances the activation of memory T cells(Reference Choi and Lillehoj39). Chicken IL-15 promoted the survival of T lymphocytes and natural killer cells and enhanced protective immunity to experimental coccidiosis when co-administered with a DNA vaccine(Reference Waldmann and Tagaya38, Reference Lillehoj, Min and Choi40). IFN-γ is a common marker of cellular immunity and high levels of IFN-γ are associated with protective immune responses to coccidiosis(Reference Lee, Lillehoj and Cho29). Administration of recombinant IFN-γ to chickens increased resistance against coccidiosis, significantly reduced the intracellular development of Eimeria parasites(Reference Lillehoj and Choi27), and showed an adjuvant effect when given with a DNA vaccine(Reference Min, Lillehoj and Burnside41). On the basis of these reports, we predict that enhanced production of these cytokines in birds that are continuously fed with a diet supplemented with CINN at a relatively low concentration may provide a novel opportunity to increase anti-coccidial immunity and reduce parasite fecundity.
In conclusion, the present results provide the first demonstration that, in general, dietary CINN enhances in vitro parameters of immunity and reduces Eimeria infection of chickens. While these effects were Eimeria species specific and were not observed across all experiments, the following key observations were reproducibly validated: dietary CINN attenuated E. acervulina and E. maxima-induced body-weight loss, decreased E. acervulina oocyst shedding, and increased E. tenella-stimulated EtMIC2 antibody responses compared with the non-supplemented control diet. Further studies are necessary to better understand the underlying immune mechanisms that are responsible for these effects and to assess the ability of dietary CINN to provide a safe and effective alternative disease control method against avian coccidiosis in commercial production facilities.
Acknowledgements
This project was partially supported by a formal trust agreement established between the Agricultural Research Service, US Department of Agriculture and Pancosma S.A. The authors thank Margie Nichols and Stacy Torreyson for their significant contribution to this research.
The present study was carried out during the sabbatical leave of Sung Hyen Lee from the National Academy of Agricultural Science, Rural Development Administration, South Korea.
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
There are no conflicts of interest.








