INTRODUCTION
Rotavirus infections cause a considerable disease burden throughout the world. The burden of rotavirus disease tends to fall predominately on children aged <5 years [Reference Djuretic1, Reference Koopmans and Van Asperen2], with an estimated half a million deaths annually attributable to rotavirus in this age group [Reference Parashar3].
Following the publication of the results of phase III trials of two oral rotavirus vaccines [Reference Ruiz-Palacios4, Reference Vesikari5], and the licensing of both by the European Medicines Agency (EMEA) [6, 7] there has been renewed interest in preventing disease due to rotavirus in the WHO European region. In trials, the vaccines provide 85–95% protection against rotavirus infection severe enough to require hospitalization, and 72–74% protection against all rotavirus infection [Reference Ruiz-Palacios4, Reference Vesikari5].
National public health authorities in the WHO European region will consider whether to introduce the new rotavirus vaccines. Each country's decision will be informed by several factors, including their burden of rotavirus disease and the cost of vaccine. Higher-income countries may wish to reduce hospitalizations and nosocomial infections through vaccination, whereas countries with lower incomes and higher mortality rates due to acute gastroenteritis may aim to reduce mortality.
Any vaccination policy for the WHO European region would benefit from an overview of the rotavirus burden. A 2006 review [Reference Soriano-Gabarro8] estimated the annual rotavirus disease burden in the 25 European Union (EU) countries at 231 deaths and nearly 90 000 admissions, but there is some evidence that a higher burden of disease exists in countries outside the EU but within the WHO European region, especially in lower-income countries [Reference Semenza9, 10].
Estimating overall and country-specific burden is difficult in lower-income countries due to the paucity of suitable published studies. Gaps in the data can be overcome by applying data for a few countries to all countries in an income group [Reference Parashar3, Reference Soriano-Gabarro8]. For countries in the WHO European region, routinely available data on mortality for the <5 years age group and the percentage contribution of gastroenteritis is available for every country, allowing country-specific estimates to be calculated. In addition, a new WHO-compiled database of hospital admissions data, based on national hospital episode statistics, is available to improve hospitalization estimates [11].
In order to inform vaccine policy advice currently being developed by the WHO Regional Office for Europe, we estimated deaths and hospitalizations due to rotavirus disease in the <5 years age group across the region, using available data from routine sources and from the literature. We focused on disease burden in children aged <5 years [Reference Djuretic1, Reference Koopmans and Van Asperen2], as this is the age group most affected by rotavirus and the age range used in comparable literature [Reference Parashar3, Reference Soriano-Gabarro8].
METHODS
Gross National Income (GNI) per capita
We obtained per capita GNI (World Bank, Atlas method, 2006 data [12]) for each country. For the burden calculations, countries were grouped according to the four income groups [low (<$905), lower-middle ($906–$3595), upper-middle ($3596–$11 115), high (>$11 115)] defined by the World Bank. All calculations refer only to children aged 0–4 years.
Contribution of rotavirus to hospital admissions for acute gastroenteritis (AGE)
A literature review was undertaken in December 2007 to obtain country-specific estimates of the percentage of hospital admissions for AGE attributable to rotavirus infection. Articles from countries in WHO European region countries published in or after 1994 were selected by searching for articles with the 52 country names as part of their descriptor (exploded MESH terms: ‘Europe’[Mesh] or ‘Europe, Eastern’[Mesh] or ‘Asia, Central’[Mesh] or ‘Israel’[Mesh] or ‘Turkey’[Mesh]), combined with the MESH terms Rotavirus, rotavirus infections, or ‘Rotavirus’ in any field. The date limit was chosen to maximize the number of countries with some literature estimates, whilst not including studies which may no longer be applicable due to changes in testing and epidemiology. Further papers were identified from the reference lists of review papers.
From the search results, we chose and obtained papers including an estimate of the percentage contribution of rotavirus to admissions for AGE in children. Papers not written in English or lacking an abstract were excluded, apart from two Russian papers [Reference Ryndich13, Reference Solodovnikov14] which were obtained and translated but were found not to contain relevant data. Papers using only nucleic acid testing were excluded as positive results may be due to incidental asymptomatic infections, rather than rotavirus being the primary cause of the AGE episode [Reference Amar15]. We also excluded papers where the period of study was <1 year, as rotavirus is a seasonal infection.
We included papers which made national estimates of the contribution of rotavirus to AGE admissions using a combination of microbiological and hospital admission data. Where papers involving children aged >5 years were included, this was noted in the results table.
For each income group, we calculated the median of all country-specific estimates of the percentage of AGE admissions attributable to rotavirus (Pr). Each country contributed only one percentage estimate; where there was more than one, the median of all article estimates was applied.
Estimating deaths due to diarrhoeal disease and rotavirus
Mortality rates for children aged <5 years (M5: defined as the total annual deaths in children aged 0–4 years divided by the annual birth rate) from 2004, and percentages of deaths in the under-5s (latest data 2000) attributable to diarrhoeal disease (Pd) were obtained from the WHO Statistical Information System (WHOSIS) [16]. The WHOSIS data file cited one source for M5 [17] and two for Pd [Reference Bryce18, 19]. UN population projections [20] were used as the source for annual births (B) per country (estimates for the period 2000–2005) and populations aged <5 years (2005 figures), except for Serbia & Montenegro, where the WHO mortality database was used as the source for the population aged <5 years [21].
The total annual deaths in children aged <5 years (D5) was calculated as the product of under-5s mortality (M5) and the annual birth rate (B):
Annual deaths due to diarrhoeal disease (Dd) were then calculated by multiplying total deaths (D5) by the proportion of deaths in this age group which were attributable to diarrhoeal disease (Pd):
As with the methods of Parashar et al. [Reference Parashar3], we assumed that the percentage of gastroenteritis deaths attributable to rotavirus was the same as the percentage of AGE hospital admissions attributable to rotavirus, on the basis that the former was a marker of severe infections which might lead to death. Country-specific estimates of rotavirus deaths (Dr) were obtained by multiplying total diarrhoeal deaths (Dd) by the median percentage contribution of rotavirus to AGE admissions for the appropriate income group:
Upper and lower limits were calculated in the same way but substituting the lowest or highest Pr in the income group (instead of the median) as appropriate.
Estimating rotavirus hospitalizations
To estimate hospitalization rates due to rotavirus, admissions for infectious intestinal disease (ICD-10, codes A00–A09) in children aged <5 years were obtained from the WHO European hospital morbidity database [11]. No data was available for low- and lower-middle-income countries. Therefore a recent literature estimate for hospital admissions due to AGE in under-5s for Uzbekistan [Reference Isakbaeva22] was used for low-income countries. The data from upper-middle-income countries was applied to the lower-middle-income countries. The total admissions were converted into an annual incidence using the under-5s population figures used for the deaths estimates. Ranges given for the AGE incidences were based on the lowest and highest reported admissions in each income group.
The median incidences of AGE admissions for each group were combined with the percentages of AGE admissions attributable to rotavirus used above, to estimate the incidence density and total admissions attributable to rotavirus in each group. The ranges given were based on the ranges of both the incidences in each group, and the literature estimates of the percentage of AGE admissions due to rotavirus.
Excluded countries
Three countries, Monaco, Andorra and San Marino, were excluded from the analysis due to an absence of published routine population and mortality data from the sources used; therefore this analysis relates to only 49/52 countries in the WHO European region. The World Bank and WHO data used are from the time when Serbia & Montenegro was a single country, so this paper uses the former total of 52 countries in the region.
RESULTS
Income group and routine mortality data
Of the 49 countries, three were classified as low income; 10 as lower-middle income; 12 as upper-middle income; and 24 as high income.
Both under-5s mortality (not shown) and the percentage of deaths attributable to diarrhoeal disease (Fig. 1), tended to be lower in higher-income groups. The within-group variation in the attributable percentages was considerably greater than for the mortality figures, with significant outliers in the low-, lower-middle- and upper-middle-income groups.
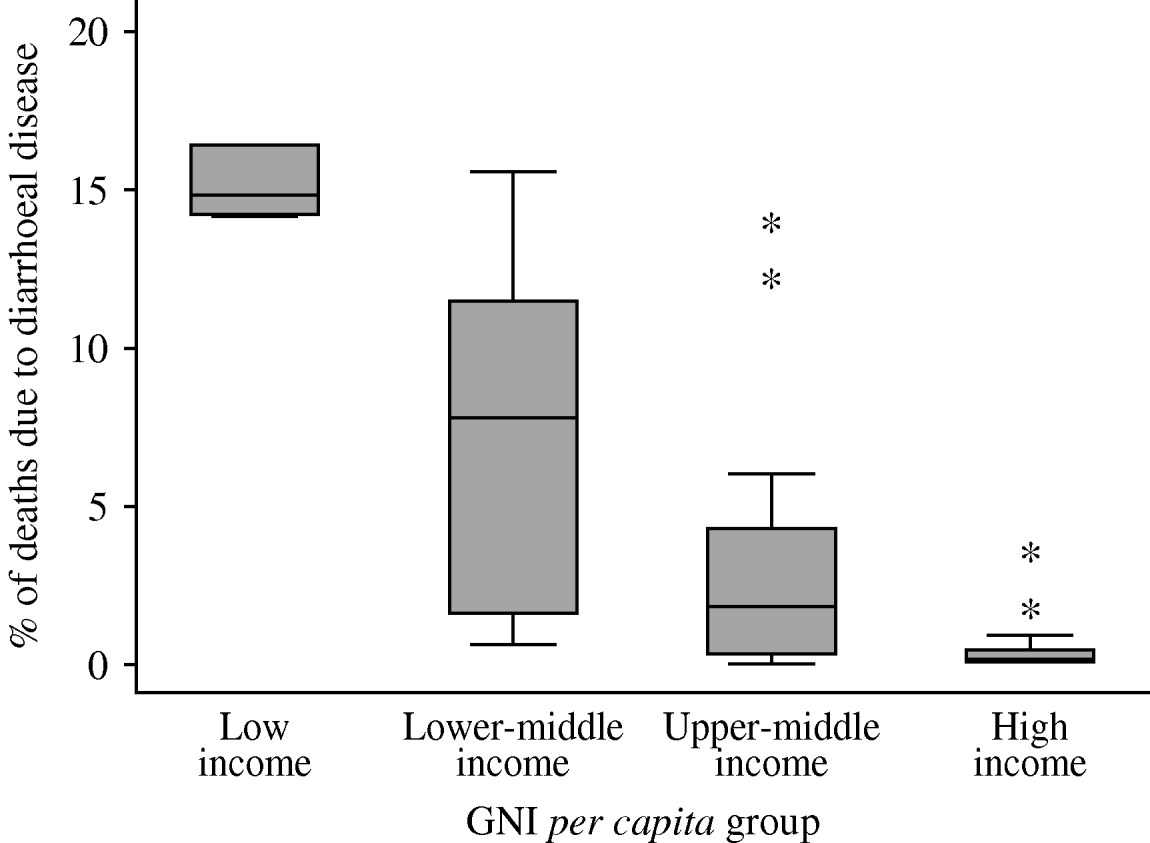
Fig. 1. Percentage of deaths in children aged <5 years attributable to diarrhoeal disease (source: WHOSIS [16]). Median and inter-quartile range (box); upper and lower adjacent values (cross-bars); and outliers (asterisks) are shown.
Percentage of hospital admissions attributable to rotavirus infection
The literature estimates of the contribution of rotavirus to AGE admissions in each country and income group are summarized in Table 1.
Table 1. Literature estimates of the percentage of acute gastroenteritis admissions attributable to rotavirus infection (children aged 0–4 yr)
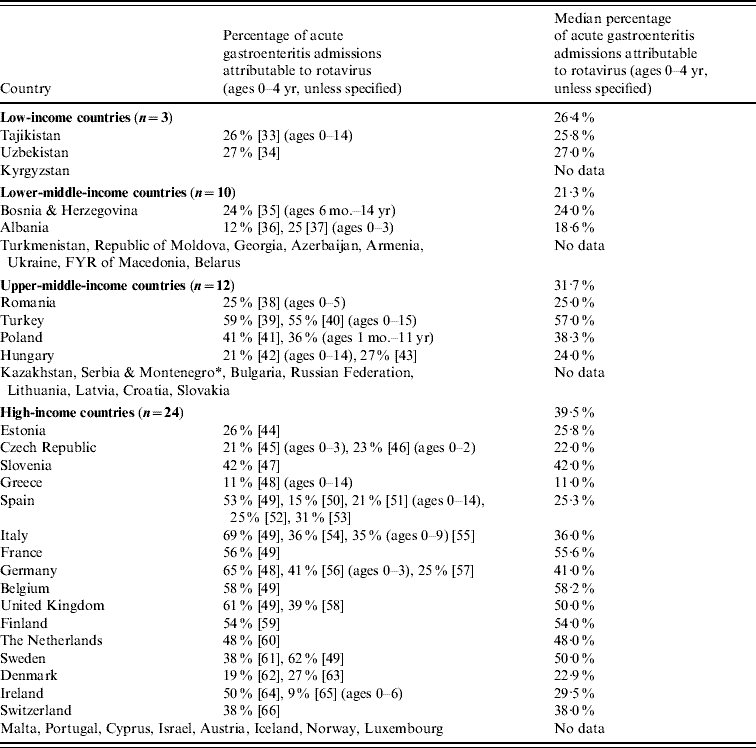
* At the time of analysis, Serbia & Montenegro was a single country.
The median estimates for upper-middle- and high-income countries were based on more published articles than for low- and lower-middle-income countries. Four of the 13 (31%) countries in the low- and lower-middle-income groups had at least one literature estimate of rotavirus contribution, compared to 20/36 (56%) in upper-middle- and high-income groups.
The median percentages of diarrhoeal disease admissions attributable to rotavirus were 26·4% (low income), 21·3% (lower-middle income), 31·7% (upper-middle income) and 39·5% (high income). The median percentages are broadly similar across the income groups, although there is considerable variation between individual studies (overall range 9–69%).
Estimated hospital admissions due to rotavirus
Table 2 shows estimates of total hospital admissions due to rotavirus. Rotavirus accounted for between 38 374 and 1 039 843 hospital admissions each year in children aged <5 years, with a median estimate of 146 287 (2·9/1000 children aged 0–4 per year). The estimated incidences are similar across the income groups, but as most children in the region live in upper-middle- and high-income countries, these groups account for the majority of hospital admissions due to rotavirus (124 743, 85%).
Table 2. Estimated annual hospital admissions in children aged <5 years, WHO European region, by World Bank income group
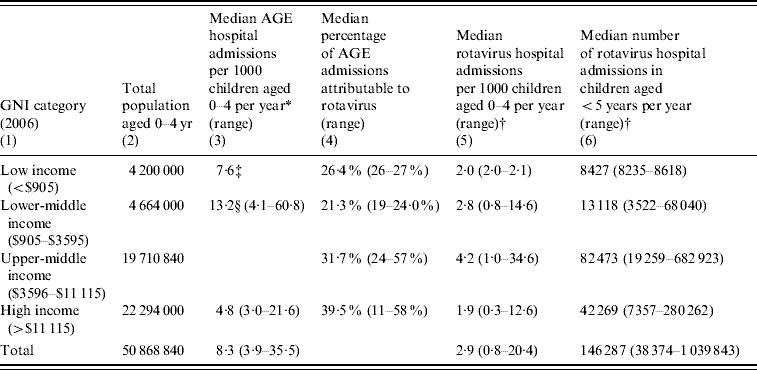
GNI, Gross national income; AGE, admissions for acute gastroenteritis.
* Source: WHO-EURO hospital data, unless otherwise specified.
† Median and range are based on the percentages in column (4), and the median and range of hospital admission incidences from column (3).
‡ Source: Literature estimate from Uzbekistan [Reference Isakbaeva22].
§ Combined figure for lower-middle- and upper-middle-income countries, based on data for five upper-middle-income countries.
Country-specific estimates of rotavirus mortality
Figure 2 shows the distribution of estimated rotavirus mortality across the WHO European region. Mortality is markedly higher in low- and lower-middle-income countries than in the two higher-income bands, with Turkey and Kazakhstan being the notable upper outliers in the upper-middle-income group. Armenia, Azerbaijan, Georgia, Kazakhstan, Kyrgyzstan, Tajikistan, Turkey, Turkmenistan and Uzbekistan had the highest estimated mortality rates (>10/100 000 per year).
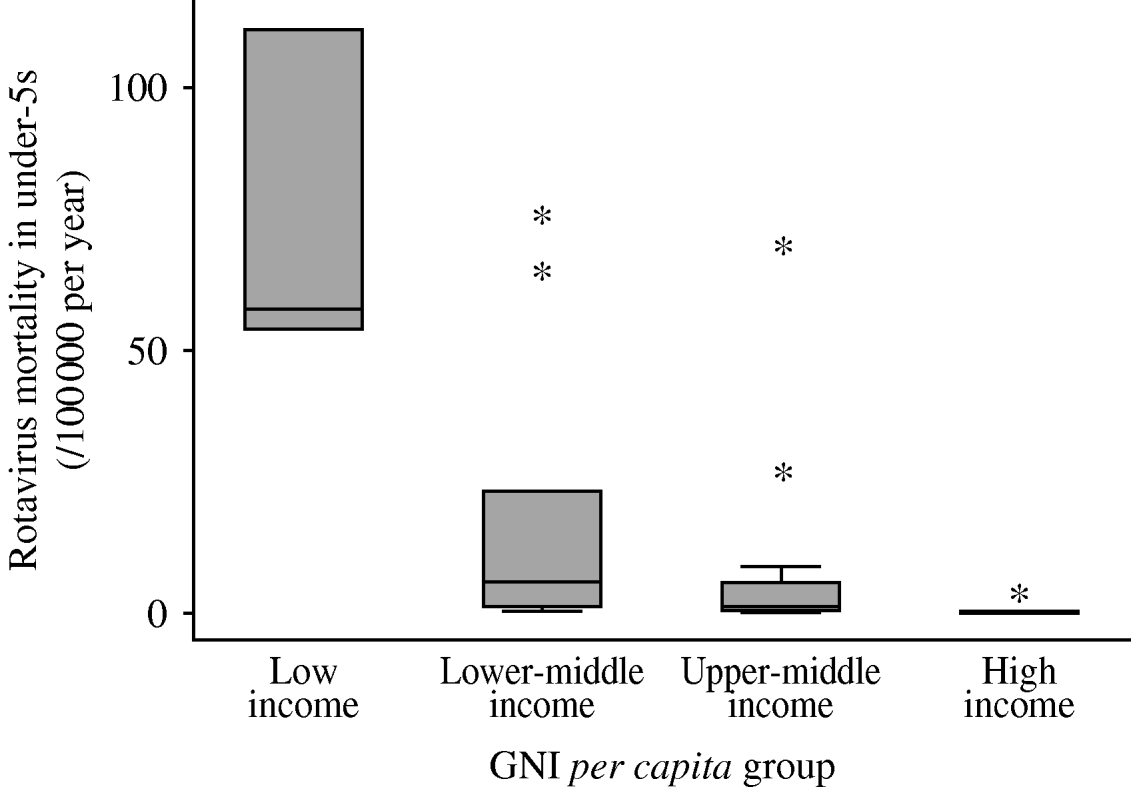
Fig. 2. Summary of country-specific estimates of mortality due to rotavirus disease in children aged <5 years, by World Bank income group. Median and inter-quartile range (box); upper and lower adjacent values (cross-bars); and outliers (asterisks) are shown.
Table 3 summarizes the total deaths due to rotavirus in each income group. There were an estimated 6550 annual deaths due to rotavirus in the region, with most occurring in the three low-income (2860) and 12 upper-middle-income (2805) countries. The overall mortality due to rotavirus disease was 12·9 deaths/100 000 per year. Mortality was highest in low-income (68·1/100 000 per year) and lower-middle-income (18·1/100 000 per year) countries.
Table 3. Estimated total deaths and mortality rates attributable to rotavirus infection in children aged <5 years, by GNI per capita category
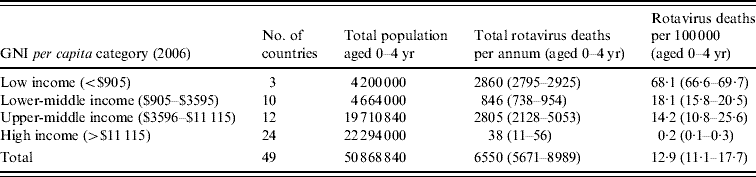
GNI, Gross national income.
The largest single contribution to the AGE and rotavirus deaths was from Turkey, a country in the upper-middle-income group, with an estimated 1700 deaths due to rotavirus (26% of the total).
Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan accounted for an estimated 4412 (range 3372–4426) deaths (67% of the total).
DISCUSSION AND CONCLUSIONS
In the WHO European region, we found that rotavirus infections cause an estimated 6550 deaths and 146 287 hospital admissions each year in children aged <5 years. The contribution of diarrhoeal disease to deaths in children aged <5 years tended to decrease with increasing GNI per capita, whereas the contribution of rotavirus to AGE admissions was similar across income groups. Most (93%) of the deaths occur in Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan and Uzbekistan, and Turkey. Rates of hospital admissions are spread more evenly across countries in the region. One in 70 children from each year's birth cohort will be admitted to hospital because of rotavirus infection during their first 5 years of life.
There are two main potential problems with the analysis: the calculation of rotavirus deaths from routine data, and the limitations of the literature estimates. The use of hospitalization as a proxy for disease leading to death may not be valid, particularly if the case-fatality of hospitalized rotavirus infections is lower than that for other causes, which would lead to overestimation of rotavirus deaths. The estimates of rotavirus deaths rely on WHO mortality and cause-of-mortality data. These figures are not all derived using the same methods, as WHO uses modelling techniques with data from multiple sources [23] to estimate mortality where routine death notification data is incomplete or inadequate. Furthermore, there are significant gaps in the literature on rotavirus burden, and the available literature varies in methods and quality. Data on the contribution of rotavirus to diarrhoeal disease deaths is lacking in one-third (1/3) of low-income countries, 8/10 lower-middle-income countries, 8/12 upper-middle-income countries, and 8/24 high-income countries. Hospital admission data is also lacking in low- and lower-middle-income countries, with only one available estimate of the incidence of rotavirus admissions [Reference Isakbaeva22]. These gaps in data are most apparent in the low- and lower-middle-income countries, often where estimated mortality is highest.
The available studies also varied in their methods and settings. Our selection criteria should have ensured a minimum quality, but variability in the location (e.g. city vs. rural) and timing may reduce the generalizability of individual studies. However, our methods using grouped country medians should produce a balanced overall estimate for each income group. Half the study estimates of rotavirus-attributable percentages were between 25% and 50%.
Due to lack of translation capacity we excluded papers not published in English, and thus may have missed literature from some countries in the eastern part of the WHO European region. It is unlikely that the inclusion of such additional material would substantially alter the results presented here, as the majority of variability in rotavirus mortality was due to the figures for under-5s mortality and the percentage thereof attributable to diarrhoeal disease – figures which were available for all countries in the study. Moreover, the abstracts we reviewed from papers thus excluded did not give any indication that the studies had produced the estimates of the percentage of AGE due to rotavirus that we sought. Finally, a parallel survey study that we undertook, which asked countries for their estimates of the attributable percentages and also for published or unpublished supporting literature, along with a search of grey literature at WHO-EURO offices, identified very few relevant additional studies, all of which were consistent with the literature cited in the present study [Reference Williams24].
However, the mortality estimates in high-income countries tally quite well with the few available country-specific data on rotavirus deaths. A recent study from France found 11 deaths due to rotaviral disease in children aged 0–3 years during a typical season, compared to 14 estimated annual deaths using our method [Reference Tran25]. The UK estimate (15 deaths) is of a similar magnitude to a published figure of 3–4 deaths (3·2–3·8) per annum [Reference Jit26]. On the other hand, the only published estimate of rotavirus mortality in a low-income country (Uzbekistan, 14/100 000 per year [Reference Isakbaeva22]) was one-quarter of our study estimate (58/100 000).
The variability of morbidity and mortality estimates within income groups suggests that this grouping method is perhaps less useful when applied to a subset of the world's countries than at a global level. However, we wished to apply and build on the methods used by Parashar et al. [Reference Parashar3] to allow comparisons to be made with papers using this standard method. Moreover, GNI per capita determines eligibility for GAVI funding [27].
We believe that these methods offer the best estimates of the current burden of rotavirus disease in the region. A recent review [Reference Soriano-Gabarro8] using an unmodified version of Parashar et al.'s methods gave an estimate of 231 rotavirus deaths in the 25 EU member states, compared to only 39 with our methods. Our country-specific figures for the diarrhoeal-disease-attributable mortality in the <5 years group are generally lower than the estimates used by Parashar and colleagues which are based on literature from a few countries applied across an income group. For example, the median attributable percentage was 9% in upper-middle-income countries using Parashar et al.'s method, compared to a median of 1·9% (range 0–14·5) in our study.
Whilst there was considerable variability in country-specific rotavirus mortality estimates, mortality tended to be higher in countries with lower per capita incomes. The three low-income countries all had mortality rates over 50/100 000 per year, and accounted for 44% of all estimated rotavirus deaths. This relationship is accounted for by the negative correlation between per capita income and both overall mortality in children aged <5 years and the percentage contribution of diarrhoeal disease to that mortality. In contrast, the percentage contribution of rotavirus was more constant across income groups.
Estimated rotavirus mortality would fall stepwise with increasing income group, were it not for the high estimated mortality in Turkey and Kazakhstan. The other 10 countries in the group have an estimated mortality rate of 2·2/100 000 per year. Turkey's high estimated mortality is the product of a high overall mortality in children aged <5 years (32/1000 live births per year) and a high percentage of deaths attributable to diarrhoeal disease (12·2%). The latter figure is similar to the 9·6% contribution to deaths in children aged <15 years from a Turkish burden of disease report [28]. High childhood mortality is particularly marked in rural areas and the eastern part of Turkey. Possible determinants include low vaccination uptake, low levels of maternal education and malnutrition [29].
The estimated 146 287 hospital admissions due to rotavirus infection in the region (population aged <5 years: 50·5 million) are broadly consistent with the 87 000 estimated in the 25 EU countries [Reference Soriano-Gabarro8] (population aged <5 years: 23·6 million). Worldwide, rotavirus accounts for an estimated 2 million hospitalizations [Reference Parashar3]. The incidence of hospital admissions for rotavirus is similar in all income groups, but absolute health-care costs will be higher in high-income countries. In one high-income country (Finland), hospital admissions accounted for around half of all costs due to rotavirus infections in those aged <5 years [Reference Takala30]. In such countries, where rotavirus deaths are rare, decisions regarding the possible introduction of rotavirus vaccine will need to be made on the basis of local cost-effectiveness analyses.
Rotavirus accounts for around one-third of deaths and hospitalizations due to diarrhoeal disease in the WHO European region. Reducing the burden of hospitalizations in the region through vaccination would require widespread vaccine introduction, but the burden of rotavirus deaths could be sharply reduced by targeted introduction of rotavirus vaccine in a few countries with high estimated mortality, not all of which are low- or lower-income countries.
If universal vaccination were introduced in Azerbaijan, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan and Turkey at very high coverage, over 80% (at 90% efficacy vs. death) of current estimated rotavirus deaths could be prevented. However, for the health systems in the countries with high estimated mortality, the cost of the vaccine is at present very high [31]. Financial support for vaccine introduction through mechanisms such as the GAVI Alliance would be helpful. Indeed GAVI has offered to subsidize the purchase of rotavirus vaccine in eligible countries [Reference Lob-Levyt32], which are (in WHO-EURO): Azerbaijan, Kyrgyzstan, Uzbekistan, Tajikistan, Georgia, Moldova, Armenia and Ukraine [27].
Rotavirus vaccination may not be the only response to the burden of rotavirus infection described here. Countries with the greatest estimated rotavirus mortalities were those with a correspondingly high mortality in the under-5s, with a significant percentage attributable to diarrhoeal disease. Most deaths due to diarrhoeal disease are preventable even after the onset of infection, and a high mortality due to diarrhoeal disease may be indicative of more general shortcomings in access, capacity and quality of health-care systems which should arguably be addressed before implementing new vaccination programmes.
Support for further studies of rotavirus burden should be targeted at those countries where estimated mortality is highest. There are currently no published direct rotavirus-specific mortality studies in the seven countries accounting for over 90% of estimated deaths. The burden of deaths due to other diarrhoeal diseases is also high in lower-income countries. Improvements in clean water, sanitation and access to appropriate treatment for diarrhoeal disease may have an equal or greater effect on overall diarrhoeal disease burden. More published studies on the burden and causes of diarrhoeal deaths in these countries would also help to determine how best to reduce the numbers of these preventable deaths.
DECLARATION OF INTEREST
None.







