Type 2 diabetes can be prevented or delayed by overall lifestyle changes including increased physical activity, decreased dietary intake of total and saturated fat, and modest weight loss in high-risk individuals, based on findings in randomised, controlled studies(Reference Tuomilehto, Lindström and Eriksson1, 2). There is still controversy over the optimal balance between carbohydrate and fat intake and the role of the quality of dietary fat in the prevention or treatment of gestational diabetes mellitus (GDM).
Most previous studies have been non-randomised comparisons of the intake of dietary fat and carbohydrate among pregnant women with normal glucose tolerance, impaired glucose tolerance and GDM. Moses et al. (Reference Moses, Shand and Tapsell3) found in an observational cohort study that a diet high in fats may increase the risk for recurrence of GDM. Increased polyunsaturated fat intake was associated with a reduced incidence of glucose intolerance during pregnancy, in an observational study of Chinese women(Reference Wang, Storlien and Jenkins4). In another observational study, saturated fat intake was independently associated with the development of gestational glucose abnormalities, especially so in the absence of conventional risk factors(Reference Bo, Menato and Lezo5). Saldana et al. (Reference Saldana, Siega-Riz and Adair6) found an association between increased total fat intake and the development of glucose abnormalities in pregnancy, in an observational study.
As part of a feasibility study, we carried out a randomised, controlled trial comparing a lifestyle intervention group with a close follow-up group of women at high risk of GDM since early pregnancy, as described elsewhere(Reference Korpi-Hyövälti, Laaksonen and Schwab7). We evaluated the effect of an intensive dietary therapy on quality of diet, weight gain and birth weight in women at a high risk of GDM.
Research design and methods
We evaluated the effect of dietary counselling by a clinical nutritionist on intake of energy, fat quantity and quality, intake of fibre and other macro- and micronutrients compared with the close follow-up group with conventional dietary advice based on national recommendations(Reference Hasunen, Kalavainen and Keinonen8), in a single session given by out-patient maternity clinics. This was an open, randomised and controlled study with two participating Finnish rural municipalities in South Ostrobothnia: Kauhajoki and Lapua. The protocol was approved by the ethics committee of South Ostrobothnia Hospital District in Seinäjoki, Finland. It was in accordance with the Helsinki declaration. All women participating in the trial gave written informed consent.
Subjects
A 2 h oral glucose tolerance test was offered to all women in the first contact with maternal healthcare units during gestational weeks 8–12. If the women had one or more risk factors (BMI>25 kg/m2, previous history of GDM or birth of child >4·5 kg, age >40 years, family history of diabetes, i.e. parents, children, siblings or grandparents) or if the venous plasma glucose concentration after 12 h overnight fasting was 4·8–5·5 mmol/l and the 2 h oral glucose tolerance test plasma glucose < 7·8 mmol/l, they were recruited to the intervention.
Of the initial group of subjects (n 102), we excluded women (n 14) who were diagnosed as having GDM already during gestational weeks 8–12. A total of twenty-eight women (n 28) who had risk factors for GDM or whose fasting venous plasma glucose was 4·8–5·5 mmol/l did not wish to participate in the trial for personal or professional reasons(Reference Korpi-Hyövälti, Laaksonen and Schwab7). We randomised sixty women, of whom three dropped out (10 %) in each group (four early miscarriages, one twin pregnancy and one woman moved away). These high-risk women were randomly assigned to the lifestyle intervention group (n 27) or to the close follow-up group (n 27) by the study physician in the Central Hospital with the use of a computed randomisation list. The healthcare nurses who scheduled the study visits did not have access to the randomisation list.
Diet counselling in the lifestyle intervention group
A clinical nutritionist gave dietary advice tailored to each subject in the lifestyle intervention group individually four times in the first and second trimesters and two times in the third trimester, as described elsewhere(Reference Korpi-Hyövälti, Laaksonen and Schwab7). Briefly, the dietary goals in this study were: carbohydrate 50–55 energy percent (E%), fat 30 E%, saturated fat < 10 E% and protein 15–20 E%(Reference Tuomilehto, Lindström and Eriksson1, Reference Hasunen, Kalavainen and Keinonen8, 9). The aim regarding the intake of dietary fibre was at least 15 g/4184 kJ (1000 kcal). Women were encouraged to eat a diet rich in vegetables, berries and fruits, and to use fat-free and low-fat dairy products, low-fat meat, soft margarines and vegetable oils (primarily low-erucic acid rapeseed oil) and whole-grain products. Recommendation for energy intake was 126 kJ/kg per d for normal-weight women and 105 kJ/kg per d for overweight women. The goal of a during-pregnancy weight gain was 12·5–18 kg for underweight women, 11·5–16·0 kg for normal-weight women and 7–11·5 kg for overweight women(Reference Hasunen, Kalavainen and Keinonen8). The women received information about the goals of eating, demands of pregnancy, meals and snacks, amount of food, supply of fibre, and amount and quality of fats by the nutritionist. The Three-Factor Eating Questionnaire was used in the beginning of pregnancy and at weeks 36–40(Reference Stunkard and Messick10). The baseline 4 d (four consecutive days, one weekend day) food record was completed before the first appointment at weeks 8–12, checked by the nutritionist, and formed the basis for dietary advice during the first session. The food record procedure was repeated before the fifth appointment at weeks 26–28 and the sixth appointment at weeks 36–40.
Diet counselling in the close follow-up group
The women were given general information by a nurse on diet and physical activity in a single session to decrease the risk of GDM during pregnancy. The advice was provided both verbally and in writing. The women returned the Three-Factor Eating Questionnaire in the beginning of pregnancy and at weeks 36–40. The 4 d food record was completed in the beginning of pregnancy and at weeks 26–28 and 36–40. Otherwise, the women were followed up in the prenatal clinic of the municipal health centre at 1-month intervals according to standard care in Finland(Reference Korpi-Hyövälti, Laaksonen and Schwab7).
Dietary assessment
Dietary assessment was carried out by using the Nutrica® nutrient calculation software (Version 2.5; Social Insurance Institute, Turku, Finland) based on Finnish nutrient composition analyses and international food composition tables(Reference Rastas, Seppänen and Knuts11).
The women returned twenty-six food records in the beginning, twenty-two at weeks 26–28 and seventeen in the end of pregnancy in the lifestyle intervention group and twenty-five food records in the beginning and at weeks 26–28 and eighteen in the end of pregnancy in the close follow-up group. The analyses were restricted to the seventeen women in the intervention group and eighteen women in the close follow-up group who returned all three food records.
Fetal macrosomia
Birth weight over 4500 g was defined as fetal macrosomia.
Laboratory assessments
A 75 g, 2 h oral glucose tolerance test was performed at weeks 8–12 as previously described elsewhere(Reference Korpi-Hyövälti, Laaksonen and Schwab7). Blood count was determined with a Sysmex XE-2100 blood count analyser (Sysmex Corporation, Kobe, Japan). Fasting erythrocyte folate, serum-B12-vitamin, serum-thyroxine and serum-thyroid stimulating hormone were all determined using a chemiluminescent microparticle immunoassay method (Abbott Laboratories, Abbot Park, IL, USA) in Central Hospital of Seinäjoki. Serum concentrations of total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG were determined using an enzymatic photometric assay (Roche Diagnostics, Basel, Switzerland) in Central Hospital of Seinäjoki. Serum sex hormone-binding globulin was determined with an immunofluorometric assay in the Laboratory MEDIX. Leptin was determined using the Linco RIA method (Linco Research, St Charles, MO, USA) in the Clinical Research Center of University of Eastern Finland.
Statistical analysis
The final analyses were conducted using SPSS for Windows version 15.0 (SPSS, Inc., Chicago, IL, USA). Macronutrients, micronutrients, the Three-Factor Eating Questionnaire and the weight of women were analysed using repeated-measures ANOVA. Further, the PUFA were analysed using the Student's t test, and corrected using the Bonferroni method. We used the Student's t test for continuous variables and the χ2 or Fisher's exact test for categorical variables. The use of spread was analysed by the McNemar test. Differences were considered significant at a two-sided P < 0·05.
The power calculation was based on a post hoc analysis of the present results. In the study period, the prevalence of GDM was 23·5 % among the women at high risk of GDM without any interventions in two neighbouring municipalities, whereas the prevalence of GDM in women with early intervention in this study was 7·4 %. Ultimately, we would have needed a total of 150 (seventy-five per group) high-risk women at a significance level of 5 % and with a power of 80 % to detect a 16·1 % difference in the incidence of GDM.
Results
Characteristics of subjects
The detailed personal characteristics and baseline macro- and micronutrient intake of the fifty-four women are presented in Table 1. At weeks 26–28, three women were classified as having GDM in the lifestyle intervention group and one in the close follow-up group. None of the women had insulin therapy. Alcohol intake was occasional in both groups during pregnancy. One woman in the intervention group and two women in the close follow-up group smoked during pregnancy.
Table 1 Baseline characteristics at the beginning of pregnancy
(Mean values and standard deviations)

E%, percentage of energy.
* P < 0·05.
† Twenty-six of the lifestyle intervention group and twenty-five of the close follow-up group returned the first food record.
‡ Student's t test for continuous and χ2 or Fisher's exact test for categorical variables.
§ College or university degree.
Eating questionnaire
There were no differences between the groups in the beginning and end of pregnancy. There was also no effect of intervention except for a slight tendency to additional cognitive restraint in the lifestyle intervention group (Table 2).
Table 2 The Three-Factor Eating Questionnaire (TFEQ)(Reference Stunkard and Messick10) during pregnancy
(Mean values and standard deviations)
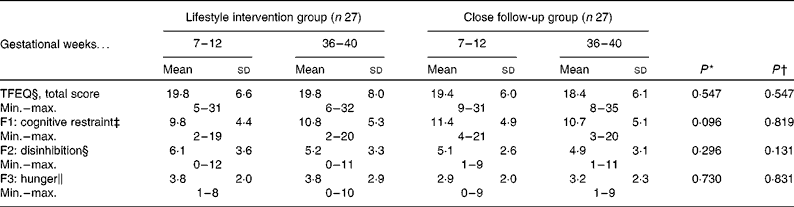
Min., minumum; max., maximum; F1, factor 1; F2, factor 2; F3, factor 3.
* P value for the change between groups, fifty-four women returned TFEQ in the beginning and nineteen (70 %) of the lifestyle intervention group and eighteen (67 %) of close follow-up group in the end of pregnancy.
† P value for the change between weeks 7–12 and 36–40, both groups combined.
‡ Max. score twenty-one out of fifty-one.
§ Max. score sixteen out of fifty-one.
∥ Max. score fourteen out of fifty-one.
Dietary intake
The following macro- and micronutrient intakes were measured: total energy, carbohydrate, fibre, total fat, saturated fat, MUFA, PUFA, 18 : 2n-6 (linoleic acid), 18 : 3n-3 (α-linolenic acid), cholesterol, protein, vitamin C, total vitamin D, folate, Ca and Fe (Table 3).
Table 3 Intake of macronutrients and micronutrients in the lifestyle intervention and close follow-up groups during pregnancy
(Mean values and standard deviations)

E%, percentage of energy.
** P < 0·01.
† DNSG, the Diabetes and Nutrition Study Group of EASD(9), DPS, Diabetes Prevention Study(Reference Tuomilehto, Lindström and Eriksson1), Ministry of Social Affairs and Health 2004(Reference Hasunen, Kalavainen and Keinonen8).
‡ P value effect of intervention between groups (repeated-measures ANOVA), seventeen of intensive lifestyle group and eighteen of close follow-up group returned all three food records.
§ Recommendation of total energy was 126 kJ/kg per d for women with normal weight and 105 kJ/kg per d for women with overweight.
The total dietary fat intake met the recommendations, ≤ 30 E% in both groups. However, the intake of SFA exceeded the recommendations ( < 10 E%) (Table 3). The intake of PUFA increased significantly in the intervention group during pregnancy (repeated-measures ANOVA, P = 0·008) and on average met the recommendations, 5·6 (sd 1·8) E% (Fig. 1(a)). PUFA intake was statistically significant and greater (t test, corrected using the Bonferroni method, P = 0·045) at the end of pregnancy. The 18 : 2n-6 (linoleic acid) intake was systematically higher in the lifestyle intervention group (P = 0·015), but there was no statistically significant intervention effect between the groups (Fig. 1(b)).

Fig. 1 Dietary intake of (a) PUFA (intervention effect, P = 0·008) and (b) 18 : 2n-6 (systematic difference, P = 0·015) during pregnancy in the intervention and follow-up groups (repeated-measures ANOVA). Values are means and standard deviations represented by vertical bars. E%, percentage of energy. ![]() , Lifestyle intervention;
, Lifestyle intervention; ![]() , close follow-up.
, close follow-up.
At baseline, 48 % of the women used vegetable oil-based spread, 37 % dairy fat-based spread and 15 % no spread in the lifestyle intervention group. The respective proportions in the close follow-up group were 54, 29 and 17 %. In the end of pregnancy, 90 % of the women in the lifestyle intervention group used vegetable oil-based spread, 5 % dairy fat-based spread and 5 % no spread. The respective proportions in the close follow-up group were 32, 42 and 26 %. The use of vegetable oil-based spread increased significantly during pregnancy in the lifestyle intervention group (P = 0·003, McNemar test). There was no statistically significant difference in the quality of the spread on the bread during pregnancy in the close follow-up group (P = 0·453, McNemar test).
The protein intake achieved the recommendations, 15–20 E% in both groups. The total carbohydrate intake met the recommendations (Table 3). However, the fibre intake remained below the recommendations >25–35 g/d throughout the study, but was at its highest in the second trimester in the lifestyle intervention group, 22·1 (sd 7·1) g/d (Table 3). The total energy intake increased in both groups from the first trimester to the second trimester (P = 0·019), but was no longer increased in the third trimester (Table 3).
The reported dietary intake of folate and Fe and especially vitamin D, only 4·8 (sd 1·8) μg, remained below the recommendations in both groups, but dietary Ca intake met the recommendations (Table 3). In the intervention group, 59 % had some vitamin supplementation in the beginning of pregnancy; most often multivitamin, containing folic acid 100 μg and vitamin D (most commonly D3) 5 μg, 1–2 tablets/d. In the close follow-up group, 33 % took vitamin supplements. Vitamin supplementation was not reported systematically during pregnancy.
Weight gain
The intensive dietary education resulted in a somewhat lower weight gain (P = 0·062, adjusted by the pre-pregnancy weight) as compared to the close follow-up group at the end of pregnancy (Table 4).
Table 4 Energy intake and weight of women during pregnancy
(Mean values and standard deviations)
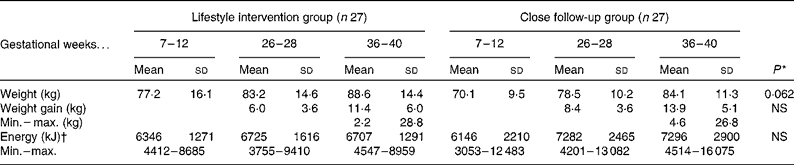
Min., minimum; max., maximum.
* P value for the change between groups (repeated-measures ANOVA adjusted by the pre-pregnancy weight).
† In the lifestyle intervention group seventeen and in the close follow-up group eighteen women were analysed.
Mean birth weight
The mean birth weight was greater, 3871 (sd 567) g, in the lifestyle intervention group (P = 0·047, adjusted by the pre-pregnancy weight of the women) compared with the close follow-up group, 3491 (sd 573) g. The ranges of birth weights were 2870–5275 g in the intervention group and 2390–5050 g in the close follow-up group. There was no difference in the gestational age: 39·8 (sd 1·2) weeks in the intervention group and 39·6 (sd 1·9) weeks in the close follow-up group. The mean birth weight was 3564 g in the period 1996–2000 in Finland(Reference Mortensen, Diderichsen and Amtzen12). There was no difference in macrosomia (P = 0·480, adjusted by the pre-pregnancy weight of the women) between the groups.
Laboratory assessments
There were no differences between the assessments of blood count, fasting erythrocyte folate, serum-B12-vitamin, serum-free-thyroxine, serum-thyrotrophin, serum sex hormone-binding globulin, total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, umbilical cord-leptin or in glucose tolerance between the groups. The only statistically significant difference was the higher number of erythrocytes (P = 0·035) in the lifestyle intervention group (data not shown). Nobody had anaemia, Hb ≤ 100 g/l. Two women in the intervention group and three in the control group had Hb ≤ 105 g/l. There was no difference observed in serum lipids, serum thyroid stimulating hormone or serum-free-thyroxin between the groups. In both groups, serum concentrations of total and LDL-cholesterol, HDL-cholesterol and TAG were higher (P < 0·001) at the end of pregnancy than in the first trimester, respectively. Serum-free-thyroxin was lower (P < 0·001) and serum thyroid stimulating hormone higher (P = 0·002) in the third trimester in both groups (data not shown). There was no difference in cord-leptin between the groups.
Discussion
A lifestyle intervention since early pregnancy with an individualised dietary counselling by a clinical nutritionist improved the quality of dietary fat during pregnancy. Weight gain of the women in the intervention group was somewhat lower (although not statistically significant) and birth weight of the infants was higher, although within normal range. The occurrence of macrosomia was similar between the groups. Dietary studies are complex during pregnancy, because the macro- and micronutrient requirements keep changing constantly. This may explain the reason for the lack of controlled dietary intervention studies.
In the present study, energy intake of the women at a high risk of GDM increased 6 % in the lifestyle intervention group and 18 % in the close follow-up group during the second trimester, but no further during the third trimester. This is somewhat lesser than what may be expected, given the 11–14 kg weight gain that occurred during pregnancy. Extra energy intake is required by pregnant women to support adequate gestational weight gain and increase in BMR, which are not totally offset by reductions in the physical exercise-related energy expenditure. In a previous study, objectively measured energy intake increased 7, 16 and 38 % in the first, second and third trimesters, respectively, in the high BMI group compared with normal-weight women(Reference Butte, William and Treuth13). Underreporting of energy consumption by self-report is well recognised, linked to increased adiposity and body size, dietary restraint and socio-economic status(Reference Hill and Dawies14, Reference Black and Cole15), and women with a high pre-pregnancy BMI are more likely to underreport nutrient intakes(Reference Olafsdottir, Thorsdottir and Gunnarsdottir16, Reference Derbyshire, Davies and Costarelli17). Overall, 65 % of women of the present study were overweight (BMI>25 kg/m2) before pregnancy. The weight gain tended to be lower in the lifestyle intervention group. Obese women, on average, gain less weight during pregnancy than women with normal weight(Reference Carmichael, Abrams and Selvin18). The women in the intervention group were less educated and overweight was more common. Only one of the women out of four weighing over 100 kg returned all three diaries. It seems probable that those women who failed to meet the aims of the intervention were most likely to refuse the reporting.
The mean birth weight in the lifestyle intervention group was greater than in the close follow-up group (P = 0·047), but macrosomia did not differ between the groups. Avoidance of macrosomia is an important goal in treating women with GDM. On the other hand, Lammi et al. (Reference Lammi, Blomstedt and Moltchanova19) showed in a population-based case–control study that the risk of early-onset type 2 diabetes decreased with increasing birth weight until 4·2 kg. Because the mean birth weight in the intervention group was well under 4 kg, the increase in birth weight may be of benefit for most offspring with respect to risk of type 2 diabetes and other chronic diseases associated with low birth weight. Consistent evidence for an influence of women's dietary composition during pregnancy on fetal growth is lacking. Moore et al. (Reference Moore, Davies and Willson20) have studied in a prospective, observational research that the E% from carbohydrate in early and late pregnancy was negatively associated, and protein intake from dairy sources was positively associated with ponderal index of the baby. Godfrey et al. (Reference Godfrey, Sheppard and Gluckman21) showed an association between the carbohydrate intake of mothers in early pregnancy with methylation of genes, predicting adiposity in children at 9 years of age. This study indicated that a mother's dietary habits during pregnancy have long-lasting effects on the child, possibly by epigenetic mechanisms(Reference Godfrey, Sheppard and Gluckman21).
Overall, the macronutrient intake in both groups met the goals of the study: carbohydrate 50–55 E%, fat ≤ 30 E% and protein 15–20 E%. We noted relatively small changes in the dietary intake, except for the increase in PUFA and 18 : 2n-6 (linoleic acid) in the lifestyle intervention group counselled by the clinical nutritionist. The vegetable oil-based spread substituted for a dairy fat-based spread in the lifestyle intervention group was the most evident explanatory factor. In the third trimester, the women in the close follow-up group reduced the intake of total fat, including unsaturated fat. Women in the intervention group maintained the intake of unsaturated fat throughout pregnancy such that the intake of unsaturated fat was statistically significant and greater (P = 0·034) than in the close follow-up group in the end of pregnancy. In the Finnish diet, the use of visible fat as a source of unsaturated fatty acids (spread, salad dressing and cooking oils) is essential in ensuring the recommended fatty acid composition of a diet. In the present study, the subjects were able to change the spread used on the bread, but other recommended changes in dietary fat intake were not adopted. Saturated fat intake remained above the dietary goal of < 10 E% in both groups, despite a transient decrease in the lifestyle intervention group and increase in the close follow-up group during the second trimester. Fibre intake remained below the recommendation of >25–35 g/d, at its best 22 g/d in the lifestyle intervention group in mid-pregnancy.
The women in the lifestyle intervention group adopted healthier eating habits. Many dietary outcomes such as intake of fibre, saturated fat, unsaturated fat, MUFA, PUFA, 18 : 2n-6 and cholesterol were closest to optimal in mid-pregnancy (Table 3). However, the women were not able to maintain changes in eating habits to the end of pregnancy, except for vegetable oil-based fat products. In the close follow-up group, total fat intake decreased (including PUFA) during the study. This result shows how difficult it is to optimise the quality of fat in the diet without professional counselling in a country where the quality of fat is traditionally more saturated than recommended. In this small sample (n 17), we could not find any factor other than education that was related to a favourable intervention response. It could be worthwhile to explore the effects of other approaches, such as educational group sessions, to achieve more persistent changes in dietary patterns.
The Three-Factor Eating Questionnaire(Reference Stunkard and Messick10) was used in this feasibility study for measuring three dimensions (cognitive restraint of eating, disinhibition and hunger) of eating behaviour. The questionnaire assists in selecting the most appropriate method of counselling for the patient. The benefit of this questionnaire was limited in the present study, because all women received informative education regardless of the results of the questionnaire. Women with a high cognitive restraint score benefit from informative education, but individuals with a high disinhibition score may be more suitable for behavioural management or peer-group approaches, especially when dealing with emotional disinhibitors such as anxiety, depression or loneliness(Reference Stunkard and Messick10). In this study, three mothers had high scores on hunger (8–9 out of 14) in the beginning of pregnancy and five mothers (8–10 out of 14) in the end of pregnancy. Hunger is by far the most important factor regulating the eating of women in Finland, based on the present study.
Even taking into account underreporting, dietary vitamin D intake, only 4·8 (sd 1·8) μg, is clearly below the recommendations (10 μg). Fish meals were random in food records and the vitamin supplementation was not reported systematically during pregnancy. Serum vitamin D concentrations were not assessed. The intakes of vitamin D and folate were below the recommendations in Finland, also in the studies of Arkkola et al. (Reference Arkkola, Uusitalo and Pietikäinen22) and Järvenpää et al. (Reference Järvenpää, Schwab and Lappalainen23). The reported intakes of folate and Fe were below the recommendations. Fasting erythrocyte folate and the blood count were normal, however, which may suggest underreporting. Food records over 4 d, which provide a reliable estimate of macronutrient intake, may nonetheless be insufficient for the estimation of micronutrient intake(Reference Basiotis, Welsh and Cronin24).
The purpose of the laboratory assessments was to show that the possible changes in the diet are not harmful to women and do not cause deficiencies of macro- or micronutrients. There were no differences between the groups in glucose tolerance or other laboratory assessments. Free-thyroxin initially increased, peaking between 9 and 13 weeks(Reference Panesar, Li and Rogers25) and then decreased; thyrotrophin mirrored changes in free-thyroxin. During pregnancy, the concentrations of total cholesterol and HDL- and LDL-cholesterol and TAG increased dramatically in both groups (P < 0·001). These are part of the natural metabolic changes in pregnancy, which seem to occur irrespective of the qualitative or quantitative changes in dietary fat intake. The implications of these changes are unclear(Reference Martin, Davies and Hayavi26), but they may be important for the fetus, especially for the development of brain and other tissues.
Conclusions
Individualised counselling by a clinical nutritionist as part of a lifestyle intervention improved the quality of dietary fat intake in pregnant women at a high risk of GDM. Birth weight was higher in the intervention group, but there was no difference in macrosomia. It may be beneficial to test other approaches, such as peer-group sessions, to achieve broader, longer-lasting changes in dietary intake.
Acknowledgements
The present study was funded by Seinäjoki Central Hospital and Kuopio University Hospital, University of Eastern Finland and the municipalities of Kauhajoki and Lapua, i.e. employers of the authors. The study was supported by EVO funding from Kuopio University Hospital and South Ostrobothnia Hospital District. The authors declare that they have no conflicts of interest. E. K.-H. participated in the design of the study, acquisition of the data, performed the statistical analysis and drafted the manuscript. U. S., D. E. L., L. N. and S. H. made substantial contributions to the conception and design of the study, and data interpretation, and helped in the drafting of the manuscript. H. L. participated in data collection and dietary counselling of the subjects. All authors read and approved the final manuscript.







