Background
Poor mental health can have a negative impact on individuals, communities and the economy, and is recognized as the third leading burden of disease in the world (Cornwell et al., Reference Cornwell, Forbes, Inder and Meadows2009; Whiteford et al., Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine and Vos2013). It is necessary to comprehensively investigate the factors associated with mental disorders to help us better understand their complexities for high-quality and targeted treatment intervention.
There is a considerable amount of epidemiological evidence that people with chronic physical conditions have poorer psychological well-being than healthy individuals (Australian Bureau of Statistics [ABS], 2012a). This association has been found in adolescents (Santos et al., Reference Santos, De Matos, Simões and Machado2015), young adults (Kornienko et al., Reference Kornienko, Kozlov and Otavina2016) and older adults (Cho et al., Reference Cho, Martin, Margrett, Macdonald and Poon2011; Pruchno et al., Reference Pruchno, Wilson-Genderson and Heid2016), and appears stronger in those with multiple chronic health conditions (Shih & Simon, Reference Shih and Simon2008). Specifically, diseases such as cardiovascular disease (Boehm & Kubzansky, Reference Boehm and Kubzansky2012; Gerber et al., Reference Gerber, King, Iverson, Pineles and Haskell2018; Rumsfeld et al., Reference Rumsfeld, Havranek, Masoudi, Peterson, Jones and Tooley2003; Scott, De Jonge, et al., Reference Scott, De Jonge, Alonso, Viana, Liu, O’Neill and Stein2013), diabetes (De Jonge et al., Reference De Jonge, Alonso, Stein, Kiejna, Aguilar-Gaxiola, Viana and Caldas-de-Almeida2014; Selvaraj et al., Reference Selvaraj, Kumar and Sujiv2015; Wändell et al., Reference Wändell, Brorsson and Aberg1997; Wiltink et al., Reference Wiltink, Beutel, Till, Ojeda, Wild, Münzel and Michal2011), respiratory conditions (Alonso et al., Reference Alonso, De Jonge, Lim, Aguilar-Gaxiola, Bruffaerts, Caldas-de-Almeida and Viana2014; Goodwin et al., Reference Goodwin, Lewinsohn and Seeley2004; Scott et al., Reference Scott, Lim, Al-Hamzawi, Alonso, Bruffaerts, Caldas-de-Almeida and De Jonge2016), gastrointestinal conditions (Engstrom, Reference Engstrom1999; Scott, Alonso, et al., Reference Scott, Alonso, De Jonge, Viana, Liu, O’Neill and Stein2013; Stewart & Berry, Reference Stewart and Berry1989) and chronic pain conditions (Benjamin et al., Reference Benjamin, Morris, McBeth, Macfarlane and Silman2000) have all been found to be linked to poor psychological well-being globally. Because disease prevalence, health systems and values of quality of life differ across time and countries, to understand the magnitude of these associations, it is important to assess associations comparatively in an Australian context.
Many factors could affect the association of physical health with mental health, and therefore standard cross-sectional studies provide no evidence for causality for this relationship. Longitudinal studies can provide some evidence of causality, but confounding remains an issue. Although previous studies have adjusted for obvious confounding factors such as age, sex, socioeconomic status, body mass index (BMI) and geographic location, they have not been able to control for genetic and many environmental confounders, such as genes and early life exposures (Whitfield & McClearn, Reference Whitfield and McClearn2005). Thus, the associations previously found may not represent a true causal relationship.
Twin study designs are valuable when considering complex associations, such as physical and mental health, because they have the unique ability to address this issue of confounding by providing a method for controlling for characteristics shared between twins (Carlin et al., Reference Carlin, Gurrin, Sterne, Morley and Dwyer2005). Because twins share genes and many environments, particularly in early life, twin within-pair differences study designs can naturally control for both measured and unmeasured factors shared between twins (Carlin et al., Reference Carlin, Gurrin, Sterne, Morley and Dwyer2005). Twin heritability studies have concluded that genetic and environmental factors influence both somatic and psychological health (Figueredo & Rushton, Reference Figueredo and Rushton2009; Hansell et al., Reference Hansell, Wright, Medland, Davenport, Wray, Martin and Hickie2012; Kaprio & Koskenvuo, Reference Kaprio and Koskenvuo2002). Accordingly, a twin study on the association between physical and mental health could help us better understand the mechanisms of this association and provide a more accurate estimate.
This research aimed to assess whether the apparent association between physical health and psychological well-being persisted after extracting the genetic and environmental factors shared between twins in a pair.
Methods
Data
This study used cross-sectional data from the Health and Lifestyle Questionnaire administered to adult twins on the Twins Research Australia Registry between 2014 and 2017 (Twins Research Australia, 2018). All active twins on the registry who were 18 or more years of age were invited via email. At the cutoff date of November 2, 2017, 1831 pairs of twins (3662 individuals) had completed the questionnaire, and data from these pairs were extracted for analysis.
Exposure
The exposures of interest were common chronic diseases in Australia (Australian Institute of Health and Welfare, 2018a), with chronic disease defined as a disease that is long-lasting with persistent effects (Australian Institute of Health and Welfare [AIHW], 2018b). Diseases were selected and grouped based on eight major groups of commonly reported diseases in Australia (AIHW, 2018b) and recent reports from the AIHW on disease burden and prevalence (AIWH, 2016, 2018a). Measurement of disease, via the questionnaire, was self-reported using a tick-box method that asked participants to only report conditions a doctor had previously diagnosed. Because of this method, some diseases were excluded based on perceived appropriateness. For example, Alzheimer’s disease was excluded due to self-reporting concerns, and cancer was excluded because this could have included previous cancers that have since been cured and are unlikely to be having a current impact on physical or mental health.
After all these considerations, 30 individual diseases in total were investigated. This article presents the results for associations of psychological distress score with any chronic disease and with each of the five main disease groups (cardiovascular disease, respiratory conditions, gastrointestinal conditions, endocrine conditions and musculoskeletal conditions). A full list of diseases, including disease group classifications, can be found in Supplementary Table S1.
Outcome
The outcome of interest was psychological distress, which was determined using the six-item Kessler Psychological Distress Scale (K6). The K6 is a validated tool for measuring an individual’s current state of psychological distress that gives a score from 6 (minimum psychological distress) to 30 (maximum psychological distress) under the Australian scoring method (Kessler et al., Reference Kessler, Barker, Colpe, Epstein, Gfroerer, Hiripi and Walters2003). Under the Australian scoring of K6, scores can be categorized in several few different ways: (1) as a binary variable, no probable serious mental illness (K6 = 6−18) and probable serious mental illness (K6 = 19−30); (2) in three categories, low psychological distress (K6 = 6–10), moderate psychological distress (K6 = 11–18) and high psychological distress (K6 = 19–30) and (3) in strata, 1 (lowest, K6 = 6), 2 (K6 = 7–13), 3 (K6 = 14–18), 4 (K6 = 19–24) and 5 (highest, K6 = 25–30) (ABS, 2012b). In this analysis, K6 was treated as a continuous variable to maintain higher statistical power (Carlin et al., Reference Carlin, Gurrin, Sterne, Morley and Dwyer2005).
Statistical Analysis
This analysis used two mixed-effects linear regression models fitted using maximum likelihood (MLE) estimation to test the association between chronic disease and psychological distress.
Model 1 treats the sample as individuals and takes into account the correlation between twin pairs by clustering on a pair identifier. The main parameter of interest is the regression coefficient βc, which represents the difference in mean psychological distress score for those that have a disease (X = 1) compared with those who do not have a disease (X = 0). βc is known as the ‘common effect’ coefficient and represents the weighted average of the within-pair and between-pair coefficients in model 2.
Model 2 is more comprehensive than model 1 because it utilizes the paired nature of the data more completely and divides estimates of association into a within-pair coefficient and a between-pair coefficient. The within-pair coefficient represents the mean difference in K6 score between the arbitrarily labeled Twin 1 and Twin 2 for a difference of 1 unit in the within-pair covariate (i.e. for twins discordant for disease). The between-pair covariate is the twin pair mean (which in this case can only take the values 0, 0.5 or 1) and the between-pair coefficient βb represents the difference in mean psychological distress score for a 1-unit difference in mean exposure between different pairs, that is, for twin pairs who are both affected compared with unaffected twin pairs. Comparison of the within-pair and between-pair coefficients highlights the extent to which the cross-sectional coefficient (which is a weighted average of the within-pair and between-pair coefficients) is confounded by genetic and environmental influences (Carlin et al., Reference Carlin, Gurrin, Sterne, Morley and Dwyer2005). The within and between-pairs model can be represented as
![]() $$E({\rm{Y}}) = {{\rm{\beta }}_0} + {\rm{\beta }}({\rm{X}} - \overline {\rm{X}} ) + {{\rm{\beta }}_b}\overline {\rm{X}} $$
, where β
w
is the within-pair estimate and βb is the between-pair estimate.
$$E({\rm{Y}}) = {{\rm{\beta }}_0} + {\rm{\beta }}({\rm{X}} - \overline {\rm{X}} ) + {{\rm{\beta }}_b}\overline {\rm{X}} $$
, where β
w
is the within-pair estimate and βb is the between-pair estimate.
Data analysis was conducted using the Stata IC version 15.0 (StataCorp, 2017).
Measured Confounders
Previous studies have identified age, sex, smoking status, BMI and socioeconomic status (measured using indicators such as education and income) as confounders of the relationship between chronic disease and mental health (Scott et al., Reference Scott, Lim, Al-Hamzawi, Alonso, Bruffaerts, Caldas-de-Almeida and De Jonge2016; Shih & Simon, Reference Shih and Simon2008; Slade et al., Reference Slade, Johnston, Oakley Browne, Andrews and Whiteford2009; Wiltink et al., Reference Wiltink, Beutel, Till, Ojeda, Wild, Münzel and Michal2011). Additionally, zygosity is often considered a confounder in twin studies. All these potential confounding variables were also included in the regression models and likelihood ratio (LR) tests were used to compare models with and without each confounder.
Ethics
Twins Research Australia and the Melbourne School of Population and Global Health’s Human Ethics Advisory Group provided ethical approval for this project (Ethics ID: 1851259.1).
Results
Overall, 1831 twin pairs (3662 individuals) completed the questionnaire. Of those, 33 twin pairs had missing data for age and 389 individuals had missing income data. After excluding all pairs where at least 1 twin had missing data, there were 1474 twin pairs (1104 monozygotic and 370 dizygotic) eligible for analysis; see Figure 1.
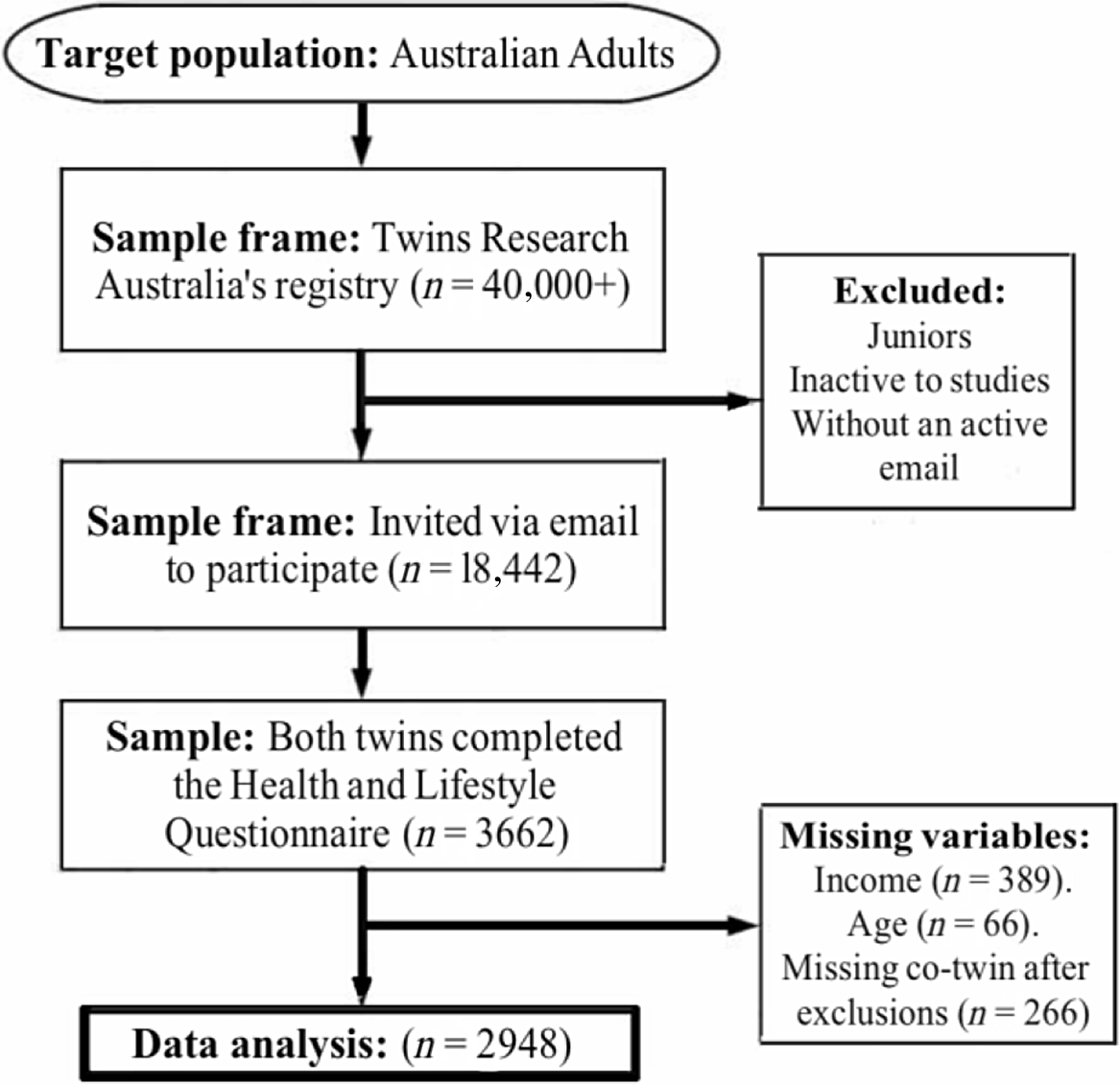
Fig. 1. Sample flowchart.
Table 1 describes the characteristics of the study sample. The median age of participants was 47 years; 2256 (76.5%) were female and 183 (6.2%) were smokers. The median K6 score was 8 (classified as low psychological distress) and 595 individuals (20.2%) had a moderate–high classification of psychological distress (K6 = 11–30). This proportion is slightly lower than that in the general population, where approximately 33% report moderate–high psychological distress (ABS, 2012b). Figure 2 illustrates the distribution of K6 in the sample, which was highly positively skewed for individuals; however, the differences in K6 between twins within a pair were normally distributed. Overall, the prevalence of diseases in the sample was similar to that in the general Australian population, with only some discrepancies for individual diseases (ABS, 2015); see Table 1.
Table 1. Sample characteristics
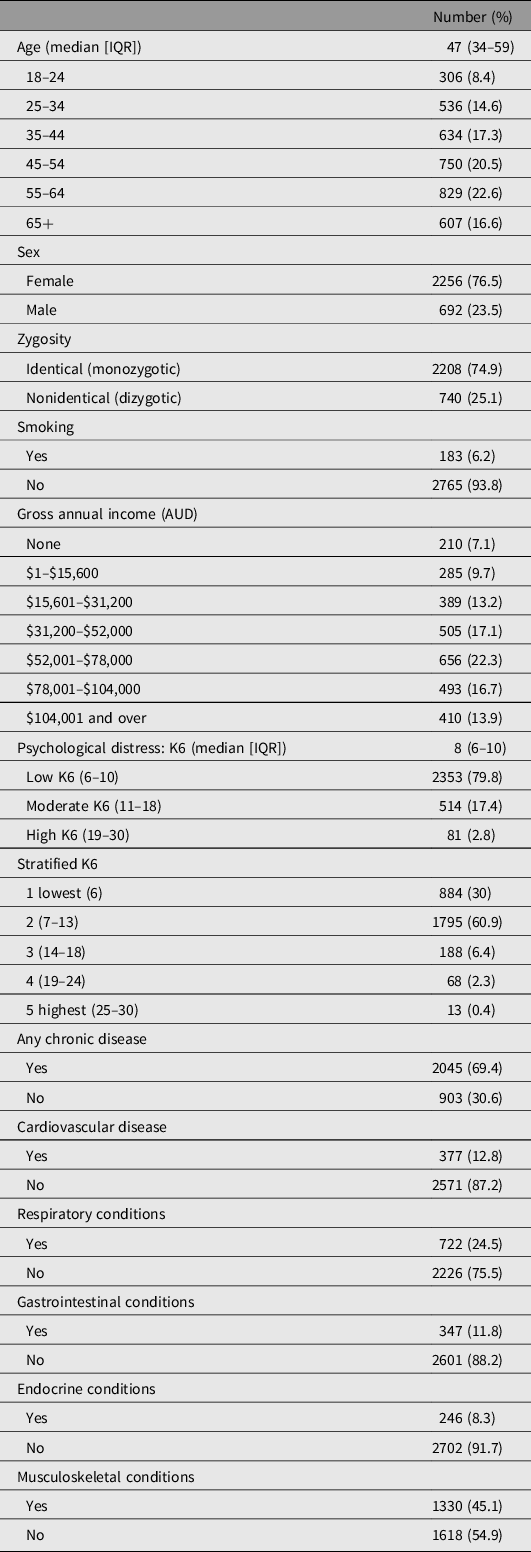
Note: IQR, interquartertile range.
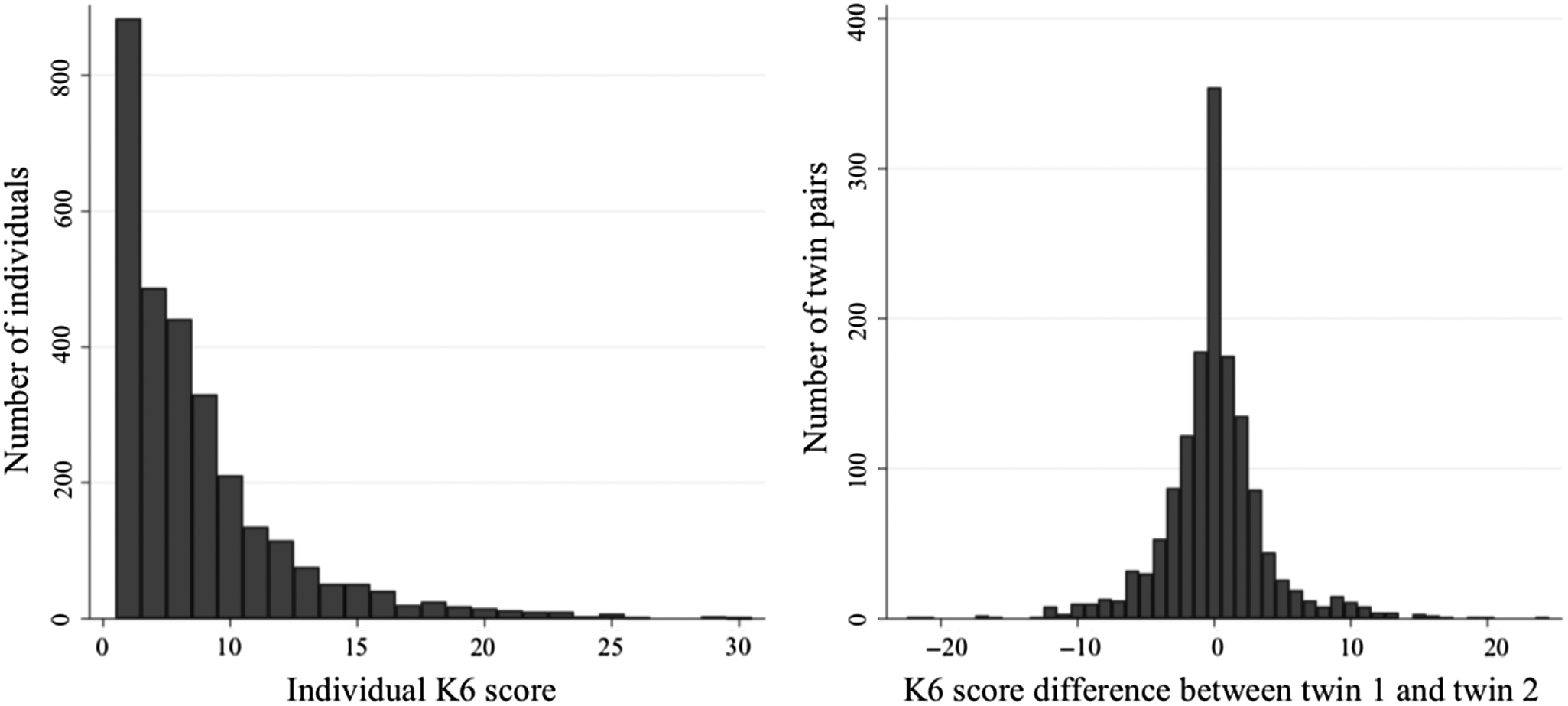
Fig. 2. Distribution of Kessler psychological distress (K6) scores in the sample.
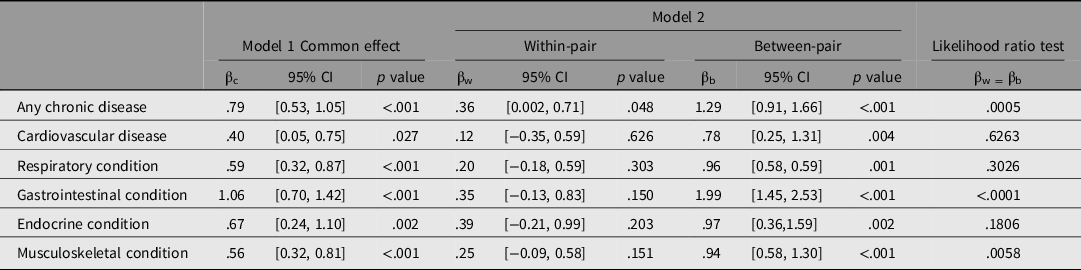
Table 2 provides the estimated regression coefficients for the association of each disease group with psychological distress. Both models adjusted for age, sex, income and smoking status; BMI and zygosity were not associated with K6, and thus were not considered confounders for this analysis (data not shown). Overall, model 1 shows moderate positive associations, indicating chronic disease is linked to higher psychological distress. Model 2 gives a more detailed overview of the relationship and shows us the extent to which shared twin genetic and environmental factors confound this association.
Table 2. Association of psychological distress (K6 score) with disease status (common, with-pair and between-pair coefficients)
Any Chronic Disease
The common effect estimate (βc) from model 1 indicates that individuals with any type of chronic disease are estimated to have a 0.79 mean increased K6 score compared to individuals without a chronic disease (95% CI [0.53, 1.05]; p < .001) (Table 2). After dividing out the shared twin factors in model 2, the within-pair estimate (βw) shows that a twin with any type of chronic disease was estimated to have a 0.36 mean increased K6 score compared to their co-twin without a chronic disease (βw = .36; 95% CI [0.002, 0.71]; p = .048; Table 2). Whereas, the between-pair estimate indicates that twin pairs that are concordant for a chronic disease have a 1.29 mean increased K6 score, compared to twin pairs where neither twin has a chronic disease (βb = 1.29; 95% CI [0.91, 1.66]; p < .001). The βw and βb estimates were shown to be statistically different, with 95% confidence intervals that do not overlap and an LR test p value of .0005, indicating little chance that the estimates are the same. This tells us that genetic and environmental factors shared between twins are having a differential confounding effect on this relationship, and after naturally controlling for these unmeasured shared-twin confounders in the within-pair analysis, we find any chronic disease appears to be weakly associated with higher psychological distress.
Cardiovascular Disease
Cardiovascular disease appeared to have the weakest effect on psychological distress in this analysis. The βc estimate showed that having cardiovascular disease was associated with an estimated 0.40 mean increase in K6 score compared with not having cardiovascular disease (95% CI [0.05, 0.75]; p = .027). Splitting out the shared twin factors again showed some evidence that genetic and environmental factors confound this relationship. The between-pair estimate was shown to be stronger (βb = .78; 95% CI [0.25, 1.31]; p = .003) than the within-pair estimate, which was not statistically different from zero in this analysis (p = .67).
Respiratory Disease
Respiratory disease showed similar findings to cardiovascular disease, where the between-pair estimate showed a moderate effect (βb = .96), the estimate from model 1 showed a smaller effect size (βc = .59) and the within-pair estimate was not significant (Table 2).
Gastrointestinal Condition
According to model 1, out of all the disease groups, having a gastrointestinal condition was most strongly associated with psychological distress (βc = 1.06; 95% CI = 0.70, 1.42; p < .001). However, when we look at model 2, we see that this strong effect was largely due to unmeasured genetic and environmental factors shared between twins. The between-pair estimate, which is biased by unmeasured factors that are shared by concordant pairs, showed that having a gastrointestinal condition was associated with an estimated 1.99 mean increase in K6 score compared to not having a gastrointestinal condition (95% CI [1.45, 2.53]; p < .001). The within-pair estimate, which is not biased by shared twin factors, gave a much smaller effect size (βw = .35) and ultimately represented null findings (95% CI [−0.13, 0.83]; p = .150).
Endocrine Condition
Similarly, model 1 showed that having an endocrine condition was moderately associated with psychological distress (βc = .67; 95% CI [0.24, 1.10]; p = .002). When separating out the unmeasured shared twin factors, this association was stronger comparing between-pairs (βb = .97; 95% CI [0.36, 1.59]; p = .002) and not significantly different comparing within-pairs (βw = .39; 95% CI [−0.21, 0.99]; p = .203).
Musculoskeletal Condition
Lastly, musculoskeletal conditions showed similar findings to other disease types, where the between-pair estimate showed a moderate effect (βb = .94), the estimate from model 1 showed a weaker effect size (βc = .56) and the within-pair estimate was close to zero (Table 2).
Sensitivity Analysis
Sensitivity analyses were conducted to test whether the results differed after (1) controlling for other potential confounders including education, BMI and zygosity; (2) by using K6 as a binary outcome of low or moderate–high psychological distress; (3) by including pairs for missing age and (4) by excluding potential outliers from the sample. No substantial differences were found in any of these analyses (data not shown). Model appropriateness was also tested by assessing the residuals of different regression models including standard least-squares linear regression with robust errors (OLS) and mixed-effects models fitted using MLE. MLE residuals were more normally distributed than OLS, thus chosen to be the superior model. A model fitted using generalized estimating equations (GEE) was also considered as GEE, suitably, does not assume normality of data (Liang & Zeger, Reference Liang and Zeger1993). The GEE model estimates did not differentiate from the MLE model, and therefore were omitted from inclusion in this paper (data not shown).
Discussion
Overall, model 1 appears to show moderate associations between chronic disease and psychological distress. The estimates in model 2 give us more detail into how twin-shared factors are affecting this relationship. The between-pair estimates in model 2 indicate strong evidence of association and are consistent with previous research, although like previous analyses of unrelated individuals, comparing between-pairs fails to account for genetic and environmental factors shared between twins. The within-pair estimates for having any chronic disease and all disease types are weaker than the between-pair estimates, which suggests the association between physical disease and psychological distress may be weaker than previously predicted. By directly comparing the within-pair (βw) and between-pair (βb) estimates in model 2, we gain an understanding of the amount of genetic and environmental confounding present in the relationship between chronic disease and psychological distress; see Figure 3.
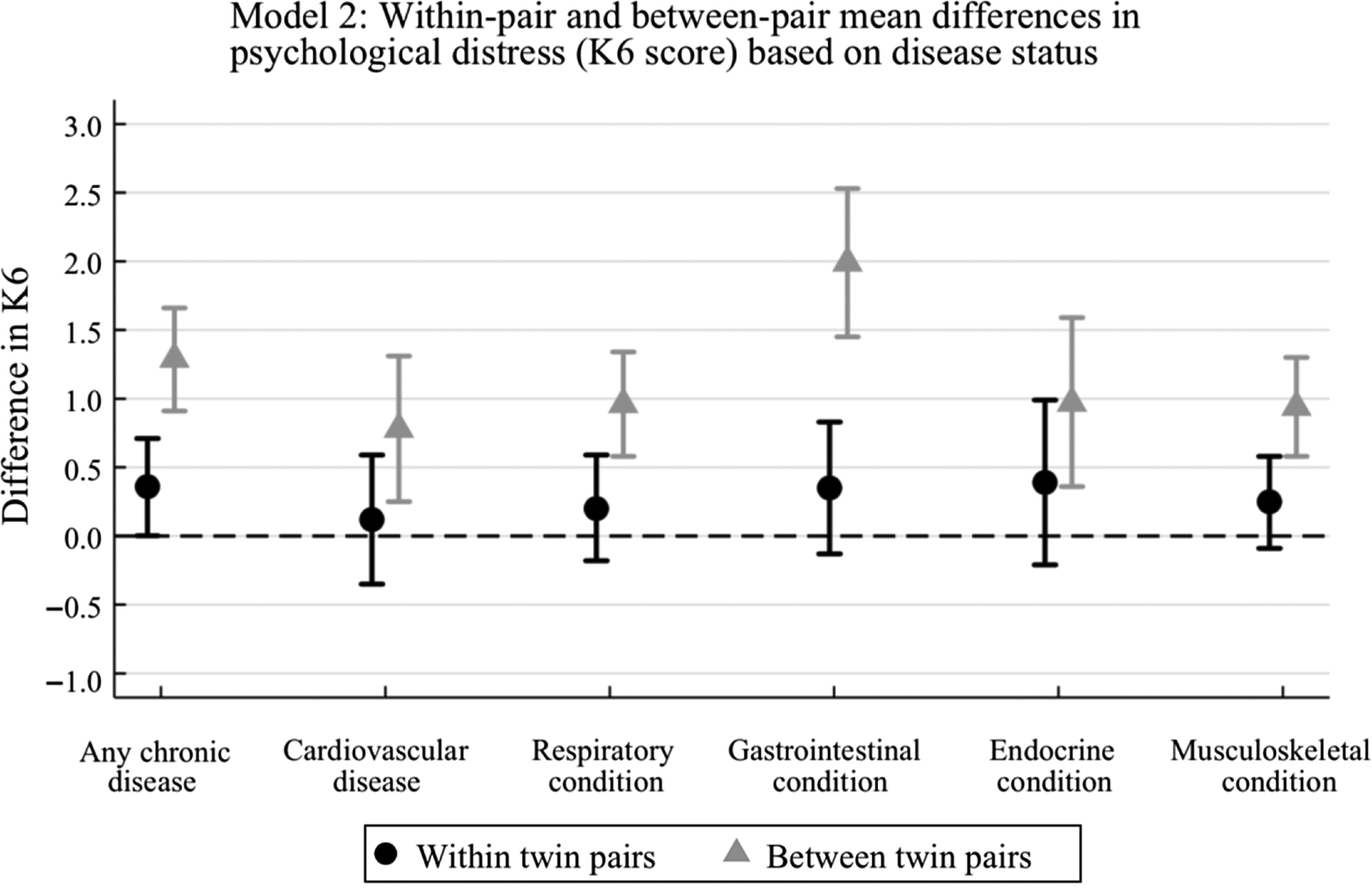
Fig. 3. Box plot of within-pair and between-pair estimates (model 2).
The between-pair estimates are larger than the within-pair estimates throughout, suggesting that there is substantial genetic and environmental confounding present in the association between chronic disease and psychological distress.
For having any chronic disease, the between-pair estimate is 1.29, whereas the within-pair estimate is 0.36, indicating that shared twin environmental and genetic factors exaggerate the relationship between chronic disease and psychological distress. The confidence intervals of each estimate do not overlap, suggesting that the estimates are unlikely to be the same. Additionally, the βw and βb estimates were shown to be statistically different with an LR test result of p = .0005. Comparatively, the common effect estimate (βc = .79) is larger than the within-pair estimate but smaller than the between-pair estimate (Table 2). This is because model 1 treats the samples as individuals but clusters on twin pair ID, thereby βc represents a weighted average of βw and βb. Given the within-pair estimate is the weakest for all coefficients indicates that factors shared between twins, such as genes and family upbringing, bias estimates of association in non-twin data.
Throughout each disease category, we can see the same trend as we saw in the ‘any chronic disease’ analysis. The within-pair estimates indicate a weaker effect than the between-pair estimates. However, due to weak evidence of within-pair associations for each disease group in this analysis, we are unable to confidently confirm the effects of different disease types on psychological distress. Each βw estimate is associated with 95% confidence intervals that cross the null and large p values, thereby indicating very weak evidence of an effect (Table 2 and Figure 3). Furthermore, for cardiovascular conditions, respiratory conditions and endocrine conditions, the 95% confidence intervals of the βw and βb estimates overlap and the LR tests show high p values, p = .63, .30 and .18, respectively. This means we cannot confidently confirm that the βw and βb estimates are different from each other.
However, for gastrointestinal conditions, we do see a significant difference when comparing the within-pair estimate (βw = .35; 95% CI [−0.13, 0.83]; p = .150) and between-pair estimate (βb = 1.99; 95% CI [1.45, 2.53]; p < .001). Figure 3 clearly depicts a larger difference in βw and βb estimates for gastrointestinal conditions compared to other disease types. The confidence intervals do not overlap and the LR test provides strong evidence against the hypothesis that the estimates are the same (p < .0001). This indicates that genetic and environmental factors shared between twins heavily influences the relationship between psychological distress and gastrointestinal diseases. Future research could explore exactly which effects shared between twins in a pair may negatively influence physical and mental health. Such factors could include genetics, but emerging research also suggests that modifiable factors, such as childhood diet could be important (Dash et al., Reference Dash, Clarke, Berk and Jacka2015).
Notably, musculoskeletal conditions show a similar magnitude change of effect size from between-pair to within-pair estimates as cardiovascular conditions, respiratory conditions and endocrine conditions. However, the results show some evidence that the βw and βb estimates are ‘statistically’ different, with an LR test of p = .0058 and the edges of the 95% confidence intervals only just meeting, giving borderline evidence of a difference. This may be due to a higher sample of people having a musculoskeletal condition than any other condition thus the analysis had higher power to detect an effect.
Overall, the results of this analysis suggest that the association between chronic disease and psychological distress may be confounded by genetic and environmental factors shared between twins, suggesting that associations observed in analyses of unrelated individuals may be partially due to confounding. The results of this analysis show that after controlling for genetic and environmental factors shared between twins, there is only weak evidence of association for the relationship between chronic disease and psychological distress.
Sample Size Consideration
Because only twins who are discordant for disease contribute to the result in the within-pair differences analysis, we need to consider the statistical power for each analysis by looking at the number of twin pairs discordant for each disease category. The numbers of twin pairs discordant for having any chronic disease, cardiovascular disease, respiratory disease gastrointestinal disease, endocrine disease and musculoskeletal disease were 463, 242, 253, 388, 164 and 524, respectively. In the within-pair differences analyses, we did see a weak association for having any chronic disease, but not for any specific disease groups. Endocrine and gastrointestinal conditions show similar effect sizes as having any chronic disease, .39 and .35 compared to .36, respectively. However, these βw estimates had wide 95% confidence intervals that crossed the null, and large p values. It is possible that small associations exist and the reason we are not seeing evidence of these is due to fewer discordant pairs per disease group.
Strengths
The design of this twin study provides a new approach to exploring the relationship between chronic disease and psychological distress. We have been able to uniquely control for measured and unmeasured genetic and environmental confounders that previous studies have failed to account for. Specifically, shared factors within twin pairs are not only twin-specific factors but include things like genes, childhood diet and lifestyle, parenting style received and one’s womb environment. The within-pair estimate extracts all these potentially confounding factors out and we ultimately get a more accurate estimate of association.
This study also appeared to have adequate power for testing the association between any chronic disease and psychological distress. Given the significance of the within-pair results despite a weak effect found suggests there was sufficient sample size.
Moreover, despite a relatively extensive questionnaire, there were minimal missing data. Pairs who had missing ages were completely random, due to a technical glitch upon extracting individual completion dates, and those that had missing income data did not appear to differ from the sample regarding association with the exposures and outcome (data not shown). Thus, it is unlikely this caused any bias in the result.
Prior research has found that physical health characteristics and health behavior of adult twins and singletons do not differ substantially in adulthood (Andrew et al., Reference Andrew, Hart, Snieder, de Lange, Spector and MacGregor2001), so results of this twin study should be generalizable to the Australian adult population. As the current study was a comparative analysis of diseases in an Australian population, it provides some understanding of the potential impact chronic disease and psychological distress have on each other in Australians.
Limitations
Despite extracting the shared genetic and shared environmental twin factors, this analysis is not free from confounding. We cannot deny that other lifestyle factors that are unshared between twins could confound the within-pair coefficients. Ultimately, this analysis cannot conclude causality.
There may be some selection bias in this sample due to the nature of recruitment. The group of people that choose to participate were mostly female (76.5%) and on average had lower psychological distress than the general Australian population (ABS, 2012a). The restricted range of individuals with medium–high psychological distress could bias results toward the null. Other biases could occur from poor self-reporting. Although questions asked participants to only report health conditions if they had been medically diagnosed, there is still potential for misclassification, which also could bias results toward the null.
Some study design aspects may limit our conclusions. The severity of disease and the strength of the twin bond were not measured. It is possible that one twin having a life-threatening chronic disease can cause distress for the other twin. Thus, consideration of disease severity could be highly important. Additionally, comorbidity was not considered in depth. There is a lot of comorbidity in our sample, making comparisons between diseases nonindependent (see Supplementary Table S1). Additional analyses were conducted to explore the potential impact of comorbidity in our sample (see Supplementary Table S2 for analyses of models that adjust for different disease types). However, to appropriately adjust for comorbidity, diseases should be considered individually rather than in groups, with epidemiological thought and understanding of disease-specific prognosis and progression.
Historically, Kessler scales have been better for reporting psychological trends in the population rather than individual changes in mental health (Kessler et al., Reference Kessler, Andrews, Colpe, Hiripi, Mroczek, Normand and Zaslavsky2002). As K6 is a proxy for psychological distress, it can be difficult to interpret what effect size changes clinically mean in terms of an individual’s mental health as there is no clinically significant effect size change in the literature. Using the ABS stratified K6 categories 1−5, a change of just 1 point can indicate a change in mental health ranking in those lower categories (ABS, 2012a). However, going from moderate distress to high distress shows a much larger range of change. Ultimately, an effect size change of 1 can have clinical significance for those with low levels of psychological distress, but is perhaps less clinically significant for those experiencing high distress. The effect size changes in these analyses appear low, which could be indicative of the lack of range of psychological distress in the sample. Ideally, K6 should be analyzed as a binary or categorical variable for interpretation ease. However, as mentioned in the methods, this limits the power of analyses.
Finally, due to the cross-sectional design of this study, we are unable to identify the causal direction of this relationship. This analysis focused on assuming chronic disease impacts psychological distress as the questionnaire measured the current state of psychological distress and asked about diseases that had previously been medically diagnosed. However, this does not exclude the possibility of psychological distress occurring before physical disease diagnosis, and in fact, contributing to the development of physical disease. A longitudinal study would be required to investigate the time course of disease in more detail.
Large-scale longitudinal and twin study designs would allow for causal inferences and should be more commonly adopted, especially when trying to understand complex disease pathways such as the mechanisms of development of mental health and chronic disease. Specifically, future research should consider adopting a longitudinal design by conducting a follow-up of the sample to improve the scientific merit of this research and provide a deeper understanding of the relationship between physical health and psychological distress.
Conclusion
Evidence that physical and mental health are associated exists. However, this analysis shows that genetic and environmental factors likely cause confounding effects which exaggerate associations in unrelated samples. It is important that future research not only identifies associations, but also investigates evidence of causal mechanisms and trajectories over time of mental and physical health.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2020.86.
Acknowledgments
This research was facilitated through Twins Research Australia (TRA). TRA receives support from the National Health and Medical Research Council through a Centre of Research Excellence Grant (ID:1079102), which is administered by the University of Melbourne. We thank all the twins who are members of TRA and completed the Health and Lifestyle Questionnaire.
Conflict of Interest
None.







