INTRODUCTION
Campylobacteriosis is an acute zoonotic gastrointestinal disease characterized by severe diarrhoea, abdominal pain, fever and nausea [Reference Heymann1]. With 65 000 laboratory-confirmed cases in 2011 Campylobacter represent the leading cause of bacterial gastroenteritis in England and Wales [2]. A wide range of wild and domestic birds and warm-blooded animals are animal reservoirs for Campylobacter, particularly farm animals, including beef and dairy cattle, sheep, pigs, and poultry [Reference Heymann1]. The large majority of human Campylobacter infections are caused by ingestion of contaminated food and water, with an infective dose as low as 500 organisms [Reference Heymann1].
Most Campylobacter infections are sporadic and outbreaks are uncommon. However, in recent years there has been a marked increase in the proportion of Campylobacter outbreaks in England and Wales linked to poultry liver pâté, rising from 12% between 1992 and 2006, to 74% between 2007 and 2009 [Reference Little3]. In 2011, 13/14 (93%) Campylobacter outbreaks reported to the Health Protection Agency (HPA; England and Wales) linked to catered events were considered to have poultry liver pâté as the vehicle for infection [4]. Campylobacter, present in 93–100% of faecal samples from farmed ducks in the UK [Reference Colles5], is also frequently found in the meat and parts of the animal [Reference Adzitey, Huda and Ali6]. Both an adequate cooking duration, and temperature, are required to inactivate Campylobacter often present in the internal tissue of duck liver, and so prevent disease [Reference Whyte, Hudson and Graham7]. Previously published outbreak reports have linked Campylobacter infection to chicken liver pâté consumption [Reference Inns, Foster and Gorton8, Reference O'Leary9]. Eating duck livers has recently also been implicated as a food vehicle in outbreaks of campylobacteriosis [Reference Unicomb10, 11].
The environmental health team of North Somerset Council received a call on 16 April 2012 from an attendee at a catered wedding held on 7 April 2012 in Somerset, UK, stating that several guests had become ill with gastroenteritis, and one had a positive stool sample for Campylobacter. An outbreak control meeting was held between North Somerset Council and the local health protection unit on 19 April 2012 where the decision was made to conduct a cohort study. The objective of this study was to identify the vehicle of infection with Campylobacter, identify any risk in food preparation, and so inform public health measures to prevent further disease.
METHODS
The study design was a retrospective cohort study. All persons that attended any of three catered events associated with the wedding event were eligible to participate; the main wedding meal, evening buffet, and a meal at a private house the following day. Members of the cohort were identified using a list provided by an organizer of the event. Staff from North Somerset Council contacted participants by telephone and administered a 126-item questionnaire investigating demographic characteristics, clinical status and food item consumption. Interviews took place between 24 and 27 April 2012. Individual food and drink items, served at any of the three catered events associated with the event were included in the questionnaire with consumption recorded as: none, <1 portion, 1 portion, ⩾2 portions.
Statistical analysis
All data analysis was performed using Stata v. 12.0 (Stata Corp., USA).
Case status was determined clinically, defined as any attendee at a catered event associated with the wedding reporting one or more symptom of diarrhoea, vomiting, or abdominal pain with onset within 10 days following the event. Participants with a positive stool culture for Campylobacter were additionally defined as confirmed cases.
Exposures, defined as ordered categorical variables, included any food item consumed at the wedding. Potential confounding variables identified a priori were age and gender.
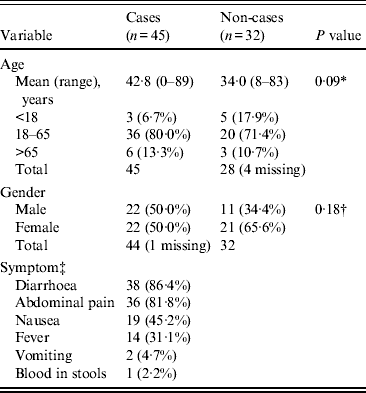
Initial descriptive analysis included calculation of the attack rate, epidemic curve, comparison of demographic characteristics in cases and controls, and reporting of clinical symptoms (Table 1).
Table 1. Demographics and clinical characteristics of cases and non-cases (n = 77)
* t test.
† χ 2 test.
‡ Some missing data.
The proportion of cases exposed to each food item was calculated. Any food item consumed by over 90% of cases was considered to be a more likely potential vehicle for infection than those consumed by fewer participants (Table 2).
Table 2. Food item exposure and relative risk of illness for foods consumed by >50% of cases, main wedding meal only

AR, Attack rate; RR, relative risk; CI, confidence interval.
* Undefined.
Univariate analysis, with calculation of risk ratios, was used to test the association between individual food item consumption, recoded as binary, and case status by means of the χ 2 test. Logistic regression was then used to build a multivariable model adjusted for confounders, for any meal attended by a significant proportion of cases. Any food item with an elevated odds ratio and P value for association with the outcome of <0·2 in univariate logistic regression was examined. A backwards stepwise procedure was employed, whereby food items were excluded from final models if they had a P value for association with the outcome of >0·1, were not implicated by environmental investigation, and their presence did not alter the odds ratio for the exposure–outcome association by >20% for any other independent variable. Age and gender were included in all final models. Exact logistic regression was used as some cells contained no observations, for example there were no cases not exposed to table water.
Any food item consumed by >90% of cases, demonstrating any evidence of an association (P < 0·1) with the outcome, or suggested by environmental sampling and biological plausibility was tested as an ordinal variable using a χ 2 test for trend for evidence of a dose–response relationship with the outcome; tests for trend did not include the unexposed stratum [Reference Tostmann, Bousema and Oliver12].
Microbiology
Efforts were made to trace the results of any stool specimens provided by study participants to their local laboratory, and to obtain samples for further characterization. On 18 April 2012, 11 days after the wedding meal, local environmental health officers sampled a subsequent batch of duck liver pâté produced at the venue, and water samples were taken from the mains supply and the bar siphon tap on 9 May 2012. All environmental samples were sent to the HPA laboratory, Porton Down, UK. Samples of duck liver pâté were cultured on Columbia blood agar, following which any isolates of Campylobacter detected underwent detailed characterization. Serotyping of isolates was performed using detection of heat-stable (HS) antigens by direct bacterial agglutination [Reference Frost13], with further subtyping performed using multi-locus sequence typing (MLST) [Reference Dingle14]; phage typing was performed according to the methods described by Frost et al. [Reference Frost, Kramer and Gillanders15]. The antibiotic resistance of any Campylobacter isolates detected was determined using an agar dilution breakpoint technique [Reference Thwaites and Frost16].
Local environmental officers visited the venue and interviewed the chef who prepared food for the event.
RESULTS
Descriptive epidemiology
Seventy-eight (88·6%) of the 88 attendees at the wedding were contacted and all agreed to complete a questionnaire; 10 attendees could not be contacted; one participant's responses lacked sufficient clinical data to ascertain a case definition. No further demographic or clinical information was available for attendees unable to be contacted. The overall attack rate was 45/77 (58·4%); four of the 45 clinically defined cases were confirmed. All 45 cases attended the main wedding meal, with only 23 (52·3%, one missing) and 17 (38·6%, one missing) attending the evening meal and meal the following day, respectively. The epidemic curve (Fig. 1) demonstrates the date of onset of symptoms for probable and confirmed cases, the median incubation time was 66 h (range 9–201 h) for all cases, and 52 h (range 26–72 h) for those also confirmed. The median duration of illness in cases was 4·5 days (range 0–20 days); five cases were still symptomatic at the time of interview (19–20 days after the wedding meal). Demographics and clinical information for cases and non-cases are presented in Table 1; cases were older than non-cases, with a higher proportion of males. The commonest symptoms in cases were diarrhoea (86·4%) and abdominal pain (81·8%).
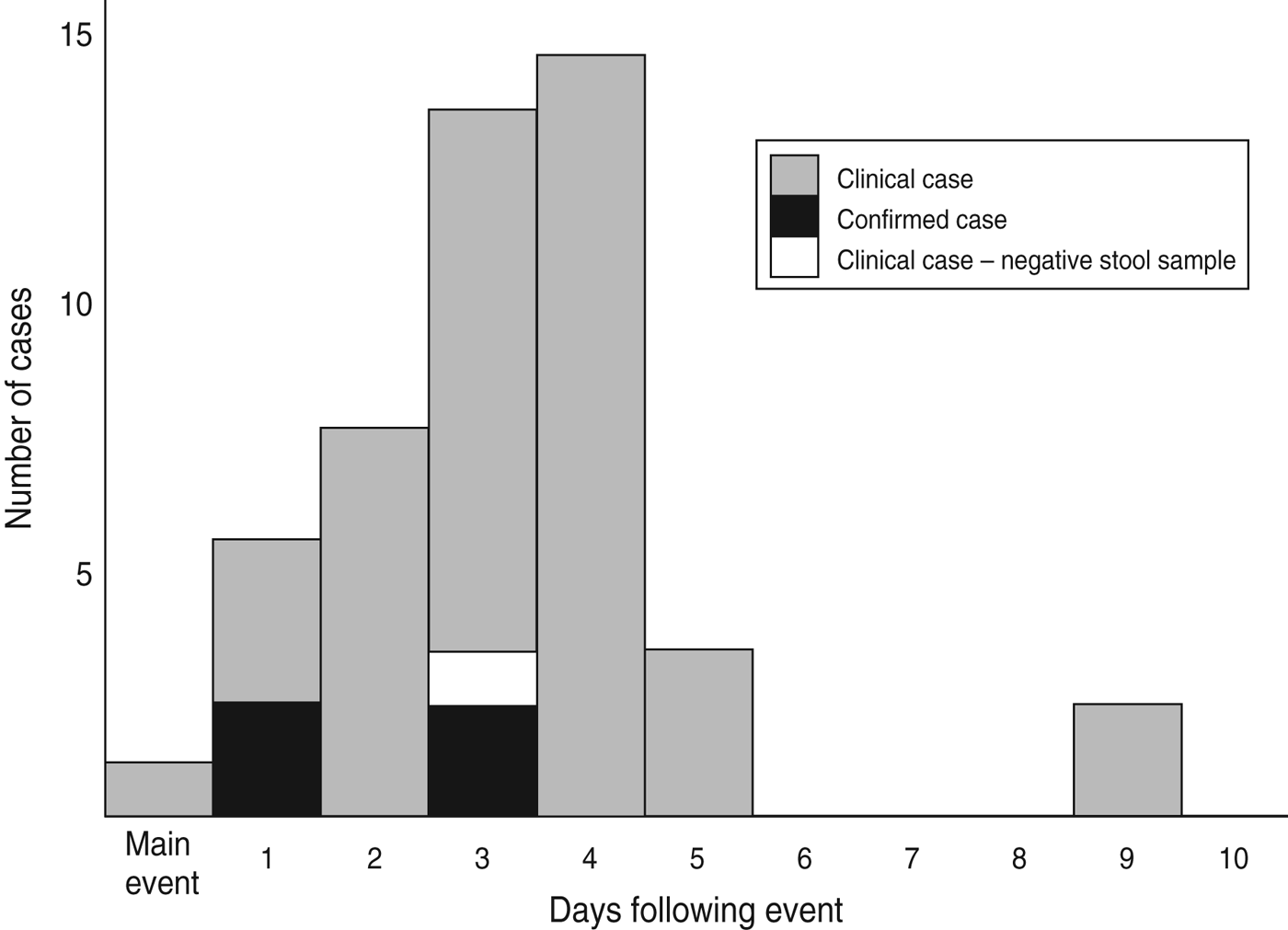
Fig. 1. Date of onset of illness by case status (n = 45).
Univariate analysis
Table 2 presents the results of univariate analysis for the association between individual food items and drinks consumed by attendees at the main wedding meal and illness; there was some evidence that consumption of duck pâté [relative risk (RR) 2·53, 95% CI 1·06–6·10], mixed leaves (RR 2·91, 95% CI 1·22–6·92), and table water (RR undefined, P < 0·01) were associated with illness. All items served at the two other meals were consumed by <45% of cases and no significant association with case status was found (data not presented). Table 3 demonstrates the attack rates stratified by dose for food items with near uniform exposure in cases. There was weak evidence of a dose–response relationship between increasing duck pâté and broccoli consumption and illness, with stronger evidence linking chicken Wellington to the outcome in this analysis.
Table 3. Dose–response relationship between number of portions of food item consumed and case status, analysis only performed for food items consumed by >90% of cases

n.a., Not available.
* Exposed only.
Multivariable analysis
The results of multivariable analysis are presented in Table 4. In an exact logistic regression model there was no association between age or gender with the outcome; the odds of illness in those exposed to table water was 18 times that of those unexposed (P < 0·001) but there was no association between duck pâté or mixed leaves consumption and outcome.
Table 4. Multivariable analysis for risk of case status, main meal only
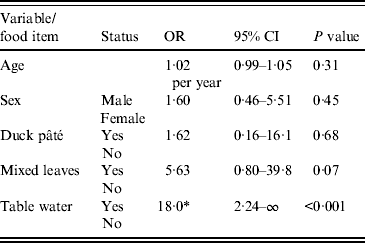
OR, Odds ratio; CI, confidence interval.
* Median unbiased estimate.
Microbiology
Environmental sampling
All three samples of duck pâté taken on 18 April 2012 demonstrated evidence of contamination with Campylobacter; sample 1 – Campylobacter jejuni HS15, ST1409, phage type (PT) untypable, resistant to ampicillin and tetracycline; sample 2 – C. jejuni HS1, sequence type (ST) – incomplete profile but distinct to ST1409, PT2, resistant to ampicillin, tetracycline and ciprofloxacin; sample 3 – C. coli HS-untypable, ST829 (ST complex 828), PT2, resistant to ciprofloxacin. In sample 1 there was an unsatisfactory aerobic colony count (1·1 × 107) according to HPA guidance, and unsatisfactory Escherichia coli levels in sample 3 [17]. Water sampling from the kitchen mains supply and bar python hose on 9 May 2012 was satisfactory; demonstrating no Campylobacter.
Stool samples
Five attendees at the wedding, all clinically defined as cases, provided stool samples; four tested positive for Campylobacter sp. and one was negative. Stool samples could not be obtained by the HPA laboratory, therefore no further speciation or serotyping was performed.
Interview with the caterer
The cooking method for the duck liver pâté was explored with the caterer; duck livers were pan-fried with onion, garlic and brandy until the livers were cooked medium to well. This was determined by touch rather than relying on a temperature probe test. A mixture of cream and butter was then brought to boiling point and this was added to a food mixer with the duck livers and the other ingredients. The pâté did not receive further cooking as the chef relied on the residual heat from the cream and the butter mixture to complete the cooking process.
DISCUSSION
This study describes an outbreak of Campylobacter following a catered event with an overall attack rate of 45/77 (58·4%). Environmental investigation strongly suggested that inadequately prepared duck liver pâté was the vehicle for infection; consistent with the findings of univariate analysis, but this food item was not an independent risk factor in multivariable models.
A recent international review estimated the mean prevalence of Campylobacter in duck meat products to be 32%, ranging from 45–83% in UK studies [Reference Adzitey, Huda and Ali6]. Campylobacter is present in high numbers in poultry livers, requiring cooking to a temperature of 70–80 °C for 2–3 min to be inactivated [Reference Whyte, Hudson and Graham7]. The cooking method used to prepare the duck liver pâté by the venue in this study was unlikely to have been sufficient to inactivate any Campylobacter present, confirmed by the presence of the bacteria in a subsequent batch prepared using a similar method in the same kitchen a week later.
No previously published studies have shown duck liver pâté to be a vehicle for Campylobacter infection, but there are outbreak data to suggest this is an established route of transmission. In 2011 two outbreaks of Campylobacter attributed to consumption of duck liver pâté at catering premises were reported to the HPA electronic foodborne and non-Foodborne Gastrointestinal Outbreak Surveillance System eFOSS [11]. Similarly in Australia, two cases of Campylobacter infection were considered to have a probable association with consumption of pan-fried duck livers proven to be contaminated with the bacteria, and a second outbreak of 67 cases (six confirmed) was associated [odds ratio 13·0, 95% confidence interval (CI) 1·9–91·5] with duck liver parfait cooked to 60°C core temperature in Western Australia in 2011 [Reference Merritt, Combs and Pingault18].
Under the UK Food Hygiene (England) Regulations 2006 there is a requirement that all food business operators put in place a food safety management system, in this particular case ‘Safer Food Better Business’ (SFBB) was in use. This system states that all liver should be cooked all the way through, as it can contain bacteria throughout the meat rather than just on the surface, and that all stir-fried meat should be temperature probed [19]. Catering practices for this event represented a clear deviation from the policy put in place locally to ensure the safe production of food.
Despite demonstrating evidence of an association with illness in both univariate and multivariable analysis it is very unlikely that the table water served at the venue was the vehicle for infection. Campylobacter has been isolated from groundwater [Reference Stanley, Cunningham and Jones20], and can contaminate private water supplies following flooding events, but is inactivated by chlorination in mains water supplies [Reference Blaser21]. The venue implicated in this outbreak had a mains water supply that was tested and did not reveal any Campylobacter. Inspection of routine HPA surveillance data did not demonstrate any increase in, or spatial clustering of, Campylobacter cases in the local area at the time of the outbreak. There was no evidence of a dose–response relationship between table water consumption and illness in this study.
The duck pâté dish was served with mixed salad leaves, which also demonstrated a univariate association with illness. Only seven participants in the study did not eat both mixed leaves and duck pâté, therefore discrimination between the causative roles of these foodstuffs was problematic. All confirmed cases ate mixed leaves and duck pâté. The salad consumed at the wedding was provided by a local supplier serving the South-West of England. Consumption of salad vegetables has been shown to be an independent risk factor for Campylobacter infection, with potential contamination by infected water or soil, or cross-contamination during food preparation [Reference Evans, Ribeiro and Salmon22, Reference Little and Gillespie23]; although four studies between 1999 and 2001 failed to demonstrate the presence of this pathogen in large samples of these food items [Reference Sagoo, Little and Mitchell24–Reference Little27]. Out of 75 Campylobacter outbreaks between 1992 and 2006 in England and Wales five were attributed to prepared salad [Reference Little and Gillespie23]. There was no increase in Campylobacter seen in the South-West region at the time to suggest a widespread outbreak linked to this producer. This study was unable to exclude the mixed salad served as a potential vehicle for infection, but a combination of knowledge from aforementioned studies and the microbiological evidence from duck pâté samples in this event makes causal association unlikely.
Limitations
The main limitations of this study arise from the near universal nature of exposure and possible measurement biases including reliance on a clinical case definition and lack of strictly contemporaneous environmental samples.
A set meal was served at the event described in this study, resulting in near uniform exposure to many food items, limiting the statistical power of the study, and increasing any bias associated with misclassification of case status. Attempts to estimate associations between food item consumption and case status in this study were limited by near universal exposure; however, we were able to demonstrate some consistency with microbiological evidence linking duck liver pâté consumption to illness by testing the dose–response relationship. χ 2 tests for trend can provide misleading results in particular situations where there are imbalances between the numbers in each strata tested [Reference Maclure and Greenland28]. The unexposed group were not included in dose–response estimations in this study as the majority of exposed cases reported a dose of ‘one portion’, which would result in the test for trend being dominated by a dichotomous difference [Reference Tostmann, Bousema and Oliver12]. Inspection of relative risks in each stratum provides an alternative means to assess dose–response.
The use of a clinical case definition in this study has the potential to lead to both differential and non-differential misclassification. Participants in this study, aware of the outbreak of illness, may have been more likely to report symptoms if they ate foods they considered a risk; attempts were made to reduce this bias by rapid administration of questionnaires after initiation of the study. Non-differential misclassification, in particular of food exposure, although more typically biasing effect estimates to the null, has the potential to lead to spurious associations when numbers unexposed are low; for example in this study a strong statistical association between table water and illness was found, whereas broccoli did not demonstrate the same, the difference between the two was one unexposed case. Five cases provided stool specimens, of which four demonstrated Campylobacter, suggesting some consistency with the clinical case definition in this limited sample; the clinical case that did not demonstrate Campylobacter suffered diarrhoea and abdominal pain, but submitted a stool sample 5 days following resolution of their symptoms. Two study participants that did not consume duck pâté were classified as probable cases, with both only reporting abdominal pain; previous Campylobacter outbreak investigations have used more specific case definitions including; diarrhoea or vomiting [Reference Inns, Foster and Gorton8]; diarrhoea or, abdominal pain and fever [Reference Mazick29]. Two cases had a date of onset 9 days after the event, one of whom was a member of an extended family group with five other cases, suggesting possible secondary transmission. The other attendee with symptom onset on day 9, a child, had abdominal pain only, and may represent a false-positive case.
In this study we were able to provide evidence that a subsequent batch of duck liver pâté cooked using the same methods and in the same venue as that served at the event contained Campylobacter, with detailed typing of isolates demonstrating the presence of three separate strains. The absence of characterization of stool samples, a likely result of locally collected specimens being discarded or unsuitable by the time of the outbreak investigation, prevented any stronger epidemiological link being made. Additional environmental sampling, including testing the same batch of duck pâté and the salad leaves served, allied to further characterization of stool specimens, may have provided further evidence from which to draw conclusions.
SUMMARY
This study demonstrates an outbreak of Campylobacter associated with inadequately prepared duck liver pâté following a catered event. Making inferences about causation in the presence of near universal exposures in this study required consideration of the limitations of statistical analysis, with the most compelling evidence provided by environmental investigation.
Postscript
Following the incident environmental health officers provided information and advice to the chef at the venue, and the owners were successfully prosecuted under UK food hygiene legislation.
ACKNOWLEDGEMENTS
We thank the staff of North Somerset Council for timely and thorough completion of questionnaires.







