Vitamin D is important to human health. Vitamin D deficiency is associated with an increase in the risk of osteoporosis, falls and fractures(Reference Mosekilde1–Reference Bischoff-Ferrari, Willett and Wong3), and epidemiological evidence has linked poor vitamin D status to a number of inflammatory, infectious, cardiovascular and metabolic disorders and with cancers(Reference Mosekilde1, Reference Adams and Hewison4, Reference Sharma, Barr and Macdonald5).
The endogenous production of vitamin D in man depends on UVB-mediated conversion of 7-dehydrocholesterol to pre-vitamin D3 and further isomerisation to vitamin D3 (25OHD) in the skin(Reference Adams and Hewison4). This depends on exposure to sunlight, and serum 25OHD levels decrease with increasing latitude and vary with season(Reference Mosekilde1, Reference Adams and Hewison4, Reference Sharma, Barr and Macdonald5).
Populations living above the Arctic Circle are characterised by no sun exposure at about mid-winter. Also, a high solar zenith angle during summer suggests a limited sun exposure during this supposedly compensatory season. This is further added to by the need for protective outdoor clothing even during the Arctic summer, defined by a mean mid-summer temperature below 10°C. Thus, very limited dermal 25OHD production is expected in Arctic residents.
The intensity of the light at sun appearance in spring is extremely high due to reflections from snow and ice. Thus, suntan and cataract are common among people with an outdoor occupation, and sun block and sunglasses are necessary in Greenland from early spring. Whether this contributes to some dermal 25OHD production remains to be settled.
The traditional Inuit diet consists mainly of sea mammals and free-living fish(Reference Sharma, Barr and Macdonald5–Reference Bjerregaard and Young8). These food items are particularly rich in vitamin D(Reference Sharma, Barr and Macdonald5) and may be an important source of 25OHD. However, the traditional Inuit diet varies with season(Reference Kuhlein, Receveur and Soueida6, Reference Andersen, Hvingel and Kleinschmidt7) and this may contribute to seasonal differences in serum 25OHD concentration.
This led us to study the influence of dietary habits and season on the levels of 25OHD in serum in population cohorts living 400 km above the Arctic Circle in North Greenland, with 1 month of darkness at mid-winter and 1 month of 24 h sun at mid-summer.
Subjects and methods
Area of investigation
Ilulissat (69°22′N) and Saqqaq (70°05′N) are situated in North Greenland, 400 and 500 km north of the Arctic Circle, respectively (Fig. 1), by the UNESCO Heritage Ilulissat Icefjord. Sun does not set from 21 May to 24 July and the polar night extends from 1 December to 13 January. Ilulissat has 4500 inhabitants, of whom approximately 90 % are Inuit (Eskimo) and 10 % non-Inuit (Caucasian Danes) populations. Ilulissat can be accessed by sea 7 months a year and has a variety of imported food items available in two major stores, supplementary to the traditional Greenlandic food items. Saqqaq is a settlement with 180 inhabitants and has one store with some market foods available, depending on access by sea and air.

Fig. 1 Map of Greenland that depicts the town of Ilulissat (69°22′N) and the settlement of Saqqaq (70°05′N) 400 and 500 km north of the Arctic Circle, respectively. Ilulissat has slightly more than 1 month of polar night and 1 month of midnight sun.
Subjects and procedures
Procedures and subjects participating in January have been described previously(Reference Andersen, Boeskov and Laurberg9). Names and addresses were obtained from the National Civil Registration System, in which every person living in Denmark, the Faeroe Islands and Greenland is recorded. A random sample was drawn and participants in Ilulissat were stratified by age, sex and place of birth (Greenland or Denmark), aiming at a balanced representation of the age groups 30–39 and 40–49 years, men and women and the three groups consisting of subjects born in Greenland and living in the town, living in the settlement and subjects who were not born in Greenland. This age range was chosen to ensure sufficient numbers of non-Inuit participants, as they are skilled labourers from Denmark who work in Greenland while in this age range. None of the participants had disease affecting bone, vitamin D absorption or vitamin D metabolism. Participants in Saqqaq (all Inuit) were matched to the Inuit participants in Ilulissat by age and sex. Data in Ilulissat were collected in spring (30 March through to 10 April 2001), summer (25 June through to 5 July 2001), autumn (25 September through to 5 October 2001) and winter (7 January through to 19 January 2002). Participants in Saqqaq were enrolled during summer 2001 and spring data were collected from 25 March through to 29 March 2002. In all, ninety-seven healthy subjects participated. A Greenlander (Inuit) was defined as an individual born in Greenland, with both parents born in Greenland.
A letter of invitation was delivered by the local hospital porter or the nursing station attendant. The investigation took place at Ilulissat hospital or at the nursing station in Saqqaq or, at request, at home visits. A physical examination was performed that included height without shoes, weight in indoor clothing and recording of major disabilities. Participants were interviewed by a Greenlandic interpreter or by one of the investigational doctors completing a questionnaire in either Greenlandic or Danish, as appropriate for the participant. Information regarding age and sex was obtained from the National Civil Registration System. Information on smoking habits (present/past/never), alcohol intake (units/week), hunting (none, leisure or trade), number of hours outdoors as an average of yesterday, daily the latest week and daily over the last month, the use of vitamin preparations each season (daily/occasional/never) and dietary habits was obtained by questionnaires. Questions were asked as written in the questionnaires.
A non-fasting blood sample was drawn from the cubital vein using minimal tourniquet. Serum was separated and kept at − 20°C until analysis.
Ethical approval by the Commission for Scientific Research in Greenland was obtained (505-63), and all subjects gave informed written consent in Danish or Greenlandic by participant choice.
Dietary habits
An interview-based FFQ was used to assess dietary habits. The questionnaire used at inclusion has been described in detail previously, and it has been validated using urinary iodine excretion as a biomarker for the intake of traditional Inuit food items(Reference Andersen, Hvingel and Kleinschmidt7). Fish and blubber from marine mammals that are rich in dietary iodine and vitamin D dominate these food items. The questionnaire asked the frequency of intake of seven traditional Inuit food items (seal, whale, wild fowl, fish, reindeer, musk ox and hare) and seven imported food items (pre-cooked meals, potatoes, vegetables, butter, cheese, eggs and fresh fruit). Six different frequency categories were given for each food item. These were daily intake, four to six times weekly, one to three times weekly, two to three times monthly, once a month or less and never. A frequency score was calculated based on the average number of days per month that each individual food item was ingested(Reference Andersen, Hvingel and Kleinschmidt7). Inuit food items scored positively and imported food items scored negatively. The sum of frequency scores for all food items consumed by each individual participant was calculated and the individual participants were categorised by quintiles of scores: diet group 1: >80 %; 2: 60–80 %; 3: 40–60 %; 4: 20–40 %; and 5: < 20 % Inuit food item scores on a scale, where 100 % was pure Inuit foods and 0 % was pure imported food. As a further check on adherence to the traditional Inuit diet, participants were asked the number of days per week that their main meal consisted of traditional Inuit food items. This was reported for each of the four seasons at inclusion and, in addition, it was asked during each data collection.
The intake of vitamin D-containing supplements was evaluated by asking the frequency of intake at each of the four seasonal data collections. Supplements were presented to one of the investigational doctors for evaluation of vitamin D content. Vitamin D-fortified foods are not available in Greenland.
Vitamin D assay
Plasma 25OHD levels were measured by a liquid-phase RIA (Gamma-B 25-Hydroxy Vitamin D RIA; Immunodiagnostic Systems Limited) that has an intra-assay precision of 5·3 % at 26 nmol/l, 5·0 % at 58 nmol/l and 6·1 % at 151 nmol/l, and an inter-assay precision of 8·2 % at 20 nmol/l, 8·1 % at 57 nmol/l and 7·3 % at 136 nmol/l (information provided by the supplier).
Statistics
Results are given as frequencies, means and medians with quartiles. Unrelated samples were compared using the Mann–Whitney U test for two groups and the Kruskal–Wallis test for more than two groups. Kendall's τ tested for trend. Related samples were compared using Friedman's test, i.e. between the four seasons. A season ratio of serum vitamin D was calculated by dividing the autumn by spring values for each participant. Explanatory variables entered in multivariate logistic regression analysis were season, diet, origin, age, sex, use of supplements, residence and weight. The dependent variable was serum 25OHD, with levels above/below 50 nmol/l. Data were processed and analysed using Corel Quattro Pro 8 (Corel Corporation) and the Statistical Package for the Social Sciences version 13.0 (SPSS, Inc.). A P value of less than 0·05 was considered significant.
Results
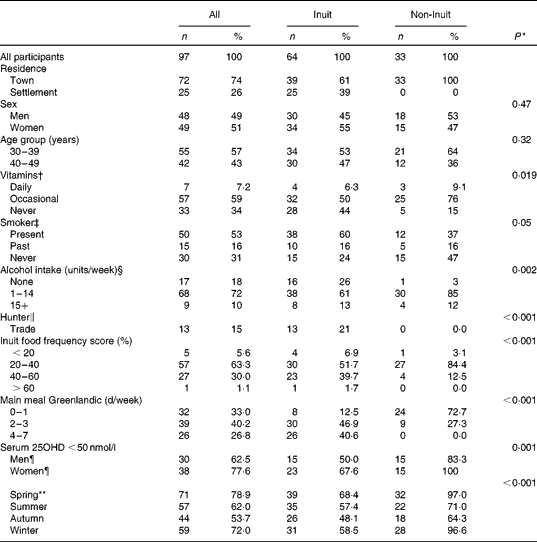
Of the ninety-seven participants enrolled, seventy participated in all four seasons, fifteen in three seasons, nine in two seasons and three participated just once. The reason for non-participation was hunting in all but one individual, who moved to Denmark. The characteristics of all ninety-seven participants enrolled are presented in Table 1. Age and sex did not differ between Inuit and non-Inuit participants, according to inclusion. Non-Inuit participants were the more frequent users of vitamin D-containing preparations, though daily use was low in both groups. Alcohol intake differed, as more Inuit participants were abstainers. Hunting was the main trade among Inuit men in the settlement and still frequent among Inuit men in the town. It did not differ between Saqqaq and Ilulissat (36 v. 25 %; P= 0·52, data not shown).
Table 1 Characteristics of participants in the survey performed in North Greenland 400 km above the Arctic Circle (Number of participants and percentages)

25OHD, 25-hydroxy vitamin D.
* χ2 test for difference between Inuit and non-Inuit participants.
† Information missing in nine participants.
‡ Information missing in two participants.
§ Information missing in three participants.
∥ Information missing in eight participants.
¶ Average of four seasonal measurements. None was below 20 nmol/l.
** P= 0·003 for difference between seasons in all participants.
Inuit food frequency score groups are shown in Table 1. Scores differed markedly between Inuit and non-Inuit participants (P= 0·022), between non-Inuit, Inuit in town and Inuit in settlement participants (P= 0·029) and with place of living in Inuit (P= 0·023) (data not shown). Also, main meal of traditional Inuit food items was more frequent in Inuit than in non-Inuit participants (Table 1), and more frequent among Inuit participants in settlement compared with Inuit participants in town (4–7 d/week in Saqqaq v. Ilulissat, 64 v. 29 %, P= 0·026) (data not shown). Days with main meal of Inuit food items were associated with food frequency scores (Kendall's τ 0·34, P= 0·001).
Average serum 25OHD levels below 50 nmol/l in individual participants was common (Table 1), but decreased with increasing intake of Inuit foods (P= 0·023) and differed between Inuit and non-Inuit (P= 0·001) participants. None had average serum 25OHD levels below 20 nmol/l. Serum 25OHD levels below 50 nmol/l was more common following the polar night than following the spring and summer with midnight sun (P= 0·003, Table 1).
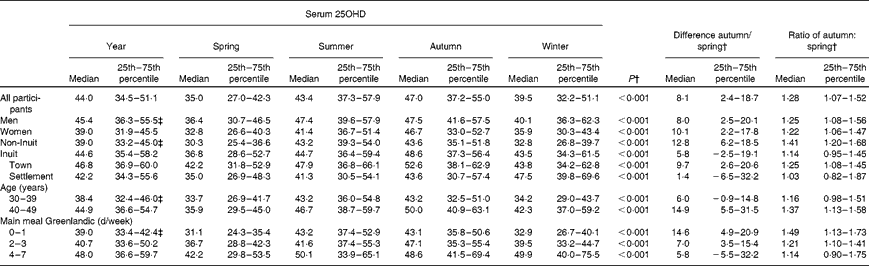
Table 2 lists average and seasonal serum 25OHD concentrations for each participant group in the sixty-three participants who attended the investigation in all four seasons and who did not take vitamin D-containing supplements. Serum 25OHD levels differed markedly with season in all groups. Overall, a rise in serum 25OHD levels was seen in spring and continued during summer, and the concentration was 28 % higher in autumn than in spring, as illustrated by the autumn:spring ratio. Sex differences in serum 25OHD levels were marked in Inuit (P= 0·013) but not in non-Inuit participants (P= 0·88). Serum 25OHD level was higher in Inuit than in non-Inuit participants (P= 0·011), and differed in Inuit v. non-Inuit men (P= 0·001) but not in women (P= 0·74). It was higher in the older age group (P= 0·023). Serum 25OHD levels increased gradually with increasing Inuit food frequency scores (P= 0·003) (data not shown) and it rose with the number of days that the main meal was prepared from local foods (Table 2, P= 0·019). The group with the lowest quintile of Inuit food frequency scores had a serum 25OHD concentration of 36 nmol/l, while it was 68 nmol/l in those having the highest quintile of Inuit food intake (data not shown). Seasonal differences tended to decrease with more frequent intake of Inuit food (P= 0·055). Still, a 14 % seasonal difference in serum 25OHD levels was seen in the group with the highest intake of Inuit foods (Table 2).
Table 2 Serum 25-hydroxy vitamin D (25OHD) (nmol/l) concentration as average of seasons and the median with 25th and 75th percentiles for each season among inhabitants of the town Ilulissat and the settlement Saqqaq in North Greenland, and the difference and ratio between autumn and spring* (Median values and 25th–75th percentiles)

* This table includes data from the sixty-three participants not taking vitamin D supplements, who participated at all four seasons.
† Friedman's test for differences between seasons.
‡ P< 0·05.
The intakes of traditional Inuit foods reported by participants at each of the seasonal visits are depicted in Fig. 2. Differences in Inuit food frequencies between the four seasons were limited but statistically significant (non-Inuit, P< 0·001; Inuit in town, P< 0·001; and Inuit in settlement, P< 0·001). When asked about food intake during all four seasons at the time of the inclusion visit, no differences in terms of traditional food intake were reported by Inuit participants in town (P= 0·10) or by Inuit participants in settlement (P= 0·79), while non-Inuit participants reported differences (P< 0·001).

Fig. 2 The reported number of days that the main meal consisted of local Inuit foods for Inuit participants in the settlement (![]() ), Inuit participants in the town (
), Inuit participants in the town (![]() ) and non-Inuit (
) and non-Inuit (![]() ) participants in each of the four seasons.
) participants in each of the four seasons.
Fig. 3 illustrates the difference between the four seasons in terms of number of hours spent outdoors during each season (Inuit, P< 0·001; non-Inuit, P= 0·012). This difference preceded a parallel variation in serum 25OHD concentrations (Fig. 3, P< 0·001).

Fig. 3 The average reported number of hours spent outdoors during each of the four seasons for the three participant groups (ordinate to the left). Vitamin D (serum 25-hydroxy vitamin D; s-25OHD) is superimposed (ordinate to the right). ![]() , Inuit settlement;
, Inuit settlement; ![]() , Inuit town;
, Inuit town; ![]() , non-Inuit;
, non-Inuit; ![]() , s-25OHD.
, s-25OHD.
Table 3 lists factors important to serum levels of 25OHD. Season remained important to serum 25OHD concentrations after adjustment for diet, origin, residence, sex, age, vitamin intake and weight. Also, diet, ethnic origin, residence and sex were important to serum levels of 25OHD in the adjusted analysis (Table 3).
Table 3 Factors important to serum 25-hydroxy vitamin D (25OHD) levels among inhabitants in the town of Ilulissat and the settlement of Saqqaq at 70°N in North Greenland(Odds ratios and 95% confidence intervals)

* Logistic regression analysis with serum 25OHD >50 nmol/l as the dependent variable. Explanatory variables: origin, residence, sex, age, vitamin intake, diet and weight.
† χ2 test for serum 25OHD < 50 nmol/l.
‡ Bartlett's test for homogeneity of variances.
§ Inuit meal < 4 times/week reference.
∥ Season was included with summer–autumn v. winter–spring (reference).
¶ P>0·05
** Non-Inuit reference.
†† Settlement reference.
‡‡ Women reference.
§§ Age 30–39 years reference.
∥∥ Not daily intake reference.
¶¶ < 80 kg reference.
Discussion
We performed repeated seasonal sampling over 1 year in populations living about 70°N, 400 to 500 km north of the Arctic Circle, with marked changes in sunlight due to having over 1 month of polar night and 1 month of midnight sun. We found differences in serum 25OHD concentrations and in the frequency of intake of local marine foods rich in vitamin D. Interestingly, serum 25OHD concentration was influenced by season and residence beyond the effect of diet, sex, ethnicity and vitamin intake.
Vitamin D is acquired through diet and skin exposure to UVB light. Dermal production is determined by length of exposure, latitude, season, degree of skin pigmentation and use of protective clothing(Reference Sharma, Barr and Macdonald5, Reference Dawson-Hughes10, Reference Webb, Kline and Holick11).
UVB exposure is absent above the Arctic Circle during the polar night. Also, a high solar zenith angle limits the intensity of light during summer(Reference Sharma, Barr and Macdonald5, Reference Dawson-Hughes10, Reference Webb, Kline and Holick11). However, spring in North Greenland is characterised by immense sunlight due to a relatively high influx of sunlight because of low humidity of the air, reflections from snow and ice and the fact that this is a high-pressure area during spring with a long line of sunny days(12). Thus, sunny weather with reflections of light could compensate for the effect of high latitude.
Early Arctic visitors described eye protectors made from sealskin used during spring and summer by the Inuit population(Reference M'Keevor13). This matches later findings of frequent cataract among Greenland Inuit(Reference Klauber14, Reference Alsbirk, Clemmesen and Kessing15), as UVB exposure increases the risk of cataract(Reference Dolin16, Reference Delcourt, Carriere and Ponton-Sanchez17). Also, dermal synthesis of 25OHD should be possible in snow-covered terrain at 70°N according to Webb & Engelsen(Reference Webb and Engelsen18). They estimated that it was possible to obtain the UV dose for adequate vitamin D synthesis in human skin at 70°N from mid-March through to late September with snow-covered ground, but emphasised that this was highly variable depending on cloudiness, ozone, surface reflection and aerosols(Reference Webb and Engelsen18). Their estimate was based on a theoretical model, but was supported by others(Reference Holick19). Also, the Danish Meteorological Office reports a UV index of 1 by 1 April and 3 by 21 June in North Greenland(20). These UV index levels are sufficient for dermal 25OHD production and may be even higher with the thinning of the ozone layer(20). Thus, there are indicators of marked UVB exposure in Greenland that support the notion of possible dermal 25OHD production 500 km north of the Arctic Circle.
Protective clothing is necessary in Ilulissat during spring, where the average ambient temperature rises from − 20°C in March to − 0·5°C in May and reaches a maximum of 7·5°C in July(12). Still, Inuit hunters reported spending up to 16 h in outdoor activity during spring and summer, and some sun exposure was evident from their marked facial sun tan. Also, arms of hunters may be exposed to the sun during working hours in spring and summer.
The local Inuit diet was important for serum 25OHD production in Inuit and non-Inuit population in the capital Nuuk in West Greenland and in rural Ammassalik district in East Greenland(Reference Andersen, Laurberg and Hvingel21). These data were collected by the same investigators (S. A., P. L.), who used the same FFQ and calculated Inuit food frequency scores using the same mathematics as this survey. However, participants in the present survey were 20 years younger and the contribution by local foods was clearly lower than in the East Greenland Inuit diet(Reference Andersen, Laurberg and Hvingel21). Still, the expected effect of Inuit diet on serum 25OHD levels was found, and the levels of serum 25OHD were as predicted from the Inuit food frequency scores(Reference Andersen, Laurberg and Hvingel21). Thus, we found that serum 25OHD levels almost doubled from the group with the lowest to the highest quintile of Inuit food frequency scores. A rough estimate based on the present data suggests an increase in serum 25OHD levels of about 4 nmol/l for each additional day with the main meal of traditional Inuit foods. This influence of marine diet on serum 25OHD concentration is in accordance with findings in other north Atlantic populations(Reference Dalgård, Petersen and Schmedes22).
The seasonal variation in serum 25OHD levels is in keeping with the findings in a previous study of Inuit and non-Inuit subjects in Denmark and south of the Arctic Circle in Greenland(Reference Rejnmark, Jørgensen and Pedersen23). They found seasonal differences in diet and in vitamin D concentrations. However, a rather crude dietary classification was used and data on exposure to ambient environment are lacking(Reference Rejnmark, Jørgensen and Pedersen23).
Serum 25OHD levels rose markedly during spring and summer, and declined in autumn and winter. Thus, we found a markedly higher risk of serum 25OHD levels below 50 nmol/l in the samples collected during and following the polar night compared with samples collected during mid-summer and autumn. This was consistent after adjusting for diet, intake of vitamin D-containing supplements, age, sex and ethnicity. The seasonal difference may be explained by dermal production of 25OHD, which is consistent with a lower level in Inuit populations in the settlement 100 km north of Ilulissat. It also fits with the exposure to immense light during spring in North Greenland.
Seasonal differences in serum 25OHD concentrations declined with higher intakes of Inuit foods. Still, a marked seasonal difference in the group with the highest intake of local foods supports the notion that dietary variations do not contribute the full explanation. Moreover, a sex difference in serum 25OHD levels was seen in Inuit participants only. This is coherent with an effect of exposure to sunlight, as Inuit men are hunters(Reference Andersen, Kleinschmidt and Hvingel24) and thus spend markedly more time outdoors than Inuit women. In fact, one in four Inuit men reported spending 8 h or more outdoors even during winter.
We did not measure dermal 25OHD production. Still, both the adjusted analyses, the serum 25OHD indexed for diet, the sex difference in Inuit participants only, the association with sunlight exposure at the four seasons, the consistent influence of residence and the presence of other indicators of UVB exposure in North Greenland support a possible dermal 25OHD production. A way to detect the contribution from the diet is by measuring vitamin D2 and D3. However, the Inuit diet consists mainly of marine mammals(Reference Andersen, Hvingel and Kleinschmidt7) with high contents of D3 that contributed by far to the majority of 25OHD concentration in Inuit participants(Reference Andersen, Laurberg and Hvingel21).
Inuit participants had a higher serum 25OHD concentration than non-Inuit participants. They also had a higher intake of Inuit foods and spent more time outdoors than non-Inuit participants. Still, the ethnic difference was confirmed in the adjusted analysis. This extends our previous finding of an influence of ethnicity on serum 25OHD concentrations(Reference Andersen, Laurberg and Hvingel21). It may be speculated that this relates to an ethnic difference in the absorption or metabolism of vitamin D.
Serum 25OHD level was measured using a RIA assay. Liquid chromatography tandem MS is considered to be the reference method and was used in another study in Greenland(Reference Højskov, Heickendorff and Møller21, Reference Højskov, Heickendorff and Møller25). Still, the findings here were supported by the fact that serum 25OHD levels were in keeping with the levels predicted from Inuit food frequency scores in a previous study(Reference Andersen, Laurberg and Hvingel21). Furthermore, the levels in the present study were used for comparison across season and groups, but not for external comparisons.
In conclusion, Inuit population in North Greenland spend up to 16 h outdoors in spring and summer and are exposed to immense light due to reflections from snow, ice and sea. The present data suggest a contribution to serum 25OHD levels beyond that of the traditional Inuit diet and vitamin D-containing supplements. This may be speculated to be caused by a dermal production of 25OHD during spring and summer as far north as 70°N in addition to the dietary sources of vitamin D.
Acknowledgements
The authors declare no conflict of interest. The present study was supported by grants from the Greenland Home Government and Karen Elise Jensen Foundation. S. A. conceived the idea, designed the study, raised the funds, collected, analysed and interpreted the data and wrote the manuscript; A. J. analysed and interpreted the data and reviewed the manuscript; P. L. conceived the idea, designed the study, raised the funds, collected and interpreted the data and reviewed the manuscript.