Although transcatheter balloon aortic valvuloplasty is preferred as the first-line treatment for critical valvular aortic stenosis in neonates, this procedure in extremely-low-birth-weight infants is still a challenge.
Case
A 438 g baby boy was born at 26 weeks of gestation. Echocardiography revealed a valvular aortic stenosis of 1.9 m/second. When he was 111 days old and weighing 890 g, he rapidly developed systemic edema. Echocardiography revealed a severe aortic stenosis of 1.9 m/second of 4.9 m/second. Although the ultrasonography revealed the femoral artery dimension of 1.6 mm was too small, the right common carotid artery dimension of 2.7 mm seemed appropriate for inserting a 4 Fr sheath. We also ensured the patency of the anterior communicating artery, which could maintain the blood perfusion even if the right common carotid artery was occluded. Because both the distances between the point of insertion of a sheath and the aortic valve and between the aortic valve and apex of the left ventricle were extremely short, it was impossible to insert the entire length of the sheath. The length of the sheath that could be inserted was estimated to be at most 25 mm when the balloon of 20 mm in length was used (Fig 1), which was shortest balloon available in our institution. Written informed consent was obtained from parents.

Figure 1. A simulative schema of balloon aortic valvuloplasty using ultrasound finding. The image of the balloon was put on this ultrasound image to know the length of sheath which can be inserted into the artery. aAo = Ascending aorta; AoV = Aortic valve; RCCA = right common carotid artery.
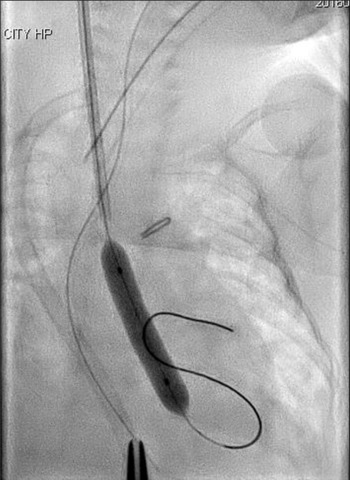
A silkworm-gut was tied on the sheath 25 mm from the tip of the sheath to prevent deep insertion, a semicircular incision was made on the common carotid artery, and a 4 Fr sheath (Terumo, Tokyo, Japan) was inserted. A 3 Fr SIM multipurpose 45° catheter (Fukuda, Tokyo, Japan) was passed through the sheath and introduced. An ascending aortography revealed an aortic valve diameter of 4.9 mm. A 4 × 20 mm TMP-Ped balloon catheter (Tokai Medical Products, Aichi, Japan) with the advantage of a short shoulder length was chosen. A 0.014′-inch Chikai guidewire (Fukuda, Tokyo, Japan) was maneuvered into the left ventricle, and the balloon catheter was positioned across the aortic valve and hand inflated immediately (Fig 2). The pressure of the ascending aorta was 55/30 mmHg, and the pull-back pressure gradient between the left ventricle and aorta was 22 mmHg. The haemodynamic condition improved significantly, and the body edema disappeared soon after this procedure. Echocardiography performed 2 days later showed no aortic valve insufficiency and a good left ventricle ejection fraction of 75%, and ultrasonography image demonstrated that there was no thrombus formation inside the right common carotid artery.
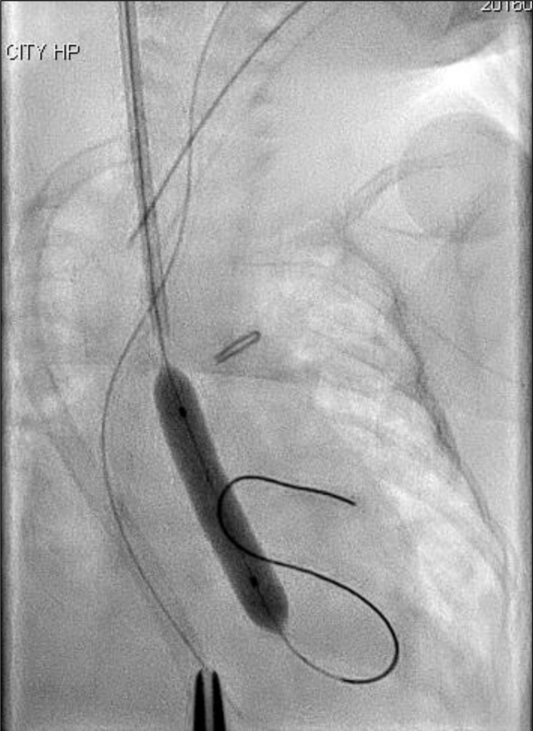
Figure 2. Balloon aortic valvuloplasty using a 4 × 20 mm TMP-Ped balloon catheter.
The second balloon aortic valvuloplasty was performed using an antegrade approach through the femoral artery when the patient was 11 months old and weighed 3.2 kg. A 7 × 20 mm Sterling balloon catheter (Boston Scientific, MA, USA) was used for an aortic valve of 7.4 mm. The pull-back pressure gradient between the left ventricle and aorta after balloon aortic valvuloplasty decreased from 89 to 35 mmHg. The patency of the right common carotid artery was confirmed by angiography without narrowing. This patient was free from any further surgical and catheter interventions for 4 years.
Discussions
Even though there have already been many reports indicating that balloon aortic valvuloplasty is the first-line treatment for critical aortic stenosis in neonates, it includes a much higher risk and difficulty for low-birth-weight infants. Although there are several reports of the balloon aortic valvuloplasty for low-birth-weight infants, all of them were done for the baby weighing 1.1–1.5 kg Reference Akin, Aykan and Karagoz1–Reference Polat4 except for one report of an 890 g infant. Reference Maschietto, Vida and Milanesi5 This was the smallest case of balloon aortic valvuloplasty using transapical approach. Reference Maschietto, Vida and Milanesi5 Therefore, our case is the smallest one which was done by balloon aortic valvuloplasty, a less invasive procedure.
The antegrade approach from the umbilical vein or the femoral vein is an alternative choice for neonates. And a transapical approach is another option of the antegrade approach. A guidewire may be passed through the severe stenotic pinhole aortic valve easier using an antegrade rather than retrograde approach. Reference Akin, Aykan and Karagoz1 However, this is not preferable for extremely-low-birth-weight infants because the success rate of the antegrade approach from the vein is low if the left ventricle volume is limited for manipulating the guide wire or catheter. Although there is a report that emphasises the transapical approach as a good choice for extremely-low-birth-weight infants, Reference Maschietto, Vida and Milanesi5 the transapical approach by medial sternotomy or thoracotomy is deemed too invasive and risky, and withdrawal may be required often because of bradycardia and hypotension. Reference Akin, Aykan and Karagoz1
The retrograde approach from the femoral artery, carotid artery, and ascending aorta can be used. Reference Akin, Aykan and Karagoz1,Reference Justino and Petit3,Reference Polat4 An approach from the ascending aorta allows only a shallow insertion of a sheath in extremely-low-birth-weight infants and there is the potential for the accidental removal of the sheath. Kimura et al overcame this problem by combining a special stainless tip with a sheath. Reference Kimura, Misaki and Kato2 However, it is preferable to avoid median sternotomy, especially for extremely-low-birth-weight infants, as much as possible. In addition, combining a special stainless tip by hand is complicated.
Another retrograde approach of femoral arterial catheterisation has a high risk of concomitant femoral injury, especially in small infants. Reference Akin, Aykan and Karagoz1,Reference Justino and Petit3 Recent data suggested that a weight of less than 4 kg poses an added risk of thrombosis. Reference Glatz, Shah and McCarthy6 And the rate of the acute arterial occlusion after femoral arterial catheterisation correlated inversely with patient weight. Reference Glatz, Shah and McCarthy6 Additionally, femoral artery occlusion is a major disadvantage especially for critical aortic stenosis neonates because they have a higher possibility of another balloon aortic valvuloplasty in the future.
Considering the neonatal carotid artery is larger in diameter than the femoral artery, Reference Gasparella, Milanesi and Biffanti7 carotid may play an important role to prevent the complications of femoral artery occlusion. Reference Justino and Petit3 And our ultrasonography image revealed that the carotid artery was larger in diameter than the femoral artery even in an extremely-low-birth-weight infant. The patency of the carotid artery after neonatal surgical cut-down is well reserved in infants. Furthermore, it has been reported that blood flow in the hemispheres remains symmetrical and does not result in neurological problems even after complete ligation of the carotid artery during extracorporeal membrane oxygenation. Reference Trittenwein, Plenk and Mach8 Balloon aortic valvuloplasty via a carotid artery should be considered as a first-line treatment for extremely-low-birth-weight infants.
Conclusion
We succeeded in performing balloon aortic valvuloplasty in an infant weighing 890 g. The transcarotid balloon aortic valvuloplasty was safe and feasible even for an extremely-low-birth-weight infant.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.