The elderly in the Chinese population has increased remarkably in the past three decades, a blessing that comes with an increased public health burden as aging coincides with an increase in comorbid chronic disorders. With the unprecedented increase in average life expectancy, researchers have sought to establish the mechanism of healthy aging, with special attention given to traits that contribute to quality of life, such as pulmonary function and muscle strength. Forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), handgrip, and five-times-sit-to-stand test (FTSST) are inexpensive, easily measurable, and preferred indicators of pulmonary function and muscle strength. FEV1, a trait that measures airflow, and FVC, a trait that measures lung volume, are the two most important and standard indicators of pulmonary function. Handgrip and FTSST are representatives of skeletal muscle strength, which is known to be associated with muscular functioning in other muscle groups and with activities of daily living (ADL). A person's ability or inability to perform ADLs is the most used measure of functional status, particularly in people with chronic disabilities and the elderly (Rantanen et al., Reference Rantanen, Avlund, Suominen, Schroll, Frandin and Pertti2002).
Aging is accompanied by a progressive decline in FEV1, FVC, and handgrip, and an increase in FTSST. Declining pulmonary function and muscle strength late in life is a major problem that severely hampers independent living and thus quality of life. Previous studies have shown that poor pulmonary function and muscle strength (Rantanen et al., Reference Rantanen, Avlund, Suominen, Schroll, Frandin and Pertti2002) is a risk for disability, morbidity, and mortality in elderly people.
In the past decades, the classical twin method has been applied to estimate the genetic and environmental contributions to phenotypes of pulmonary function and muscle strength in Western countries, including FEV1, FVC, handgrip, and FTSST. The heritability estimates of FEV1 and FVC were 32–67% and 26–56% (Ghio et al., Reference Ghio, Crapo, Elliott, Adams, Hunt, Jensen and Afman1989; Givelber et al., Reference Givelber, Couropmitree, Gottlieb, Evans, Levy, Myers and O'Connor1998; Hukkinen et al., Reference Hukkinen, Kaprio, Broms, Viljanen, Kotz, Rantanen and Korhonen2011; Ingebrigtsen et al., Reference Ingebrigtsen, Thomsen, van der Sluis, Miller, Christensen, Sigsgaard and Backer2011; Klimentidis et al., Reference Klimentidis, Vazquez, de Los Campos, Allison, Dransfield and Thannickal2013; McClearn et al., Reference McClearn, Svartengren, Pedersen, Heller and Plomin1994; Redline et al., Reference Redline, Tishler, Rosner, Lewitter, Vandenburgh, Weiss and Speizer1989; Wilk et al., Reference Wilk, Djousse, Arnett, Rich, Province, Hunt and Myers2000; Xu et al., Reference Xu, Yang, Chen, Wang, Jin, Fang and Weiss1999), respectively in adult twins, with environmental factors explaining a modest part of the variance in most of the studies.
Genetic factors accounted for 57% of the variance in repeated chair stands performance of the lower extremity strength among elderly male twins (Carmelli et al., Reference Carmelli, Kelly-Hayes, Wolf, Swan, Jack, Reed and Guralnik2000) and for 22–52% of the total variance in handgrip strength (Arden & Spector, Reference Arden and Spector1997; Carmelli & Reed, Reference Carmelli and Reed2000; Frederiksen et al., Reference Frederiksen, Gaist, Petersen, Hjelmborg, McGue, Vaupel and Christensen2002; Reed et al., Reference Reed, Fabsitz, Selby and Carmelli1991). However, a multivariate genetic analysis showed a much more modest additive genetic effect (14%) in older female twins aged 63–76 years (Tiainen et al., Reference Tiainen, Sipila, Alen, Heikkinen, Kaprio, Koskenvuo and Rantanen2004). Univariate analyses in this study even suggested that the CE model showed the best fit for handgrip strength, suggesting no genetic effect on handgrip.
In contrast to Western countries, far less is known about the genetic and environmental influences on aging-related indicators of pulmonary function and muscle strength in the rapidly aging Chinese population. This article reports results from the first wave of data on pulmonary function and muscle strength in Chinese middle- and old-aged twins as part of a comprehensive investigation on genetic and environmental regulation on aging indices or traits.
Methods
Study Participants
Sample collection was conducted through the Qingdao Twin Registry (QTR), the first twin registry in China (Li et al., Reference Li, Gao, Lv, Cao, Zhan, Yang and Hu2006; Li et al., Reference Li, Gao, Yu, Lv, Cao, Zhan and Hu2013) established at Qingdao Center for Disease Control and Prevention (Qingdao CDC; Duan et al., Reference Duan, Ning, Zhang, Wang, Zhang, Tan and Pang2013; Pang et al., Reference Pang, Ning, Unger, Johnson, Wang, Guo and Lee2006). We recruited the twin samples through QTR, local CDC, community, and media publicity from 2012 to 2013. Twins unable or unwilling to cooperate, suffering from pulmonary and skeletal muscle disease, professional athletes, and incomplete twin pairs were excluded from sampling. We included a total of 379 complete adult twin pairs, including 240 monozygotic (MZ) twins (114 MZ males [MZM], 126 MZ females [MZF]) and 139 dizygotic (DZ) twins (41 DZ males [DZM], 39 DZ females [DZF], 59 opposite-sex DZ [OSDZ]) aged 51.8 ± 7.6 years (40–80 years old). The participants included came from all social classes and from different occupations, so they did not represent a specific socio-economic status. Zygosity was identified using DNA markers with 99.9% correct assignment (Becker et al., Reference Becker, Busjahn, Faulhaber, Bahring, Robertson, Schuster and Luft1997; Tomsey et al., Reference Tomsey, Kurtz, Kist, Hockensmith and Call2001) at a laboratory at the Qingdao Blood Center.
Institutional Review Boards of Qingdao CDC approved the study. We conducted the study in accordance with the ethical principles of the Helsinki Declaration. All volunteer twin participants gave the informed consent and then completed the questionnaire and physical examination at Qingdao CDC.
Measurements of Physical Function
FEV1 and FVC (liters) were measured by the electronic hand-held spirometer (Micro 0102) to assess pulmonary function. Every participant performed two maneuvers while standing, according to the standard procedure of spirometry. Duplicate tests were made and the best trial data were used for analysis. Handgrip strength was measured in kilograms by using an electronic dynamometer (WCS-100, Nantong, China). Participants were asked to squeeze the handle as hard as possible in a standing position with the arm straight down. Handgrip strength was assessed as the maximum of six attempts with three tests on each hand. The FTSST (seconds) was adopted to measure the lower limb strength. The participants were asked to fold their arms across the chest and rise from the chair and then sit down fully as fast as they could without pause. The time spent to complete the test was recorded using a stop watch.
The spirometer, dynamometer, and stop watch were calibrated every morning before measurement.
Statistical Analysis
Epidata3.1 (www.epidata.dk) was used to input and correct the data. Because the distributions of four variables were skewed, the Box-Cox transformation was applied to ensure normal or approximately normal distribution. The Box-Cox transformation was done using the free R package car (http://cran.r-project.org/web/packages/car/index.html). In the model fitting, age, sex, and age*sex were included as the covariates to adjust for their effects on pulmonary function and muscle strength. Because smokers and non-smokers did not differ significantly on pulmonary function, data were not corrected for smoking status (Finkel et al., Reference Finkel, Reynolds, Emery and Pedersen2013). Twin correlation was measured by calculating the intra-class coefficients (ICCs) and compared between MZ and DZ. ICCs were estimated using twinlm in the R package mets (http://cran.r-project.org/web/packages/mets/index.html). Phenotypic correlation among the four indicators was assessed by cross-twin, cross-trait correlation (CTCTC) calculated using R package car (http://cran.r-project.org/web/packages/car/index.html).
Twin Modeling
We fitted classical univariate twin models to decompose the total variance of every indicator of pulmonary function and muscle strength into the components of additive genetic (A), common environmental (C), dominant genetic (D), and unique environmental (E) variances by constructing the full ACE model or the ADE model. The ADE model was only taken into account when the ICC of MZ twins was more than twice as large as that of DZ twins. The E term includes any measurement error and can therefore never be zero. Following the parsimony principle, nested models, such as AE, CE, DE, E were fitted by dropping the relevant variance components. Statistical significance of nested models was tested by a likelihood ratio test and we chose the best-fitting model based on the parsimony principle and the Akaike's Information Criterion (AIC; Akaike, Reference Akaike1987). In addition to the univariate twin modeling described above, we also fitted bivariate twin modeling to assess the genetic and environmental correlations between indicators of pulmonary function and muscle strength. The CTCTC was estimated before fitting the bivariate model and compared across zygosity to provide indication of genetic mediation between the two traits of interest. Twin models were fitted by R package mets for the univariate analysis and Mx (http://www.vcu.edu/mx) for the bivariate analysis. The level of statistical significance was set at p >.05.
Results
In Table 1, we show the descriptive statistics together with results from fitting linear models to each measurement for the effects of age, sex, and age*sex. We found that age had a significant effect on the four phenotypes. There was a strong pattern of decline in FEV1, FVC, and handgrip whereas FTSST increased with age, as indicated by the highly significant negative and positive regression coefficients, respectively. For sex, we found lower FEV1, FVC, and handgrip in females than in males (males coded 1 and females 2). The FTSST did not differ between females and males. There were significant age–gender interactions in favor of females, meaning that females outperformed males in FEV1 and handgrip. Table 1 also has ICCs for all measurements for MZ and DZ twins estimated separately. Overall, MZ twins had significantly higher ICCs than that for DZ twins for all items (p >.05) except FVC. This provided a first indication of genetic influences on FEV1, handgrip, and FTSST.
TABLE 1 Descriptive Statistics and Linear Regression for Covariates

Note: males coded 1 and females coded 2. *p >.05.
Because all the ICCs in MZ were less than twice those in DZ, we fitted the full ACE and its nested models (AE, CE, E) to each of the four indicators of pulmonary function and muscle strength. We analyzed the variance components and heritability estimates after adjustment for all covariates. The most parsimonious model for FEV1, handgrip, and FTSST was the AE model, while FVC had the CE model as its best-fitting model (Table 2). There were significant additive genetic influences on FEV1, handgrip, and FTSST. In the AE models, moderate heritability (55–60%) was estimated with 95% CIs well beyond zero. In the CE model for FVC, the common environment contribution was about 50%. All the four phenotypes appeared to be similar with regard to the magnitude of effects of the unique environmental factors (40–50%).
TABLE 2 The Full and the Best-Fitting Models for Pulmonary Function and Muscle Strength Measurements Adjusted for Age, Sex, and Age*Sex

Note: A = Additive genetic influence, C = Shared environmental influence, E = Unique/non-shared environment influence, -2LL = twice the negative log-likelihood; df = degrees of freedom, χ2 = difference of χ2 value, Δdf = change in degree of freedom; p = χ2 test in model fitting; AIC= Akaike's information criterion, AE model is nested within the ACE model, the ACE model is the fully saturated model. The best-fitting models (shown in bold type) were chosen on the basis of a change in χ2 not representing a significant worsening of fit (for a change of df of 1, the statistically significant change in χ2 is 3.84).
We found that FEV1, FVC, and handgrip had significant differences in CTCTC between MZ and DZ twins (Table 3). CTCTCs were higher in MZ than in DZ twins except between handgrip and FTSST, implying potential genetic involvement in the correlations of pulmonary function and muscle strength. So, we additionally fitted the bivariate twin models to estimate the genetic and environmental correlations for the indicators of pulmonary function with muscle strength. Similar to the univariate analysis, both full and nested models were fitted for best-fitting model selection, with the nested models fitted by dropping the genetic and environmental components in the covariance between every combination of these four indices. In Table 4, we show the estimated genetic and environmental correlations in the full and best-fitting models for the combinations of pulmonary function with muscle strength. The correlations between FEV1 and FVC were best fitted by the ACE model. FEV1, FVC, as well as handgrip and FTSST had the AE model as the best-fitting model. FEV1, as well as FVC and handgrip, had the CE model as the best-fitting model. The genetic correlation between FEV1 and FVC was r G = 1.00 [95% CI: 0.87, 1.00], the moderately negative genetic correlation between FEV1 and FTSST was r G = −0.33, [95% CI: −0.68, −0.14], between FVC and FTSST was r G = −0.42 [95% CI: −1.00, −0.13]. We found no significant genetic correlation between handgrip and pulmonary function, or FTSST. FEV1 and FVC also presented a high positive common environmental correlation, with r C = 1.00 [95% CI: 0.74, 1.00], and between FEV1 and handgrip, r C = 1.00 [95% CI: 0.31, 1.00], and moderately common environmental correlation between FVC and handgrip, with r C = 0.44 [95% CI: 0.17, 1.00]. Furthermore, FEV1 and FVC showed high unique environmental correlation, with r E = 0.76 [95% CI: 0.71, 0.81], mild unique environmental correlation between handgrip and FEV1, r E = 0.17 [95% CI: 0.06, 0.28], and FVC, r E = 0.14 [95% CI: 0.03, 0.25], as well as FTSST, r E = −0.13, [95% CI: −0.25, −0.01], with positive or negative directions, respectively. The estimates on common and the unique environmental correlations for the other combinations were all zero.
TABLE 3 Phenotypic Correlations (Cross-Twin, Cross-Trait Correlation) Between Pulmonary Function and Muscle Strength
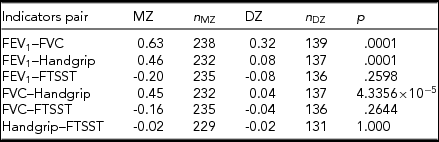
TABLE 4 Estimated Genetic and Environmental Correlations Between Indicators

Note: r G, = genetic correlation between traits; r C = shared environmental correlation between traits; r E = non-shared environmental correlation between traits.
*p > .05.
Discussion
We have conducted the first twin study on aging-related indicators of pulmonary function and muscle strength in a population-based sample of healthy adult Chinese twins. The current study demonstrated that there was strong evidence of genetic influence on FEV1 and indices of muscle strength in middle- and old-aged twins. Our analysis obtained a heritability of more than 50% for FEV1, handgrip, and FTSST. For FVC shared and unique environmental factors, both explained the same proportion of total variance of 50%.
The results indicated that about 55% of the variance in the level of FEV1 in a middle- and old-aged Chinese population may be explained by genetic factors. However, no significantly genetic contribution of FVC was estimated. Pulmonary function has been found previously to have a heritable basis in Western countries. Our estimates of heritability were similar to previous studies (Klimentidis et al., Reference Klimentidis, Vazquez, de Los Campos, Allison, Dransfield and Thannickal2013) but the estimate of genetic component of FVC was different from the 26% found by Ingebritsen et al. (Reference Ingebrigtsen, Thomsen, van der Sluis, Miller, Christensen, Sigsgaard and Backer2011). The reason may lie in the different social, cultural, and environmental factors between China and Western countries or the relatively small sample size in the present study. Further investigation of genetic mechanisms with respect to pulmonary function is needed.
Our results indicated a similar heritability of handgrip strength as that of Frederiksen et al. (Reference Frederiksen, Gaist, Petersen, Hjelmborg, McGue, Vaupel and Christensen2002) of 52% but higher than that of Arden and Spector (Reference Arden and Spector1997) of 30%. It has been observed previously that the relative contributions of genetic and environmental effects to handgrip strength may change over the years. For example, in a study performed by Carmelli and Reed (Reference Carmelli and Reed2000), the heritability of handgrip strength decreased from 35% to 22% during 10 years follow-up, whereas the shared environmental component increased from 39% to 45% in male twins aged 63 years old on average at the baseline. We considered that the relatively high heritability of handgrip in the present study could be due to the relatively younger participants compared with those of Western studies. The current study estimated a genetic contribution to FTSST, which was comparable to 57% reported by Carmelli et al. (Reference Carmelli, Kelly-Hayes, Wolf, Swan, Jack, Reed and Guralnik2000).
The same level of genetic (55–60%) and unique environmental (40–45%) contributions were found for FEV1, handgrip, and FTSST. Approximately equal contribution of genetic (58–60%) and unique environmental factors (40–42%) was estimated between handgrip and FTSST, the two indicators of muscle strength. We were surprised to see that the non-shared environmental effects on all four phenotypes of pulmonary function and muscle strength were also quite similar (40–50%) in the univariate analysis of the present study. Environmental effects were primarily dominated by unique and stochastic effects rather than to common exposures of both twins. Measurement error would have inflated the relative contribution of the non-shared environmental effects. Only FVC presented a significant common and unique environmental influence, which exerted approximately equal impact on FVC according to the estimates. No role of the shared environment at all was found for FEV1, handgrip, and FTSST.
The genetic correlation expresses the extent to which two measurements of pulmonary function and muscle strength reflect the same set of genes. Study of the genetic correlation can facilitate exploration of pleiotropic genetic variants. The present study showed that FEV1, FVC, and FTSST may share the same or similar sets of genes but with a positive or negative direction. The bivariate genetic analysis showed that the significantly genetic correlation between FTSST and FEV1 as well as FVC was moderate but in the opposite direction, which was in line with the corresponding CTCTC estimate, suggesting that the pulmonary function and FTSST may share a similar genetic basis that moderately regulated the elevated FEV1, FVC, and decreased FTSST at the same time and vice versa. As the decreased FTSST meant enhanced lower limb strength, we thought that the underlying genes may have positive pleiotropic effects on both pulmonary function and lower limb strength. We found no significant genetic correlation between handgrip and the three other indicators, implying handgrip may be dominated by its own genetic factors. Interestingly, we found that FEV1, FVC, and handgrip may share the same or similar common environmental factors. In contrast to the genetic correlation, we observed that handgrip and the other three indicators presented mild unique environmental correlations, indicating that handgrip, FEV1, and FVC, as well as FTSST, may share similar unique environmental factors.
Improved knowledge of the genetic basis of pulmonary function and muscle strength could lead to a better understanding of the genetic architecture of these traits. Recent genome-wide association studies have identified several loci that are associated with pulmonary function and are biologically plausible candidates (Tang et al., Reference Tang, Kowgier, Loth, Soler Artigas, Joubert, Hodge and Cassano2014). A polymorphism of the IGF2 gene has been observed to have an effect on adult handgrip strength among men (Sayer et al., Reference Sayer, Syddall, O'Dell, Chen, Briggs, Briggs and Cooper2002). The results of the present study implied that pulmonary function and lower limb strength may be regulated by the same or a common set of genes. However, the specific genes regulating these indicators remained to be defined by molecular genetic studies.
A strength of our study was the evaluation of four representative aspects of aging-related pulmonary function and muscle strength at the same time. However, it is necessary to point out the limitations of our study. Compared with published studies from Western countries, the sample size was relatively small, which resulted in less reliable heritability estimates of phenotypes of pulmonary function and muscle strength. Another limitation is the relatively young age of our twin samples and the large span of age covered (40 years). Although age was adjusted in the twin modeling, the non-linear pattern, if any, of age-dependent change in pulmonary function and muscle strength could have been averaged out and could also serve to reduce power of the study.
Acknowledgments
This study was supported by National Natural Science Foundation of China (31371024). All the authors contributed significantly to the study. X.T., C.X., Y.W., J.S., H.D.: sample collection, conduct of the study, drafting the manuscript; D.Z., B.J., Z.P.; design of the study, data preparation; S.L., Q.T.: data analysis strategy, interpretation, manuscript revision.
Conflict of Interest
None.






