West Nile virus (WNV) is a zoonotic flavivirus borne by mosquitoes (genus Culex) which often causes an asymptomatic infection, and a febrile illness in 20% of affected patients with varying degrees of fatigue, headache, myalgia, and arthralgia. Reference Mostashari, Bunning and Kitsutani1 Severe illness, such as meningitis or encephalitis, occurs in less than 1% of cases, Reference Mostashari, Bunning and Kitsutani1 with an increased risk in older, diabetic, or immunocompromised patients. Reference Nash, Mostashari and Fine2 WNV is associated with ocular manifestations, of which chorioretinitis is the most common, especially in patients with neuroinvasive disease. Reference Khairallah, Ben Yahia and Ladjimi3,Reference Hasbun, Garcia and Kellaway4 Herein, we describe three patients who presented with WNV ocular involvement in Montreal, Canada during the 2018 outbreak where 49 people were infected. 5
Our goal is to bring attention to the pathognomonic ocular findings of WNV. Neurologists should be aware that WNV causes specific ocular manifestations and thus, if WNV is suspected in patients, ophthalmology consultations should be requested early for fundoscopy, which can be performed even in an intensive care unit (ICU) setting. Neurology can also specifically request that presence of pathognomonic retinal findings be sought out, that is, linear punched-out chorioretinal lesions extending toward the periphery.
A 62-year-old immunocompetent diabetic Caucasian male was admitted to the ICU for meningoencephalitis (Case 1). Head imaging was non-contributory. He was treated empirically with broad-spectrum antibiotics and acyclovir. Cerebrospinal fluid analysis revealed leukocytosis (99 cells/µL, 59% lymphocytes), elevated glucose (5.7 mmol/L), and elevated protein (2.00 g/L) compatible with aseptic meningitis. Testing was negative for bacterial and viral pathogens with WNV antibodies pending.
An ophthalmology consultation was requested on hospital day 16 for new onset of decreased vision in the left eye (OS). Best-corrected visual acuity (BCVA) was 20/30-1 in the right eye (OD) and 20/50-2 OS with left relative afferent pupillary defect. Dilated fundus examination (DFE) revealed bilateral punched-out, cream-colored chorioretinal lesions (Figure 1A), cystoid macular edema OS, and no evidence of vasculitis (Figure 1(1)). Overall, the clinical picture was highly suspicious of WNV chorioretinitis, which was soon confirmed with positive WNV serum IgM and IgG. Topical prednisolone acetate 1% and nepafenac 0.1% were started OS for the macular edema.

Figure 1: Color fundus photography and fluorescein angiography (FA) of the right eye of Case 1 at (A) presentation, (B) 2 weeks, and (C) 5 months follow-up showing the characteristic linear distribution of chorioretinal lesions extending radially from the optic nerve and following the trajectory of the retinal nerve fibers. (A) Acute lesions are deep, round, cream-colored lesions around 250 µm and exhibit early hypofluorescence (blockage) and late hyper-fluorescence (staining). (B) Subacute lesions are more well-defined with variable central atrophy and a creamy halo; these target lesions showcase central hypofluorescence and a hyperfluorescent peripheral ring on FA. (C) Chronic lesions are atrophic with well-defined borders and variable pigmentation; these are hyperfluorescent throughout the FA showing a window defect and staining. (1) Color fundus photography of the left eye at presentation showing subtle acute lesions. (2) Montage color fundus photography of the left eye at 2 weeks showing occlusive vasculitis with vascular sheathing and intraretinal hemorrhages.
Two weeks later, BCVA OS further deteriorated from 20/50-2 to 20/70 + 2. Repeat DFE revealed better-defined chorioretinal lesions bilaterally (Figure 1B) and a new diffuse occlusive retinal vasculitis OS (Figure 1(2)). Treatment with 40 mg of oral prednisone daily was started, tapering slowly over 2 months. Two months later, the occlusive vasculitis had resolved with BCVA 20/25 + 2 OD and 20/50 + 2 OS, and the chorioretinal lesions were cicatricial (Figure 1C).
A 46-year-old female was admitted to the ICU for meningoencephalitis with severe polyneuropathy (Case 2). Past medical history included systemic lupus erythematosus under immunosuppressants. Cerebrospinal fluid analysis revealed leukocytosis (137 leucocytes/µL, 88% neutrophils), normal glucose (3.0 mmol/L), and elevated protein (1.41 g/L). Testing was negative for bacterial and viral causes with California serogroup viruses pending. Magnetic resonance imaging showed bithalamic T2 and fluid-attenuated inversion recovery signal anomalies including a focal lesion in the left thalamus with diffusion restriction. As per the neuro-radiologist, these bithalamic anomalies were compatible with an encephalitis caused by flaviviruses. WNV IgM came back positive several days later.
An ophthalmology consultation was requested to evaluate for the presence of ocular features of WNV. DFE revealed multifocal cream-colored chorioretinal lesions bilaterally. She received intravenous immunoglobulin and corticosteroids, which proved ineffective. Her condition deteriorated, and after a protracted course, the patient passed away.
A 41-year-old otherwise healthy woman who noticed decreased vision and floaters OS was found to have mild-to-moderate inflammation and superior chorioretinal lesions with intraretinal hemorrhages and vascular sheathing OS (Case 3). She reported a 2-week history of fever, malaise, and night sweats, followed 1 week later by a maculopapular rash. Prednisolone acetate 1% drops every hour were initiated and tapered over 4 weeks to four times daily. The patient improved and was evaluated at an academic uveitis clinic 6 weeks later. BCVA was 20/30 OD and 20/25 OS. There was limited anterior chamber inflammation bilaterally. DFE OD was unremarkable, while DFE OS revealed mild vitritis and multiple deep, yellow-white outer retinal lesions (Figure 2). Positive results for WNV IgM, WNV IgG, and plaque reduction neutralization testing confirmed WNV, while the remaining posterior uveitis workup was negative.
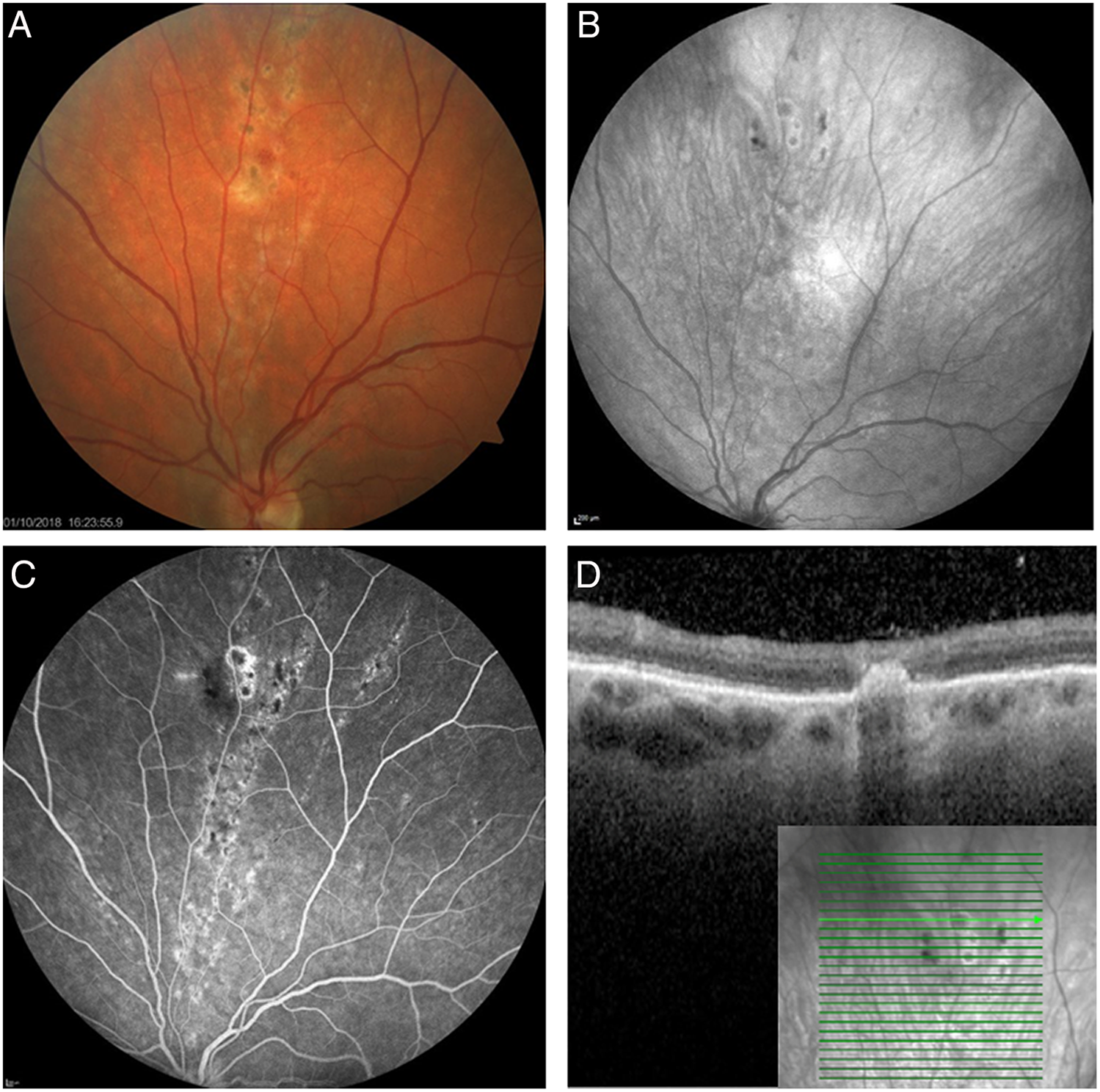
Figure 2: Ancillary testing of typical chorioretinal lesions from Case 3: (A) color fundus photography showing the punched-out, round lesions with a creamy halo that extend in a linear distribution along the path of retinal nerve fibers toward the periphery; (B) fundus autofluorescence showing subtle central hypofluorescence of lesions; (C) fluorescein angiography at 5 minutes showing peripheral hyperfluorescent late staining around hypofluorescent lesions; and (D) spectral domain optical coherence tomography through a lesion showing a hyperreflective focus located in the outer retina and the sub-retinal pigment epithelium which spares inner retinal layers.
As a systemic illness, WNV usually presents with non-specific findings. Reference Mostashari, Bunning and Kitsutani1 It can be difficult to diagnose and requires a high degree of suspicion. Diagnostic delay while awaiting confirmatory results can lead to unnecessary investigations and treatments. However, ocular findings can help establish the diagnosis. WNV can cause posterior uveitis with chorioretinitis characterized by pathognomonic, linear punched-out lesions extending toward the periphery. Reference Khairallah, Ben Yahia and Ladjimi3,Reference Yahia and Khairallah6 These are found early with an average delay of 10 days between systemic symptoms onset and examination. Reference Khairallah, Ben Yahia and Ladjimi3 In contrast, confirmatory testing for WNV may take up to 3 weeks depending on local laboratory capacities. These lesions follow the retinal nerve fiber layer suggesting WNV enters the eye through contiguous transmission from the central nervous system via the optic nerve. Reference Khairallah, Ben Yahia, Attia, Zaouali, Ladjimi and Messaoud7 They occur in 25% of patients with serology-proven WNV infections and in up to 80% of patients with neurologic manifestations. Reference Khairallah, Ben Yahia and Ladjimi3,Reference Hasbun, Garcia and Kellaway4 Occlusive retinal vasculitis occurs more rarely, with few cases reported in the literature, and can result in permanent visual loss. Reference Garg and Jampol8 WNV chorioretinitis may be asymptomatic and is usually self-limited. Reactivation of WNV-associated ocular involvement after resolution of the systemic disease has been reported. Reference Beardsley and McCannel9 The visual prognosis of WNV chorioretinitis is variable. Cicatricial chorioretinal lesions may produce visual scotomas. Reference Garg and Jampol8 Diagnosing chorioretinitis can also be relevant for rehabilitation as they are associated with dependence in activities of daily living and lower quality of life. Reference Hasbun, Garcia and Kellaway4
In summary, WNV infection can lead to severe neuroinvasive disease and is associated with pathognomonic chorioretinitis. Therefore, patients presenting with neurological manifestations, such as meningoencephalitis and acute flaccid paralysis, in WNV endemic regions or during outbreaks, should undergo a DFE. This may provide valuable diagnostic information as well as early recognition of visually significant pathology and limit further tests or empirical treatments. Finally, WNV patients require a consultation in ophthalmology in the acute setting for diagnosis and care as well as for ongoing monitoring of delayed-onset manifestations and sequelae.
Acknowledgments
The authors would like to acknowledge Mariam T. Ibrahim for her presentation of one of these cases at the Canadian Ophthalmological Society Annual Meeting, 2019.
Statement of Authorship
MH: conception and design of the work, analysis of data, original draft, final approval of manuscript. CB: conception and design of the work, acquisition and analysis of data, critical revision, final approval of manuscript. MB: acquisition and analysis of data, critical revision, final approval of manuscript. KO: analysis of data, critical revision, final approval of manuscript. MJA: conception and design of work, analysis of data, critical revision, final approval of manuscript.
Conflict of Interest
No conflicting relationship exists for any author.




