Introduction
Tuberculosis (TB) causes inestimable suffering and claims millions of lives worldwide. Multidrug-resistant tuberculosis (MDR-TB), defined as resistance to at least two first-line anti-TB drugs (isoniazid and rifampicin), has become a significant threat to global public health [1]. The extent and burden of MDR-TB vary significantly but the burden of disease is highest in resource-poor countries [Reference Ormerod2, Reference Uplekar3]. Patients who have a previous history of TB treatment present a higher risk of developing MDR-TB, indicating the failure of TB control programmes [Reference Engel4, Reference Sharma and Mohan5]. In 2018, an estimated half a million new cases of rifampicin-resistant (RR-TB) and MDR-TB worldwide with a prevalence varying from 3.4% to 18% among new and previously treated cases respectively [1]. Globally, 30 countries accounted for more than 90% of the MDR-TB cases [Reference Falzon6], and more than half originated from Russia, India and China [1]. In Africa, the MDR-TB prevalence was estimated to be 2.5% and 12% among new and re-treatment cases, respectively [1]. Ethiopia is one of the 27 high MDR-TB burden countries and ranks third in the continent with an estimated 2100 annually notified MDR-TB cases [7, 8]. The prevalence of MDR-TB in Ethiopia reported in 2018 was 0.71% among new, and 16% among re-treatment cases [1]. There have been progressive improvements worldwide in the scaling up of MDR-TB case detection and treatment enrolment in recent years [Reference Falzon6]. In Ethiopia, the Programmatic Management of Drug-resistant TB (PMDT) was started in 2009 in response to the first 45 MDR-TB patients identified at St. Peter's TB Specialist hospital [9]. Globally, as of 2018, the World Health Organisation (WHO) has reported that nearly 50% of MDR-TB patients were successfully treated but there were still high rates of mortality and loss of patients to follow-up [1]. Overall, the high death and default rates are generally cited as the major obstacles in achieving better cure rates [1, 10, Reference Glaziou11]. As the death of a patient during TB treatment signifies a failure of the programme, the accurate determination of the cause of death is the first step towards designing appropriate and timely interventions to prevent premature deaths. Identifying and effectively addressing risk factors for death among MDR-TB patients is therefore necessary to improve treatment outcomes. As such information remains limited, this study aimed to identify the rate and predictors of mortality among MDR-TB patients in the Amhara region of Ethiopia through retrospective survey data from September 2010 to December 2017.
Methods
Study design and setting
A retrospective study was conducted in three selected treatment-initiated centre hospitals of the Amhara region, namely the University of Gondar, Borumeda, and Debre-Markos Referral Hospital which together provide clinical services for approximately 90% of MDR-TB patients in the region. All isolates from TB-positive individuals were screened for rifampicin resistance at baseline using the rapid drug susceptibility testing (DST) technique in the GeneXpert system (manufactured by Cepheid, located in California, America) or the line probe assay (LPA) (manufactured by the Hain Lifescience GmbH based in Nehren in the district of Tübingen, Germany). Isolates from all patients with confirmed RR/MDR-TB were tested using the LPA for core second-line drugs before or within 1 week of treatment initiation with the MDR-TB regimen. All patients with isolates resistant to first- and second-line anti-TB drugs were further evaluated using culture and phenotypic DST [7]. All patients with MDR-TB were treated in the hospital setting under medical supervision. Patients requiring treatment at the beginning or any stage of care were admitted to the hospital. The recommended commonly used drugs for the treatment of MDR-TB in Ethiopia were bedaquiline (Bdq), levofloxacin (Lfx), moxifloxacin (Mfx), linezolid (Lzd), clofazimine (Cfz), cycloserine (Cs), amikacin (Am), capreomycin (Cm), delamanid, prothionamide (Pto), pyrazinamide (Z), isoniazid high dose (HHD) and ethambutol (E). Three MDR-TB treatment regimens in Ethiopia are recommended based on patients' clinical and laboratory characteristics. The first is the all-oral longer regimen of Bdq-Lfx-Lzd-Cfz-Cs for 18–20 months; the second is a shorter regimen of Am-Mfx-Pto-Cfz-Z-HHD-E/5 Mfx-Cfz-Z-E for 9–12 months. The last is an individualised regimen comprising at least 4–5 likely effective drugs based on their availability, the susceptibility of the isolate, and clinical expert decision for 18–24 months. This regimen is tailored to the specific case scenario of each patient.
Data collection and variables of the study
Time to death was the response variable; explanatory variables were socio-demographic characteristics (sex, age, residence, marital status, occupation and educational status), behavioural factors (smoking and alcohol drinking), clinical characteristics (baseline body mass index (BMI), HIV co-infection, MDR-TB category, co-morbidities, lung complications, radiological findings, time of sputum culture conversion, number of previous TB treatments, TB type and functional status).
Death was defined as a documented death of a patient while on MDR-TB treatment. Patients who were cured, treatment completed, treatment failed, lost to follow-up, transferred out and on treatment at the end of the study, were considered as censored. A new TB case was defined as a patient who had no prior anti-TB treatment or had been treated for less than 1 month. A previously treated case was one who had been treated for TB for more than 1 month, other than for chemoprophylaxis. BMI was defined as low if <18.5 kg/m2 and normal if ⩾18.5 kg/m2. The ability to self-care was a patient who was ambulant and able to perform daily activities; patients unable to meet these criteria were categorised as lacking self-care.
Sputum culture conversion was evidenced by two consecutive TB- negative cultures sampled at least 30 days apart; the date of the first sample was taken as the date of conversion. Two consecutive positive cultures, taken at least 30 days apart, represented ‘culture reversion’ [7]. WHO clinical stages 3 and 4 were considered as a marker of advanced severe TB or advanced HIV [12].
Patients and the public were not involved in the design and conception of the study and it was not possible to communicate the results to patients owing to the retrospective nature of the study.
Data collection and analysis
Data were collected using a data extraction check-list. This was prepared in English after reviewing the treatment registration logbook and patient follow-up charts, as well as computer databases. After checks for consistency and completeness, data were entered into Epi-data version 3.1 and exported to Stata version 14.0 for analysis. Summary statistics were used to describe demographic, behavioural and clinical data. The incidence rate was calculated by dividing the number of total deaths by the total person-years of the follow-ups. The life table and Kaplan–Meier curves were used to estimate the overall survival time and supplemented by a log-rank test to compare the survival curves of different exposure groups. Model comparison was performed using the smallest values of the Akaike information criterion and Bayesian information criterion. Both bi-variable and multivariable Weibull regression with the gamma frailty model was fitted to identify predictors of mortality. Variables with a P-value <0.2 in the bi-variable analysis were fitted to the multivariable analysis, and variables <0.05 in the latter analysis were considered statistically significant.
Results
Baseline socio-demographic and behavioural characteristics
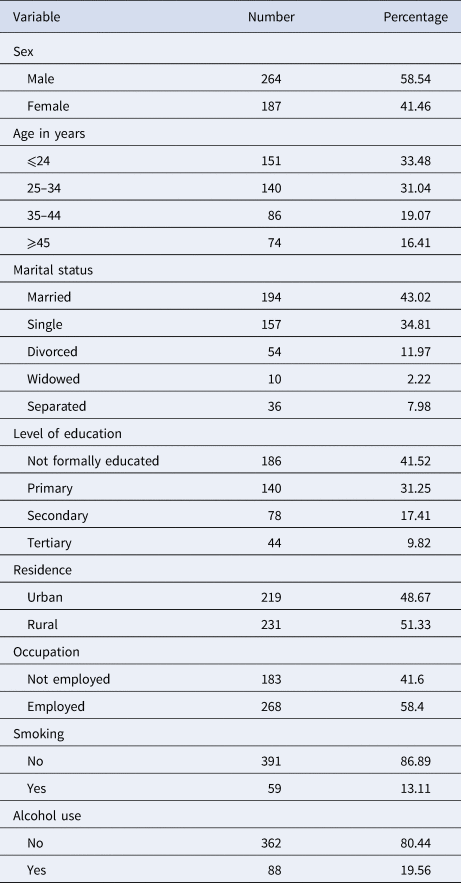
A total of 451 patient records from the three referral hospitals were eligible for analysis. Of these, 58.54% were males; the mean patient age was 31.29 (±12.02) years, 80.49% were Orthodox Christians followed by Muslims (18.18%). The great majority (94.24%) were from Amhara Regional State most of whom (51.22%) were rural dwellers (Table 1).
Table 1. Baseline socio-demographic and behavioural characteristics of MDR-TB patients in the Amhara region, September 2010–January 2017
Baseline clinical characteristics
The majority (92%) of patients presented with pulmonary TB. Most (72%) of the participants had a baseline BMI of <18.5 kg/m2 94% of patients had a history of prior TB treatment, most of whom (62.3%) had received first-line anti-TB drugs on more than one occasion. One-hundred and eighteen of 451 patients (26.2%) were HIV positive, with a median CD4 count of 179 (interquartile range (IQR): 91, 279), and 63.9% were in WHO clinical stage 3 at the commencement of MDR-TB treatment. Of the total HIV co-infected patients, 108 (91.5%) were on antiretroviral treatment (ART) at the beginning of the MDR-TB treatment. A total of 367 patients had baseline chest X-rays of which 49.9% and 34.1% had evidence of cavitation and infiltration, respectively (Table 2).
Table 2. Clinical characteristics of MDR-TB patients at the University of Gondar Comprehensive Specialised Hospital, September 2010–January 2017
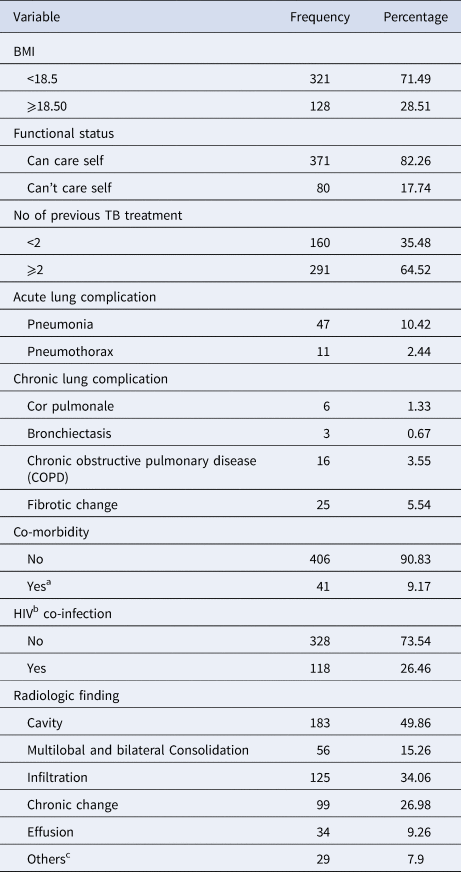
a Diabetes mellitus, hypertension, bronchial asthma, COPD, chronic kidney disease, epilepsy, cardiac diseases, deep venous thrombosis and gangrene, depression, visceral leishmaniasis, toxic goitre, rheumatoid arthritis, cholelithiasis, cervical cancer and glaucoma.
b HIV (human immunodeficiency virus).
c Hilar lymphadenopathy, normal, cardiomegaly, empyema and fungal ball.
Time to death of MDR-TB patients on treatment
Study participants were followed for a total of 7541 person-months (628 person-years), with a median follow-up time of 20 months (IQR: 12, 22). A total of 230 (51%) patients were cured, 22 (4.9%) completed treatment, 46 (10.2%) died, 32 (7.1%) were lost to follow-up, 105 (23.38%) remained on treatment, 12 (2.7%) were transferred out and 4 (0.9%) had treatment failure. The incidence rate of mortality was 7.42 (95% confidence interval (CI) 5.56–9.91) per 100 person-years observation. The cumulative probability of death at the end of 6 months, 1 year, 2 years and 2.5 years were 6.2% (95% CI 4.31–8.9), 9.0% (95% CI 6.65–12.2), 11.1% (95% CI 8.41–14.6) and 14.8% (95% CI 8.74–24.56), respectively (Fig. 1). The mean survival time for patients who couldn't self-care was 14 months, compared with 26 months for those who could self-care at the time of death; the difference was strongly significant (P-value <0.0001) (Fig. 2).
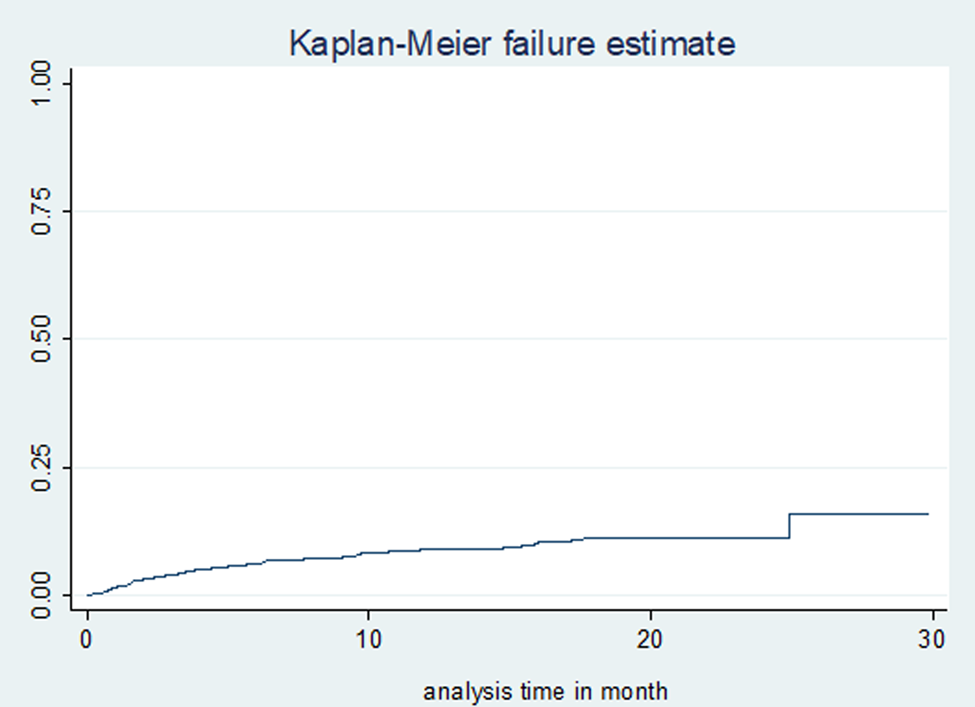
Fig. 1. Kaplan–Meier failure (death) estimates of MDR-TB treatment in Amhara Regional State, September 2010–January 2017.
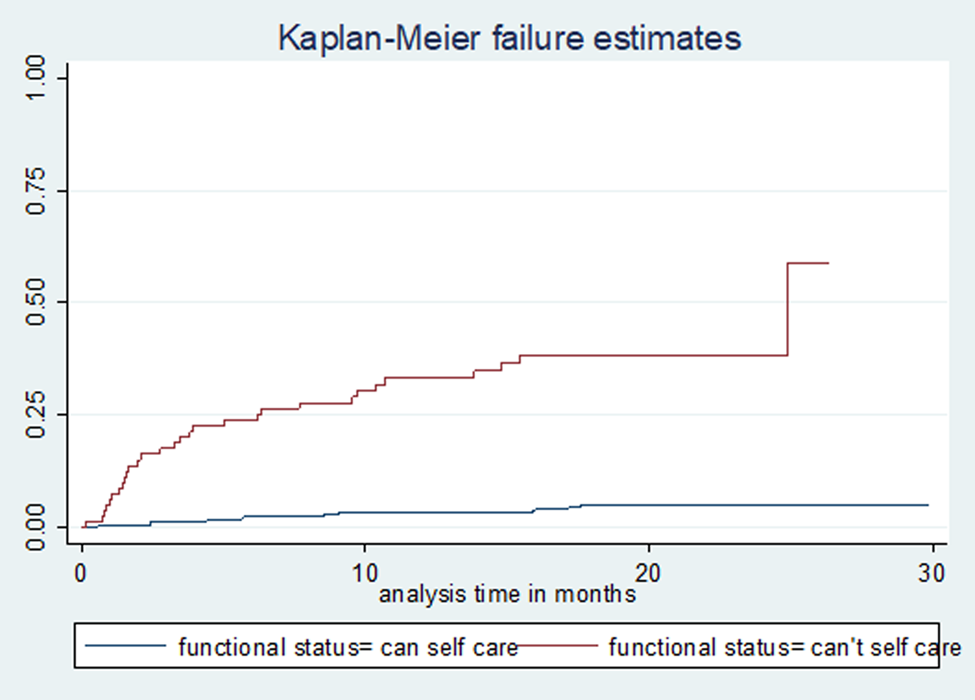
Fig. 2. Kaplan–Meier failure (death) estimate curve by functional status in Amhara Regional State, September 2010–January 2017.
Predictors of mortality
The bi-variable Weibull regression with gamma frailty analysis showed that age, sex, BMI, inability to self-care, acute and chronic lung complications, occupation, functional status, co-morbidity, consolidation and effusion on baseline chest X-ray, were associated with mortality. However, in the multivariable model, advanced age, patients who couldn't self-care, presence of co-morbidity, BMI < 18.5 kg/m2, acute lung complication and multi-lobar and bi-lateral consolidation remained significant predictors of mortality (Table 3).
Table 3. Bi-variable and multivariable Weibull regressions with gamma frailty model for predictors of death among MDR-TB patients at Amhara Regional State, September 2010–January 2017
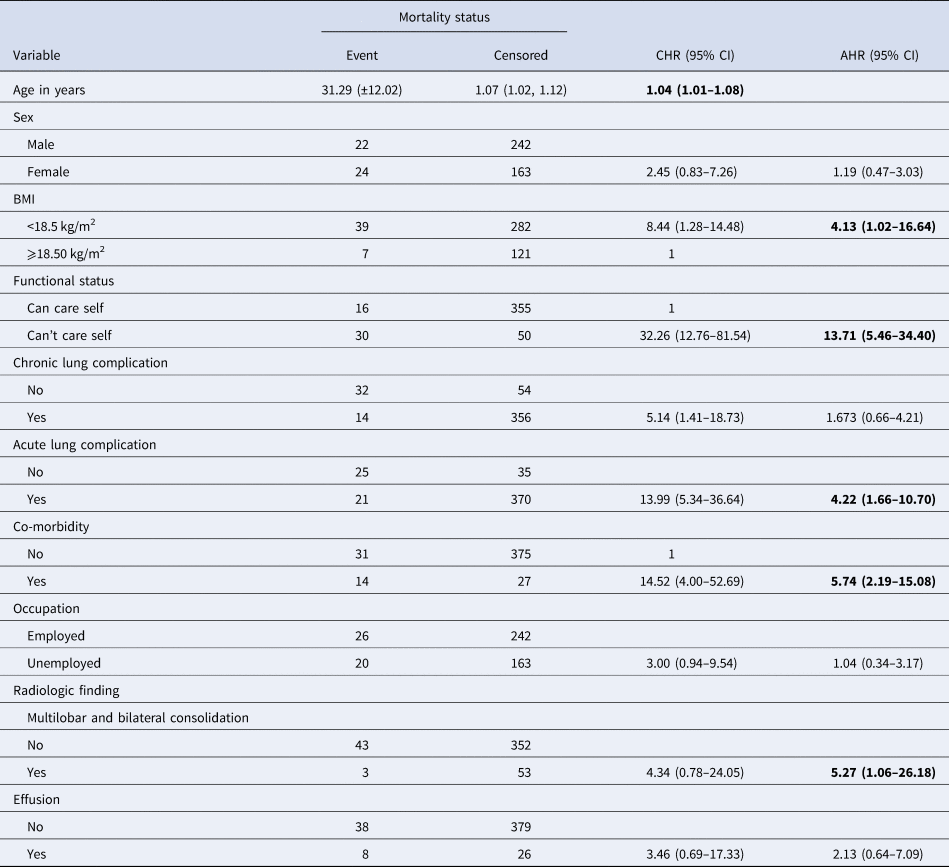
As age increased by 1 year the hazard of death was increased by 4% (adjusted hazard ratio (AHR) = 1.04, 95% CI 1.01–1.08). Patients who couldn't self-care had a 14.71-fold higher hazard of death compared to those who could self-care (AHR = 13.71, 95% CI 5.46–34.40). For patients with co-morbidities, the hazard of death was 5.74-fold higher than those who had no co-morbid factors (AHR = 5.74, 95% CI 2.19–15.08). Similarly, a BMI < 18.5 kg/m2 was associated with a 4.13-fold hazard increase compared to those above 18.5 kg/m2 (AHR 4.13, 95% CI 1.02–16.64); acute lung complications were 4.22-fold higher compared with others (AHR 4.22, 95% CI 1.66–10.70) and those patients with multi-lobar and bilateral consolidation had a 5.27-fold higher hazard of death compared with no consolidation (AHR 5.27, 95% CI 1.06–26.18).
Discussion
In this study, the incidence of mortality was 7.42 (95% CI 5.56–9.91)/100 person-years follow-up during treatment and most patients died early on the initiation of treatment. This finding accords with earlier reports from Ethiopia [Reference Girum, Tariku and Dessu13, Reference Eshetie14] but our incidence was considerably higher than that found in Eastern Europe (1.094/100 person-years) [Reference Balabanova15]. This might be due to the early detection of TB cases in Europe (46%), than in Ethiopia (8.6%). Several factors, no doubt, contribute to the latter statistic and include better health care systems with advanced supportive patient management, higher socio-economic status and fewer co-morbidities such as malnutrition and HIV in Europe. The higher death rate in Ethiopia might also be attributed, in part, to the constitution, and duration, of the current treatment regimen used in Ethiopia. In particular, the use of drugs such as Mfx have reported higher efficacy for TB in Europe compared to the lower efficacy of Lfx, commonly prescribed and used in Ethiopia. Indeed, some evidence suggests that patients treated with Mfx have a better treatment outcome and earlier conversion to negative cultures [Reference Ahmad16, Reference Chien17]. There is no comprehensive DST programme for first- and second-line TB drugs in the Amhara region; consequently, all patients are put on standardised regimens (i.e. constructed for the majority of patients) instead of tailored regimens based on susceptibility test results of individual patients which have been shown to achieve more successful outcomes [Reference Riccardi18]. Moreover, putting MDR-TB patients for more than 20 months on treatment would likely increase severe adverse events which can contribute additionally to the risk of death [Reference Schnippel19]. A recent study in our region showed the median time to commencement of treatment after a diagnosis of MDR-TB was 8 days [Reference Tefera20] which falls short of international standards in developing countries, and so may play a role in the high death rate [Reference Cazabon21]. A number of other factors including older age, inability to self-care, co-morbid conditions, acute lung complications, low BMI and multi-lobar or bilateral lung consolidation also contributed to mortality.
Our finding of an association between increasing age and death has been noted by a number of studies across the world [Reference Balabanova15, Reference Ahmad22–Reference Nair24]. This could be linked to the risk of chronic diseases in the elderly and general physical deterioration along with impaired immunity [Reference Manesen23, Reference Nair24]; the latter has been associated with high mortality among MDR-TB patients [Reference Millet25, Reference Vasankari26]. Other contributory factors may include the reluctance of the elderly to consult healthcare facilities leading to delayed presentation and diagnosis compared with younger patients [Reference Millet25, Reference Vasankari26].
Patients who couldn't care for themselves were at a higher risk of mortality than those who could. This finding was consistent with a study from Russia [Reference Velasquez27] and was not surprising as such patients could be bedridden with other advanced diseases and are also likely to be on several medications which could result in drug interactions and poor compliance with anti-TB medication due to a high pill burden. The severity of TB may also result from infection with highly virulent strains, suboptimal host response and/or extended disease duration culminating in a significantly higher risk of death [Reference Chiang28]. It is widely recognised that the management of MDR-TB in critically sick patients is challenging and compounded by pharmacokinetic issues such as poor gastric absorption, high rates of organ dysfunction and drug toxicity [Reference Heysell29] often necessitating admission to intensive care [Reference Girum, Tariku and Dessu13], which are lacking in our study region. Alcohol abuse has long been associated with poor TB treatment outcomes [Reference Johnston30]. A number of patients who couldn't self-care also consumed alcohol in this study. Furthermore, the majority were co-infected with HIV, had low BMI, increased co-morbidities and clinical complications all of which would accelerate their mortality [Reference Girum, Tariku and Dessu13, Reference Ahmad22]. Medications given for co-morbid conditions may interact with MDR-TB drugs and overlap in toxicity, often leading to poor compliance by patients [Reference Girum, Tariku and Dessu13, Reference Limenih and Workie31]. For example, the management of MDR-TB is similar for both diabetic and non-diabetic patients and it is recognised that diabetes patients have worse TB treatment outcomes due to the induction of poor glucose control by TB itself [Reference Jimenez-Corona32]. Additionally, glucose control drugs such as metformin may induce more pronounced gastrointestinal side-effects when co-administered with the anti-TB agents ethionamide and para-aminosalicylic acid [Reference Scheen and Paquot33].
HIV co-infection is not associated with mortality in our study. The possible explanation might be the integration of TB-HIV service and all of our patients are on ART. Studies also show response to DR-TB treatment did not differ with HIV infection with access to and earlier ART, which resulting in the slow progression of DR-TB and limiting with advanced HIV [Reference Manesen23, Reference Seifert34].
Patients with acute lung complications had a higher risk of death as a consequence of infection of the lower respiratory tract leading to the accumulation of inflammatory exudate in the alveoli resulting in reduced oxygen exchange and respiratory insufficiency. MDR-TB patients with clinical complications also experience longer recovery times and poor response to TB-medications [Reference Limenih and Workie31, Reference Zhang35]. The relationship between a low BMI and risk of death has been noted in several studies worldwide [Reference Nair24, Reference Chiang28, Reference Seifert34, Reference Tang36–Reference Benova38] and has been strongly linked to poor nutritional status, which was evident in over 70% of our patient cohort. To address this issue all patients in Amhara region received food packages obtained from the global fund but they are not very adequate. Economical support and early nutritional intervention has been shown to reduce the mortality of MDR-TB patients [Reference Chiang28, Reference Wai39], and in resource-limited settings, profound undernutrition and gastrointestinal toxicities have been noted during MDR-TB treatment [Reference Meressa37]. Malnutrition is also a predisposing factor for impaired host immunity to TB. Such patients might also lack essential micronutrients rendering them more susceptible to life-threatening diseases [Reference Nair24, Reference Seifert34]. It follows that weight gain is a useful interim marker for favourable TB treatment outcomes [Reference Benova38].
We found that patients with pulmonary consolidation at baseline were at a greater risk of death than those without consolidation, a finding previously reported from China [Reference Tang36]. The presence of a multi-lobar and bilateral consolidation on a chest X-ray shows a severely damaged lung due to which the patient may not be able to get enough oxygen that can cause damages to other body organs. As the penetration of drugs into highly damaged lungs is poor, patients do not respond to treatments and the infection worsens with a higher hazard of death. The other possible explanation is the lack of surgery for patients having cavitary and or lobar consolidation, with optimal medical follow-up and therapy in the management of MDR-TB in the Amhara region. The WHO 2018, PMDT guideline indicates that MDR-TB patients with surgical management appear to have a superior outcome, lower mortality rate and reduced recurrences compared with medical therapy alone [40].
The key strength of this study was the incorporation of a multi-centre site and employing an appropriate statistical model for better estimation of risk factors. However, some important variables such as the specific cause of death, patient compliance with therapy and knowledge of the drug-resistance patterns of patient isolates are lacking due to the nature of the data collected during routine clinical practice. The 105 participants classified as on-treatment had no equal follow-up time as for the remainder of MDR-TB patients and were included in the final regression analysis thus potentially lowering the number of deaths which may impact the HR. The overall mortality rate in this study population was high making it difficult to meet the target of the end-TB strategy aimed at reducing 95% of TB-related mortality by 2035.
In conclusion, the lack of data on the specific cause of death brings into question whether the factors predictive of death are indeed linked to MDR-TB or other variables. Except for age many of the independent risk factors identified were modifiable. It is, therefore, time to think about nutritional support, early detection and management, and shorten the duration of the MDR-TB treatment regimen to improve the chances of meeting the goal of markedly reducing the incidence and mortality of TB in Ethiopia.
Acknowledgements
We thank the University of Gondar Comprehensive Specialised hospital, Borumeda, Debre-Markos specialised hospitals and administrative staff for their cooperation and permission to conduct the study. We are also grateful for the data collectors who participated in this study for their commitment without which the study would not have materialised.
Author contribution
Conceptualisation, validation, writing the original draft and writing review and editing: all the authors; data curation: GMK, YAG, TTA, ATT, KST and TYA; formal analysis: GMK, ATW, YAG, ATT, KST and TYA; funding acquisition and resource: GMK: software: GMK, YAG, KST and TYA; methodology: GMK, ATW, YAG, ATT, KST and TYA; supervision: ATW, YAG, KST and TYA and visualisation: YAG and TTA.
Financial support
The study was funding by the University of Gondar for graduating Master's student thesis work.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards and consent to participate
Ethical clearance was obtained from the Institutional Review Board of the University of Gondar, College of Medicine and Health Sciences, Institute of Public Health. Informed consent of patients was deemed not necessary as data were entirely secondary and no access to meet the study subjects. However, a permission letter was obtained from the Amhara Regional Health Bureau, hospital administrations and MDR-TB focal persons. The checklist was kept securely in locked cabinets and the database was password protected.
Data availability statement
Data will be available upon a reasonable request from the corresponding author.








