Although a substantial literature exists concerning the potential association of lithium with reduced suicide mortality, very few studies have examined lithium's potential influence on non-suicide mortality. Many organ systems are exposed to lithium, Reference Morton, Sonne and Lydiard1,Reference Frost and Messiha2 and lithium produces diverse physiological effects. Some of these effects are potentially beneficial (for example leukocytosis, Reference Carmen, Okafor and Ike3 reduced heart rate Reference Colton and Manderscheid4 and neurogenesis Reference Manji, Moore and Chen5 ), whereas others are potentially hazardous (for example renal Reference Hicks6,Reference Gitlin7 and thyroid insufficiency, Reference Bocchetta and Loviselli8 QTc prolongation Reference Colton and Manderscheid4 or arrhythmias Reference Weintraub, Hes, Rotmensch, Soferman and Liron9–Reference Wolf, Ranade, Molnar, Somberg and Mosnaim11 ). A limited number of studies, primarily Reference Norton and Whalley12–Reference Angst, Stassen, Clayton and Angst19 but not exclusively Reference Cipriani, Pretty, Hawton and Geddes20 non-randomised, have examined associations between lithium treatment and non-suicide mortality. These studies are consistent with the possibility that lithium might reduce mortality risk in psychiatric patients. Determining the effects on non-suicide mortality of lithium or other psychiatric treatments is clearly important, especially since patients with serious mental illness are at particular risk for premature mortality. Reference Kilbourne, Morden, Austin, Ilgen, McCarthy and Dalack21–Reference Zivin, Ilgen, Pfeiffer, Welsh, McCarthy and Valenstein24
We conducted a nationwide cohort study of the US Veterans Health Administration's (VHA) detailed clinical databases, employing two methods intended to increase the likelihood that observational studies will yield results similar to randomised trials: high-dimensional propensity score (hdPS) matching and intent-to-treat estimates. The hdPS matching permit inclusion of particularly detailed information concerning potential confounding while facilitating the assessment of the balance in these potential confounders that is achieved between treatment groups. Intent-to-treat estimates enhance interpretation of results by allowing assessment of whether benefits during active treatment are negated by risks upon discontinuation. Employing these approaches, we investigated whether initiation of lithium was associated with reduced non-suicide mortality compared with initiation of valproate, a treatment that has largely replaced lithium in many countries. Reference Young and Hammond25–Reference Shulman, Rochon, Sykora, Anderson, Mamdani and Bronskill28
Method
Data sources
Demographic characteristics, in-patient and out-patient mental and non-mental health treatment records, and out-patient pharmacy prescription data were obtained from the VHA National Psychosis and Depression Registries. Reference Blow, Valenstein, Austin, Khanuja and McCarthy29 (These registries are linked, de-identified healthcare databases of all VHA patients nationwide since 1997 with at least one psychotic or depressive disorder diagnosis). This study was approved by the Institutional Review Boards of the Bedford and Ann Arbor Veterans Affairs Medical Centers.
Study cohort
Incident users Reference Ray30 (≥6 months of no lithium or valproate use but with recent VHA utilisation) receiving at least one out-patient prescription for lithium or valproate from April 1999 to December 2008 were identified (online Fig. DS1). A broad cohort of patients with mood or psychotic diagnoses in the 30 days prior to medication initiation was examined since the limited prior literature concerning lithium and mortality is not restricted to bipolar disorder (online supplement DS1). Reference Coppen, Standish-Barry, Bailey, Houston, Silcocks and Hermon13,Reference Muller-Oerlinghausen, Ahrens, Grof, Grof, Lenz and Schou15,Reference Angst, Stassen, Clayton and Angst19,Reference Cipriani, Pretty, Hawton and Geddes20
Patients with possible non-psychiatric indications for valproate or lithium (epilepsy, migraine or cluster headache, or neuropathy diagnoses in the past 30 days; dementia medication use in the past 180 days; cancer, dementia, skull fracture diagnosis, traumatic brain injury diagnosis or treatment, home care, or hospice care in the past year; or any nursing home residence or in-patient rehabilitation in the past 2 years) were excluded. Patients were also excluded if they initiated lithium or valproate on an ‘as needed’ basis or both medications simultaneously, or resided outside the USA.
Exposure determination
Receipt of lithium or valproate was defined by a patient filling an out-patient prescription for these medications. For the intent-to-treat analysis, all patients filling an initial out-patient prescription were followed until the end of the follow-up (i.e. 90, 180 or 365 days) or death. Secondary analyses stratified follow-up time by whether patients were still receiving initial treatment. Patients were considered ‘as-initially treated’ until a ≥15-day gap occurred between out-patient prescriptions (adjusting for early refills) or upon initiation of the other mood stabiliser (i.e. lithium or valproate). ‘Former users’ consisted of patients after initial treatment discontinuation until follow-up time was censored upon treatment resumption, treatment switching (for example patients receiving valproate as initial treatment who switch to lithium), death or the end of follow-up. We will use the term ‘former users’ to designate patients within the period of time during which they have stopped their initial treatment and were not exposed to either lithium or valproate. (‘Former user’ exposure time was censored if the patient resumed either medication.) Given that this ‘former user’ follow-up period is free from exposure to either medication studied, former users have been advanced as a potential index of residual baseline confounding and/or selection occurring during treatment. Reference Hernan and Robins31,Reference Cooper, Habel, Sox, Chan, Arbogast and Cheetham32 However, risks among former users can be more fully conceptualised as representing the sum total of effects of residual confounding and selection along with any persistence of effects from active treatment, and any risks produced upon treatment discontinuation (online supplement DS2). This last category of ‘discontinuation-associated risks’ would include effects such as rebound mania or depression.
Outcome
Date and cause of death was obtained from National Death Index files for 1999–2009. Reference Cowper, Kubal, Maynard and Hynes33 This study was limited to non-suicide mortality, with follow-up time for patients dying of suicide censored at suicide death.
Propensity score modelling
In total, 948 covariates derived from VHA databases were included in an initial propensity score model generally following the hdPS approach Reference Schneeweiss, Rassen, Glynn, Avorn, Mogun and Brookhart34,Reference Patorno, Bohn, Wahl, Avorn, Patrick and Liu35 (online supplement DS3). These covariates included potential risk factors for both non-suicide and suicide mortality Reference Wyss, Girman, Locasale, Alan Brookhart and Sturmer36 (including demographic characteristics, diagnoses, general VHA mental and non-mental health services utilisation, Reference Schneeweiss, Rassen, Glynn, Avorn, Mogun and Brookhart34 admissions to hospital, clinic use, occurrence of diagnostic testing, current and recent prescriptions, recent injuries and diagnosed suicide attempts, and state-level and VHA-hospital subsystem mortality risk Reference Selim, Berlowitz, Fincke, Rosen, Ren and Christiansen37 ) (online Table DS1 and online supplement DS4), often with multiple indicator variables to allow for non-linear covariate–mortality relationships. An ‘outcome-focused’ propensity score was then derived limiting covariates to the 523 covariates with substantial associations with outcome Reference Brookhart, Schneeweiss, Rothman, Glynn, Avorn and Sturmer38 (i.e. ±20% change in non-suicide mortality). Reference Patrick, Schneeweiss, Brookhart, Glynn, Rothman and Avorn39 Further details of how this covariate restriction was implemented are provided in online supplement DS4. This outcome-focused propensity score, intended to limit unintended amplification of confounding that remained uncontrolled, Reference Brookhart, Schneeweiss, Rothman, Glynn, Avorn and Sturmer38,Reference Pearl40,Reference Brooks and Ohsfeldt41 provided the basis for the results reported here. Results from analyses using the initial propensity score are provided in online supplement DS5. The results from the two models are generally similar but differ in a few important details, such as the time periods for which significant associations are detected.
Statistical methods
The propensity score was calculated using logistic regression. Patients initiating lithium and valproate were 1:1-matched (online supplement DS6) using callipers of 0.2 standard deviations of the propensity score logit, Reference Austin42,Reference Faries, Leon, Haro, Obenchain and Institute43 resulting in 99.3% matching of lithium-initiated patients. Balance in covariates between treatment groups was assessed using standardised differences (equivalent to Cohen's d effect sizes, with a difference of >0.10 indicating significant imbalance). Reference Austin, Grootendorst and Anderson44
Statistical significance was determined using techniques that reflected matching (stratified Cox regression with sandwich variance estimators) for the primary intent-to-treat analyses and the secondary as-treated analyses. Ordinary Cox regression was used for the secondary former user analysis (since matching was not preserved for this analysis). All analyses except standardised differences were performed using SAS, version 9.3. Standardised differences were calculated using Microsoft Excel 2010.
Results
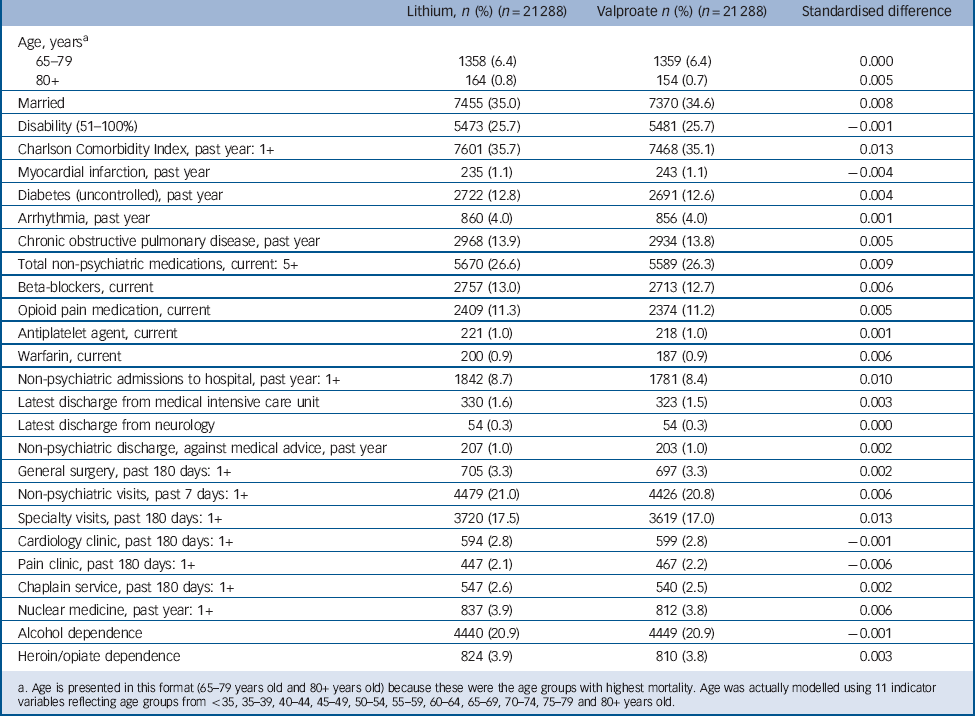
The incident user cohort of 93 162 patients initiating lithium or valproate was generally balanced (i.e. standardised differences between the treatment groups <0.10) Reference Austin, Grootendorst and Anderson44 in virtually all non-mental health and mental health covariates studied, even prior to matching. After matching, substantial additional balance was achieved between treatment groups (n = 21 288 patients per group). As an end result, hdPS matching achieved a very close balance (standardised differences <0.019) for each one of the 523 covariates included in the outcome-focused analysis. Table 1 and 2 indicate the standardised differences in the matched cohort for two categories of covariates: (a) those few variables with a substantial imbalance (≥0.10 standardised difference) between treatment groups initially (8 of 523 covariates, or 1.5%, Table 1), and (b) a number of additional covariates with well-established or highly plausible relationships with non-suicide mortality (Table 2). These additional covariates include age, disability status, recent number and types of hospital admissions, diagnoses, medications, and attendance at certain out-patient clinics. Comparison of the unmatched and hdPS-matched treatment effect estimates indicate that hdPS-matching reduced effect sizes in a direction consistent with reducing baseline confounding biasing against valproate (i.e. reducing differences that placed patients initiating valproate at higher intrinsic risk of non-suicide mortality) (online supplement DS7).
Table 1 Characteristics of patients initiating lithium and valproate for initially substantially imbalanced covariates (initial standardised difference ≥0.10) (propensity-score matched sample)

| Lithium, n (%) (n = 21 288) | Valproate n (%) (n = 21 288) | Standardised difference | |
|---|---|---|---|
| Gender, female a | 2932 (13.8) | 2952 (13.9) | −0.003 |
| Bipolar I disorder, past 30 days | 9630 (45.2) | 9719 (45.7) | −0.008 |
| Other psychosis, past 30 days | 251 (1.2) | 261 (1.2) | −0.004 |
| Post-traumatic stress disorder, past year | 4858 (22.8) | 4849 (22.8) | 0.001 |
| Other mood stabiliser(s), current | 2963 (13.9) | 2894 (13.6) | 0.006 |
| Prior mood stabiliser treatment | 7573 (35.6) | 7473 (35.1) | 0.010 |
| Mild liver disease, past year | 1795 (8.4) | 1708 (8.0) | 0.015 |
| Angiotensin converting enzyme inhibitor, current | 2772 (13.0) | 2765 (13.0) | 0.001 |
a. See text for a discussion of the gender imbalance.
Table 2 Characteristics of patients initiating lithium and valproate for select additional variables with lesser initial imbalances (propensity-score matched sample)
| Lithium, n (%) (n = 21 288) | Valproate n (%) (n = 21 288) | Standardised difference | |
|---|---|---|---|
| Age, years a | |||
| 65–79 | 1358 (6.4) | 1359 (6.4) | 0.000 |
| 80+ | 164 (0.8) | 154 (0.7) | 0.005 |
| Married | 7455 (35.0) | 7370 (34.6) | 0.008 |
| Disability (51–100%) | 5473 (25.7) | 5481 (25.7) | −0.001 |
| Charlson Comorbidity Index, past year: 1+ | 7601 (35.7) | 7468 (35.1) | 0.013 |
| Myocardial infarction, past year | 235 (1.1) | 243 (1.1) | −0.004 |
| Diabetes (uncontrolled), past year | 2722 (12.8) | 2691 (12.6) | 0.004 |
| Arrhythmia, past year | 860 (4.0) | 856 (4.0) | 0.001 |
| Chronic obstructive pulmonary disease, past year | 2968 (13.9) | 2934 (13.8) | 0.005 |
| Total non-psychiatric medications, current: 5+ | 5670 (26.6) | 5589 (26.3) | 0.009 |
| Beta-blockers, current | 2757 (13.0) | 2713 (12.7) | 0.006 |
| Opioid pain medication, current | 2409 (11.3) | 2374 (11.2) | 0.005 |
| Antiplatelet agent, current | 221 (1.0) | 218 (1.0) | 0.001 |
| Warfarin, current | 200 (0.9) | 187 (0.9) | 0.006 |
| Non-psychiatric admissions to hospital, past year: 1+ | 1842 (8.7) | 1781 (8.4) | 0.010 |
| Latest discharge from medical intensive care unit | 330 (1.6) | 323 (1.5) | 0.003 |
| Latest discharge from neurology | 54 (0.3) | 54 (0.3) | 0.000 |
| Non-psychiatric discharge, against medical advice, past year | 207 (1.0) | 203 (1.0) | 0.002 |
| General surgery, past 180 days: 1+ | 705 (3.3) | 697 (3.3) | 0.002 |
| Non-psychiatric visits, past 7 days: 1+ | 4479 (21.0) | 4426 (20.8) | 0.006 |
| Specialty visits, past 180 days: 1+ | 3720 (17.5) | 3619 (17.0) | 0.013 |
| Cardiology clinic, past 180 days: 1+ | 594 (2.8) | 599 (2.8) | −0.001 |
| Pain clinic, past 180 days: 1+ | 447 (2.1) | 467 (2.2) | −0.006 |
| Chaplain service, past 180 days: 1+ | 547 (2.6) | 540 (2.5) | 0.002 |
| Nuclear medicine, past year: 1+ | 837 (3.9) | 812 (3.8) | 0.006 |
| Alcohol dependence | 4440 (20.9) | 4449 (20.9) | −0.001 |
| Heroin/opiate dependence | 824 (3.9) | 810 (3.8) | 0.003 |
a. Age is presented in this format (65–79 years old and 80+ years old) because these were the age groups with highest mortality. Age was actually modelled using 11 indicator variables reflecting age groups from <35, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, 75–79 and 80+ years old.
It should be noted that the gender ratio of our sample (Table 1) differs substantially from that found for bipolar disorder in the general population because our sample is a Veteran sample, primarily made up of men. It is particularly important to note that the hdPS-matching procedure did not substantially change the prevalence of male or female gender in the final study cohort compared with the initial sample, or the prevalence of any of the other included covariates. Rather, the matching procedure led to the selection of a set of patients initiating valproate who were very similar to those initiating lithium. For instance, in the original study cohort prior to matching 13.9% of patients initiating lithium and 9.4% of patients initiating valproate were women. In the final cohort, 13.8% of patients initiating lithium and 13.9% of patients initiating valproate were women.
Impersistence with treatment was very common even within 180 days, but rates of treatment impersistence were highly similar between the treatment groups: approximately 76.5% of patients initiating lithium and 76.1% of patients initiating valproate did not persist with initial treatment for 180 days (online Table DS4).
Overall survival was greater among patients initiating lithium, with 274 deaths over 365 days observed among the lithium intent-to-treat cohort, compared with 296 deaths among the valproate intent-to-treat cohort. Greater differences (71 v. 101 deaths, respectively) were observed between the as-initially treated cohorts. Survival curves for the intent-to-treat and as-initially treated analyses are provided in Fig. 1.
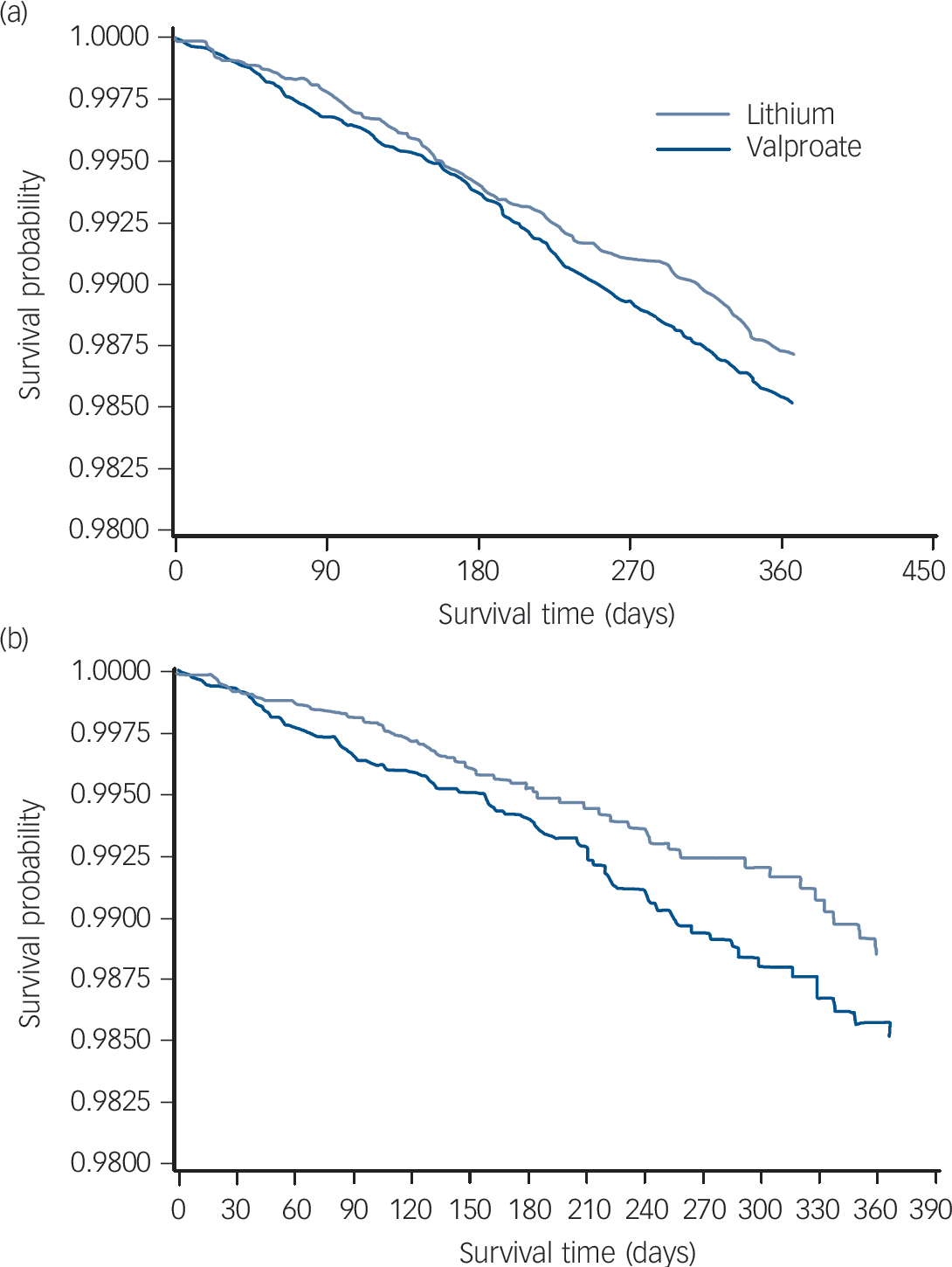
Fig. 1 Survival curve of lithium and valproate treatment for (a) intent-to-treat cohort and (b) as-treated patients.
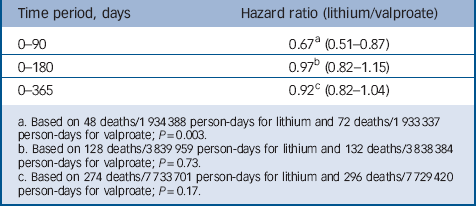
Table 3 provides the primary, intent-to-treat analysis results, indicating that lithium was associated with substantially reduced mortality risks over 0–90 days (hazard ratio (HR) = 0.67, 95% CI 0.51–0.87), the period of greatest medication persistence, but not 0–180 days (HR = 0.97, 95% CI 0.82–1.15) or 0–365 days (HR = 0.92, 95% CI 0.82–1.04).
Table 3 Risk of non-suicide mortality (intent-to-treat cohort)
| Time period, days | Hazard ratio (lithium/valproate) |
| 0–90 | 0.67 a (0.51–0.87) |
| 0–180 | 0.97 b (0.82–1.15) |
| 0–365 | 0.92 c (0.82–1.04) |
a. Based on 48 deaths/1 934 388 person-days for lithium and 72 deaths/1 933 337 person-days for valproate; P=0.003.
b. Based on 128 deaths/3839 959 person-days for lithium and 132 deaths/3838 384 person-days for valproate; P=0.73.
c. Based on 274 deaths/7 733 701 person-days for lithium and 296 deaths/7 729 420 person-days for valproate; P=0.17.
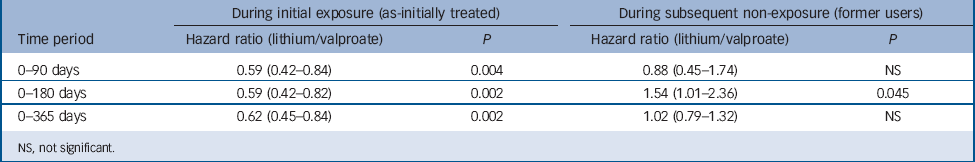
Secondary analyses by treatment status (Table 4) reveal large and significant associations with non-suicide mortality during active lithium treatment compared with valproate treatment over all time periods. Hazard ratios were consistently and considerably lower during the period of likely active use of lithium compared with likely active use of valproate (as-initially treated hazard ratios ranging from HR = 0.59, 95% CI 0.42–0.82 to HR = 0.62, 95% CI 0.45–0.84). However, significantly increased non-suicide mortality was also observed among lithium former users over 0–180 days (HR = 1.54, 95% CI 1.01–2.37), although not other time periods.
Table 4 Risk of non-suicide mortality (stratified by exposure status)
| During initial exposure (as-initially treated) | During subsequent non-exposure (former users) | |||
|---|---|---|---|---|
| Time period | Hazard ratio (lithium/valproate) | P | Hazard ratio (lithium/valproate) | P |
| 0–90 days | 0.59 (0.42–0.84) | 0.004 | 0.88 (0.45–1.74) NS | |
| 0–180 days | 0.59 (0.42–0.82) | 0.002 | 1.54(1.01–2.36) 0.045 | |
| 0–365 days | 0.62 (0.45–0.84) | 0.002 | 1.02 (0.79–1.32) NS | |
NS, not significant.
Table 5 indicates no significant intent-to-treat associations existed between treatment and specific categories of causes of death at 365 days. Therefore, the observed hazard ratios, modestly above or below the null for each cause of death, possibly represent simple chance fluctuations. The mortality categories with associations the closest to statistical significance, were cardiovascular disease and deaths from all other causes. Upon further examination, these categories were also the only categories to have significant (all other causes, HR = 0.50, 95% CI 0.28–0.91) or borderline significant (cardiovascular disease, HR = 0.60, 95% CI 0.36–1.01) associations among as-initially treated individuals.
Table 5 Risk of non-suicide mortality by cause (intent-to-treat cohort)
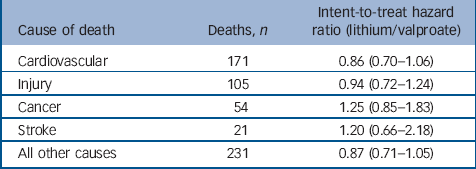
| Cause of death | Deaths, n | Intent-to-treat hazard ratio (lithium/valproate) |
|---|---|---|
| Cardiovascular | 171 | 0.86 (0.70–1.06) |
| Injury | 105 | 0.94 (0.72–1.24) |
| Cancer | 54 | 1.25 (0.85–1.83) |
| Stroke | 21 | 1.20 (0.66–2.18) |
| All other causes | 231 | 0.87 (0.71–1.05) |
Discussion
Main findings
In a nationwide cohort study of 42 576 VHA psychiatric patients initiating lithium and valproate, significant intent-to-treat associations of lithium initiation with lower mortality risk were observed over 0–90 days but not over 0–180 days or 0–365 days. In contrast, secondary analysis of patients who persisted with treatment, indicated strong associations with reduced mortality for lithium, compared with valproate, treatment across all time periods. However, the reduction in the intent-to-treat association from 0–90 to 0–180 days is very rapid, and our secondary analysis indicates significantly increased mortality risk associated with discontinuing lithium, compared with valproate, over 0–180 days of treatment. Both findings suggest a possible increase in mortality risk associated with lithium, compared with valproate, among patients who discontinue the medication (online supplement DS8). These findings, combined with the possibility that at least some level of confounding (most likely biasing towards worse outcomes in patients initiating valproate) may remain in our analyses, prevent firm conclusions concerning whether the initiation of lithium is associated with a net mortality benefit or harm. Regardless of this uncertainty, however, one clear and important clinical recommendation results from this mortality study: once lithium is initiated, persistence with lithium treatment should be monitored and, if clinically reasonable, maintained.
Confounding
Risks associated with lithium discontinuation could, in theory, potentially limit, eliminate, or even exceed mortality benefits associated from active lithium treatment when compared with valproate. Determining whether this occurs is difficult even though the intent-to-treat estimates significantly favour lithium treatment from 0–90 days and numerically, although not significantly, favour lithium treatment over longer time periods. Although intent-to-treat estimates are intended to reflect the balance of benefits and harms among all patients, regardless of whether continuing on or discontinuing treatment, even relatively small amounts of confounding biasing against valproate could alter risk–benefit judgements. Propensity score matching can achieve very close balance on measured factors (and did so here), however, factors that are either incompletely modelled or unmeasured in the analysis are intrinsically not able to be balanced by propensity score methods. Such factors might include provider tendencies to prescribe valproate to individuals who are more severely ill within the categories of illness that are balanced through the propensity score matching. Thus, not only does random error (as indicated by the lack of statistical significance) limit interpretation of the intent-to-treat hazard ratios after 90 days, but so does possible residual confounding (online supplements DS8 and DS9).
Although our hdPS successfully achieved close balance on a large variety of important potential confounders, some degree of remaining confounding is plausible. Risk estimates indicating greater mortality risk among patients initiating valproate in the unmatched sample (online supplement DS7) become less pronounced after hdPS-matching, suggesting overall confounding initially biases against valproate. Also, the former-user hazard ratio for patients initiating, then stopping, lithium over 0–90 days compared with patients initiating, and then stopping, valproate, is <1.0 (although random error cannot be excluded). (‘former users’ are those patients no longer receiving either mood stabiliser, thus, this ‘former user’ period represents time not exposed to either lithium or valproate). This observation is consistent with the possibility that, if residual confounding does exist to at least some degree, then patients initiated on lithium were likely at lower baseline mortality risk on average than patients initiated on valproate. Finally, even though significantly increased mortality risk is observed among lithium former users from 0 to 180 days, the possibility of some confounding against valproate persisting throughout the analysis is suggested by the subsequent sharp decrease in former user risks from 0–180 to 0–365 days (central estimate HR = 1.54 changing to HR = 1.02) (online supplement DS8). Although, again, random error cannot be excluded, if any confounding does generally bias against valproate, this suggests that the mortality risk associated with patients who discontinued lithium over 0–180 days compared with patients who discontinued valproate may be even greater than indicated.
Nevertheless, even if confounding biasing against valproate exists, the effect estimates for active lithium treatment compared with active valproate treatment are of such size (central estimate HR = 0.59–0.62) as to command attention. Such as-initially treated estimates are often the only type of effect estimate typically provided in non-randomised treatment studies. In this study, these ‘as-initially treated estimates may provide only part of the important information to consider, given the possibilities of confounding and discontinuation-associated risks. Nevertheless, even if some level of confounding persists after matching, it is unclear whether such confounding persists to such an extent to fully account for the significant intent-to-treat association observed early during follow-up, or as-initially treated associations observed throughout follow-up. This becomes a question for further research. Not focused upon here, but also a potential contributor to the secondary analysis results is differential selection of patients, once they have initiated treatment, to stop one medication (e.g. lithium) compared with the other medication (e.g. valproate). Such differential selection could occur from either providers or patients relying on different reasons when deciding to discontinue their initial treatment, depending on whether that initial treatment was lithium or valproate. This possibility is consistent with some, but not all, of the study findings (online supplement DS10).
Importance of treatment persistence
Regardless of these uncertainties, one clear clinical recommendation can be made: once initiated, persistence with lithium treatment should be monitored and, if clinically indicated, maintained. Whether or not the predominant association of lithium treatment (compared with valproate) with mortality is one of lower mortality risks during active treatment or higher mortality risks during lithium discontinuation, in either case maximising persistence with lithium treatment would be of clear benefit. Treatment persistence would both minimise potential risks upon lithium discontinuation and maximise potential benefits resulting from active lithium treatment (online supplement DS11).
Findings from other studies
Our results are generally consistent with a limited prior literature. A clinical trial meta-analysis of both placebo and comparator-controlled trials reported significant reductions in overall mortality with lithium treatment (HR = 0.42, 95% CI 0.27–0.81); however, restriction to non-suicide mortality from active comparator trials results in the findings being informed by just 4 deaths among lithium recipients and 12 deaths among comparator recipients (see Cipriani et al Figures 2 and 4 Reference Cipriani, Pretty, Hawton and Geddes20 ). Non-randomised studies of lithium's effects on non-suicide mortality are few but generally indicate reduced risks with active lithium treatment, although they typically lack active comparators, intent-to-treat designs or detailed controls for potential confounding. Reference Norton and Whalley12–Reference Angst, Stassen, Clayton and Angst19 Our study is, to our knowledge, the first non-randomised study to examine non-suicide mortality risks associated with lithium discontinuation in a study with an active comparator, but is consistent in a general sense with limited prior uncontrolled studies. These studies observed that lithium discontinuation is a high-risk period for overall mortality. Reference Muller-Oerlinghausen, Wolf, Ahrens, Glaenz, Schou and Grof45,Reference Bocchetta46 Our study's findings potentially may also be broadly consistent with prior randomised Reference Christodoulou and Lykouras47 and non-randomised Reference Suppes, Baldessarini, Faedda and Tohen48,Reference Faedda, Tondo, Baldessarini, Suppes and Tohen49 literature indicating that lithium discontinuation substantially increases the risk of mood episodes. These risks for mood-episode recurrence were sufficiently pronounced that one author reviewing these studies even recommended that lithium not be initiated unless a patient was likely to complete 2 years of continuous treatment. Reference Goodwin50 Finally, our conclusions concerning the importance of persistence with lithium treatment are generally consistent with multiple prior studies reporting substantial lithium treatment impersistence. Reference Scott and Pope51–Reference Kessing, Sondergard, Kvist and Andersen57
Future directions
In our judgement, this study clearly establishes high-priority clinical and research agendas. The data from this study clearly suggests a need for clinical systems and providers to encourage patients to continue with their lithium treatment once it is initiated. A limited literature exists concerning psychosocial interventions that might help accomplish this task. Reference Lolich, Vazquez, Alvarez and Tamayo58 In addition, two trials that have included group psychoeducation on the importance of medication treatment and/or adherence for successful management of bipolar disorder have shown superior outcomes to standard care. Reference Colom, Vieta, Sanchez-Moreno, Palomino-Otiniano, Reinares and Goikolea59,Reference Kessing, Hansen, Hvenegaard, Christensen, Dam and Gluud60 Our data also supports monitoring patients closely upon discontinuation when feasible, a practice already recommended in some guidelines to limit mood-episode recurrence. Reference Goodwin61 Finally, some approaches such as gradual discontinuation Reference Faedda, Tondo, Baldessarini, Suppes and Tohen49 have been proposed to limit the adverse psychiatric effects of lithium discontinuation.
From a research perspective, this study establishes a need for further non-randomised studies to elucidate the balance of risks and harms associated with lithium initiation. This might include evaluating cohorts with different demographics, treatment persistence rates, or psychiatric or non-psychiatric comorbidities. If additional methodological innovations could be combined with the approaches used here, further reductions in confounding or selection during treatment might result. For instance, if valid instrumental variables can be identified, these might reduce confounding from imperfectly measured or unmeasured factors. Marginal structural models might reduce the impact of differential selection during treatment, and allow the impact of additional treatments commenced during follow-up to be evaluated. Research consortiums have been recently developed to rapidly conduct research along such lines; Reference Chao and Urbano62 our findings suggests that the mortality effects of lithium and comparators should be a high-priority target for these consortiums. Such non-randomised research could also help determine the need for subsequent randomised trials, although the acceptability of such trials is uncertain. The need for additional, rapidly initiated research arises from the obvious health relevance of even small differences in non-suicide mortality between these commonly used medications.
Limitations
Study limitations include our lack of in-patient prescription information, lack of serum medication levels as an alternative method to assess persistence with treatment and the inherent inability to completely model potentially important covariates such as admissions to hospital (online supplement DS12). A few variables found to be important in past mortality studies (income and ethnicity) that are sometimes poorly measured in Veterans Affairs data were not included in the outcome-focused propensity score. Although available medical information was extensively represented, this information was only present for treatment received at the VHA. Although we employed multiple methods to attempt to balance the treatment groups in VHA medical utilisation (including indicators such as the presence and number of recent non-psychiatric medications, overall visits and specialist visits a patient received), this lack of outside healthcare data may be particularly relevant for patients receiving emergency care (which is more likely to occur at the nearest available hospital) or for older patients with Medicare. We also did not rebalance our treatment groups during follow-up for time-varying factors such as the use of other medications through methods such as marginal structural models, although the treatment groups were closely balanced on a very extensive set of psychiatric and non-psychiatric medications present at treatment initiation. Given that a very large majority of patients had stopped or modified their initial treatment by 365 days, we did not examine patient outcomes occurring over longer than 365 days in our primary analyses. A follow-up period of 1 year Reference Kales, Valenstein, Kim, McCarthy, Ganoczy and Cunningham63–Reference Soyka, Apelt, Lieb and Wittchen65 or even briefer Reference Wang, Schneeweiss, Avorn, Fischer, Mogun and Solomon66,Reference Kales, Kim, Zivin, Valenstein, Seyfried and Chiang67 is fairly standard for studies examining overall or cause-specific mortality in relation to psychiatric medication initiation, even in cohorts with greater medication adherence. Nevertheless, to the degree that either lithium or valproate is associated with health risks or benefits that accrue over >1 year of treatment, the impact of these risks and benefits upon mortality will not be reflected in this study.
As a pilot analysis, we extended the follow-up time we investigated from 0–365 days to 0–1095 days (3 years), and found this made minimal difference in the results (intention-to-treat HR = 0.93, 95% CI 0.87–1.00, P = 0.04, as-initially treated HR = 0.64, 95% CI 0.48–0.87, P = 0.004), although the intent-to-treat findings were now significant at the 0.05 level. Very little of the additional intent-to-treat follow-up time, however, relates to patients still receiving initial treatment, and very little of the as-initially treated follow-up time relates to treatment exposure after the first year of treatment.
Patients with several major mental health diagnoses were included to achieve sufficient power (for example depression, bipolar and psychotic diagnoses). Although the psychiatric diagnoses were each balanced closely between treatments by hdPS-matching, this may have introduced some heterogeneity in the associations between treatments and mortality. Suicide deaths, which some studies have reported as strongly influenced by lithium treatment Reference Baldessarini, Tondo, Davis, Pompili, Goodwin and Hennen68,Reference Goodwin, Fireman, Simon, Hunkeler, Lee and Revicki69 and/or its discontinuation, Reference Baldessarini, Tondo and Hennen70 may have been miscoded to some extent as accidents/injuries, resulting in an outcome not completely specific for non-suicide mortality. Studies of overall mortality have also been criticised in general for their lack of specificity. Reference Ray71 However, an overall non-suicide mortality focus for this study appears appropriate, given that lithium and valproate affect so many organ systems that a priori cause-specific hypotheses are difficult.
One potential limitation that can affect some non-randomised cohort studies are biases arising from inclusion of patients either in the midst of treatment (‘prevalent users’) or who have been exposed to either study agent in the past (‘past users’) (online supplement DS13). We sought to minimise these potential biases through an ‘incident first-user design’, whereby we only examined patients during their first use of either lithium or valproate after achievement of a 6 month or longer ‘clean period’ of no exposure. However, a small, extremely similar fraction of patients in each treatment group did have record of past (but not recent exposure) to lithium or valproate (12.0% v. 11.9%, respectively). Excluding these individuals produced little change to the effect estimates (365-day intention-to-treat HR = 0.89, 95% CI 0.82–1.03, P = 0.11; 365-day as-treated HR = 0.66, 95% CI 0.46–0.95, P = 0.02). Unfortunately, it is not possible to similarly control for the possibility that some uncertain number of patients may have received one of these medications prior to 1999 (when VHA medication dispensing began to be electronically recorded), or received either medication from outside sources (either recently or in the past). To reduce the concern about recent outside use of lithium or valproate, we only included the patients that we judged were particularly unlikely to have unrecorded outside use by requiring all patients to have more than a year of prior VHA system use. In addition, almost all patients (approximately 95%) had already been using VHA pharmacy services as well.
The rates of treatment impersistence among patients initiating lithium and valproate were quite high (median time to discontinuation of initial treatment approximately 90 days). Possible reasons for this high rate of treatment impersistence may relate to the high rates of psychiatric comorbidities (for example post-traumatic stress disorder), substance use disorders Reference Manwani, Szilagyi, Zablotsky, Hennen, Griffin and Weiss72 and homelessness in our Veteran sample compared with other patient samples. However, the treatment impersistence rates appear to be consistent with those observed in the only other incident cohort from a broad sample of US patients that we were able to identify. Johnson & McFarland reported a median time to discontinuation of the first episode of treatment with lithium of only 72 days in a US Health Maintenance Organization sample. Reference Johnson and McFarland56 In contrast, findings from a non-US incident cohort (a nationwide sample from Denmark) indicated higher rates of persistence with lithium treatment (median time to discontinuation of 181 days). Reference Kessing, Sondergard, Kvist and Andersen57 However, the authors note that their sample may have had less severe mental illness than many cohorts, since >50% of the lithium prescriptions were apparently initiated by general practitioners, not psychiatrists. Reference Kessing, Sondergard, Kvist and Andersen57
Although methodological work in comparative effectiveness research has been steadily advancing, it has not been determined whether outcome-focused propensity scores should be favoured over larger propensity scores in all circumstances. Of note, the results given here for the outcome-focused model and in online supplement DS5 for the initial model are generally consistent in many aspects. These aspects include a significant intent-to-treat difference between lithium and valproate at 90 days and substantial effect sizes for as-treated and former users that are almost uniformly consistent in direction of effects, although not always identical in significance. There are a number of reasons, both theoretical and specifically from the data itself, that suggest the outcome-focused approach, designed to limit unintended amplification of confounding, is likely less biased and more valid (online supplement DS5). However, because these methods are still being refined, a definite conclusion cannot be reached.
Our study examines a US Veteran sample, so its generalisability to non-Veteran samples is uncertain. (For instance, 86% of our sample is male. To the extent that the association of lithium or valproate might be modified by a patient's gender, the results here might differ from those observed in patient samples with a more typical gender distribution. Investigating such a possibility, however, will likely require even larger patient samples than examined here). Perhaps less obviously, intent-to-treat estimates (essential for developing a full view of the possible risks and benefits of treatment) produce potential limitations to generalisability. For intent-to-treat estimates to likely generalise to other patient samples, that patient sample would need to also exhibit a similar rate of treatment persistence. If active lithium treatment does have a genuine association with lower mortality risks, however, then cohorts with greater treatment persistence than the patients studied here would generally be expected to show greater benefits. Nevertheless, given that residual confounding appears to bias in the direction of patients being initiated on lithium being at some degree of lower mortality risk, the significant risks observed after lithium discontinuation over 0–180 days (albeit time-limited, in that they are no longer significant over 0–365 days) are actually the mortality findings least likely to be attributable to baseline confounding.
Indirect v. direct effects of lithium on mortality
Whether any non-suicide mortality differences between lithium and valproate are primarily as a result of their direct psychiatric effects (stabilising mood and/or destabilising mood on discontinuation), indirect effects on physical health (for example mood stability possibly leading to better adherence to medical treatment) or direct effects on physical health (both medications affect many organ systems) remains to be elucidated. The possibility of associations between lithium treatment and mortality operating through lithium's effect on psychiatric status might unify the observations of decreased risks associated with active treatment and increased risks associated with discontinuation, given lithium's established impact upon mood in both circumstances. Of note, some recent randomised Reference Geddes, Goodwin, Rendell, Azorin, Cipriani and Ostacher73 and non-randomised Reference Kessing, Hellmund, Geddes, Goodwin and Andersen74 studies have suggested lithium may have greater efficacy in bipolar disorder than valproate. In the BALANCE trial, the valproate treatment arm underperformed both the lithium–valproate combination and lithium alone treatment arms. Reference Geddes, Goodwin, Rendell, Azorin, Cipriani and Ostacher73 In Denmark, lithium was found to be associated with fewer subsequent admissions to psychiatric hospital than valproate. Reference Kessing, Hellmund, Geddes, Goodwin and Andersen74
Implications
This cohort study of US VHA patients observed significantly reduced non-suicide mortality among all patients initiated on lithium compared with valproate over 0–90 days but not beyond this period. Furthermore, significant associations were observed in opposite directions in secondary analyses: reduced mortality associated with individuals receiving lithium treatment, and increased mortality associated with individuals discontinuing lithium treatment (over 0–180 days), relative to valproate. This pattern suggests a potential dual nature to the mortality risk associations observed between lithium and valproate: mortality benefits associated with active lithium treatment that potentially exceed or are exceeded by counterbalancing risks associated with lithium discontinuation. Intrinsic uncertainties common to non-randomised studies (for example confounding), despite our efforts to minimise them, preclude a definitive judgement of whether lithium, compared with valproate, initiation was associated with net mortality benefit or net harm. One clear and important clinical conclusion nevertheless emerges: once lithium treatment has been initiated, patients and providers should strive to maximise persistence with lithium treatment when feasible and clinically indicated. Such a conclusion results regardless of whether lithium is associated with benefits during active treatment or harms after discontinuation. Given the problem of premature mortality in patients with serious mental illness, Reference Kilbourne, Morden, Austin, Ilgen, McCarthy and Dalack21–Reference Zivin, Ilgen, Pfeiffer, Welsh, McCarthy and Valenstein24 the potential mortality differences between lithium and valproate should immediately become a greater research and clinical focus.
Funding
This work was financially supported by a Health Services Research and Development Service (HSR&D) Career Development Award (CDA-09-216; E.G.S), by funding from the VHA HSRD Center for Healthcare Organization and Implementation Research and by technical and programming support from the VHA Serious Mental Illness Treatment, Resource, and Evaluation Center, Ann Arbor, Michigan. Databases were constructed in part through funding from VHA HSR&D MRP 03-320 and the VHA Serious Mental Illness Treatment, Resource, and Evaluation Center. The sponsor had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Acknowledgements
We would like to thank two anonymous reviewers whose comments significantly improved this manuscript. The work reported here served as partial fulfilment of the requirements for E.G.S.'s PhD thesis.









eLetters
No eLetters have been published for this article.