Nutrition and health are known since ancient times to be intimately linked; however, the vital influence of micronutrients on immune response was clearly established only in the last quarter of the 20th century. The understanding of adequate trace element nutrition is a key requirement to designing better nutrition that protects animals and humans against infections. The current paradigm to evaluate the status of trace element deficiencies is to characterise some effector activities in leukocytes from various tissues such as blood, bone marrow and lymphoid organs. Among others, copper and iron have so far attracted much attention, as attested by recent reviews describing the relationship between trace elements and immune functionsReference Brock and Mulero1–Reference Cunningham-Rundles, Ahrn, Abuav-Nussbaum and Dnistrian4.
Trace elements in the biological functions of immune cells
The availability of powerful tools in analytical cell biology and molecular genetics has facilitated efforts to identify specific cellular and molecular functions of trace elements in the maturation, activation and functions of host defence mechanisms. Immune cells, like all other types of cell, require an adequate supply of trace elements for the structure and function of metalloproteins that participate in housekeeping processes such as energy production (e.g. iron for cytochromes a, b and c, NADH and succinate dehydrogenases; copper for cytochrome c oxidase in the mitochondrial electron-transport chain) and to protect the cell against highly toxic reactive oxygen species (e.g. copper for superoxide dismutase and iron for catalase). Moreover, the continuous generation of immune cells in bone marrow and the clonal expansion of lymphocytes in response to antigenic stimulation require the availability of sufficient micronutrient for the synthesis of deoxyribonucleotide precursors by ribonucleotide reductase and for the various nucleotidyl transferases that are required for DNA replication and cell division, respectively. In addition, trace elements are also required to maintain the activity of a number of enzymes that directly participate in the defence processes. The best example of such a role is the need for haem iron for myeloperoxidase-dependent generation of hypochlorous acid, which is a microbiocidal factorReference Hampton, Kettle and Winterbourn5. Copper and zinc, together with selenium, are linked in cytosolic defence against reactive oxygen and nitrogen speciesReference Klotz, Kröncke, Buchczyk and Sies6. Undoubtedly there will be additional discoveries of metalloenzymes that are required for the normal development and reactivity of immune cells.
Trace element status can affect primary lymphoid organs including the thymus. However, the mechanism of thymus atrophy in both copper and iron deficiency is unknown. Recently, cell cycle analysis showed decreases in thymocyte proliferation, but not increases in apoptosis, as a possible cause of thymic atrophy in iron-deficient miceReference Kuvibidila, Porretta, Baliga and Leiva7. This finding differs from that observed during zinc deficiency, where apoptosis occurs in the thymus in parallel with increases in plasma cortisol levelsReference Fraker, King, Laakko and Vollmer8. It is unknown whether iron-deficient mice have elevated plasma cortisol levels. It has been established that the decrease in circulating T lymphocyte number is likely to be secondary to thymic atrophy. Concentrations of blood mononuclear cells from rat spleen are diminished by copper deficiencyReference Bala, Failla and Lunney9. Iron deficiency also decreases the number of peripheral blood T lymphocytes where T-helper and T-suppressor cells are very sensitive to limited iron availabilityReference Santos and Falcao10–Reference Mullick, Rusia, Sikka and Faridi12. Recently, our group found decreases in the numbers of naive T-helper (CD4+CD45RA+) and T-cytotoxic (CD8+CD73+) cells in blood from iron-deficient subjects (unpublished data). This finding suggests that iron is also required for the regeneration of new CD4+T lymphocytes and maintenance of T cytolytic processes.
Micronutrients may potentially influence some processes of nonspecific immunity by modulating inflammatory cell functions. For instance, the effect of copper deficiency on phagocytic cells, particularly neutrophils and macrophages, is well detailed in the literature. Functions of neutrophils include travelling to the site of infection, adhering to the endothelium and transmigration across the endothelium, where they are involved in phagocytosis and killing of foreign invaders by activation of the respiratory burstReference Karimbakas, Langkamp-Henken and Percival13. Copper deficiency causes a decrease in the number of circulating neutrophils, a condition termed neutropaenia. This condition is observed in copper-deficient animalsReference Koller, Mulhern, Frankel, Steven and Williams14 and humansReference Heresi, Castillo-Duran, Munoz, Arevalo and Schlesinger15. Cellular copper status, respiratory burst, and candidacidal activity (yeast-killing ability) of peritoneal macrophages have been shown to decrease in severely copper deficient ratsReference Babu and Failla16. However, only limited understanding exists about the exact mechanisms triggered by inadequate trace element nutrition that affect macrophage function. Decreased phagocytosis by polymorphonuclear cells in iron deficient (ID) patients has recently been reportedReference Bergmann, Hertzel, Pinchasi, Straussberg, Djaldetti and Bessler17. One possible mechanism is decreased ATPase activity in phagocyting cells resulting in altered membrane fluidity and less capability to engulf foreign particles, a process similar to that observed in rat and human ID red blood cellsReference Ifere, Ifon, Ebong and Umoh18
The effects of mineral deficiency on acquired immunity can be further demonstrated by examining the response of lymphocytes to T cell mitogens (blastogenesis). An impaired proliferative response has been reported in copper- and iron-deficient animals and humansReference Bala, Failla and Lunney9, Reference Kelley, Daudu, Taylor, Mackey and Turnlund19. Blastogenic activity appears to be a sensitive marker of marginal trace element status and is one of the cell-mediated immune parameters that responds to mineral repletion. One of the possible mechanisms by which iron deficiency impairs lymphocyte proliferation is by reducing the translocation or activation of protein kinase C (PKC)Reference Kuvibidila, Kitchens and Baliga20. Additional evidence of a role for iron in lymphocyte signalling pathways comes from activation studies of phosphatidyl inositol-4,5-bisphosphate (PIP2), a precursor of second messengers. Decreased PIP2 hydrolysis in activated spleen cells from Fe-deficient mice has been reportedReference Kuvibidila, Baliga, Warrier and Suskind21.
Trace element status also affects the synthesis and secretion of cytokines that modulate the activities of immune and other cell types. Copper deficiency attenuates both interleukin (IL)-2 mRNA expression and protein secretion in activated Jurkat human T cell linesReference Hopkins and Failla22 by inhibiting transcription of the IL-2 geneReference Hopkins and Failla23. However, there is a need for human trials before IL-2 production can be considered a potential marker of copper status. We recently reported that copper intake increased IL-2 production by blood cells from healthy subjects with low to normal plasma ceruloplasmin levelReference Munoz, Lopez, Olivares, Pizarro, Arredondo and Araya24. Kuvibidila and co-workersReference Kuvibidila and Warrier25 demonstrated that iron deficiency in mice induces an imbalance in the in vivo secretion of Th1 and Th2 cytokines. Dietary iron restriction is associated with low serum levels of interferon (IFN)-γ IL-12 and IL-10, which return to normal after iron repletion. Human studies have shown decreased levels of serum IL-2 and IL-6 in iron-deficient childrenReference Sipahi, Akar, Egin and Cin26, Reference Ekiz, Agaoglu, Karakas, Gurel and Yalcin27. These observations suggest that iron deficiency alters the balance between pro- and anti-inflammatory cytokines, a change that may affect innate and cell-mediated immunity.
Human iron deficiency is a well-known public health problem that predominantly affects young children and women of childbearing age in developing countriesReference Ramakrishnan28. The relationship between iron and immunity is complex, and has been reviewed by us and othersReference Brock and Mulero1, Reference Walter, Olivares, Pizarro and Munoz29, Reference Oppenheimer30. Studies conducted by our group have focused the attention on the multifunctional cytokine tumor necrosis factor-alpha (TNF-αReference Munoz, Rios, Lopez, Schlesinger and Nunez31, Reference Munoz and Olivares32, since this mediator plays important roles in both immunity and iron metabolism. We recently evaluated different TNF-α expression forms by activated blood mononuclear cells from adult women with ID due to chronic genital bleeding not associated with neoplasiaReference Lopez, Rios, Schlesinger, Olivares, Nunez and Munoz33. Both the secretion and mRNA levels of the cytokine, but not its membrane expression, were significantly lower in ID subjects compared to the controls (Fig. 1). These data suggest that TNF-α secretion is transcriptionally regulated and the impaired secretion in cells from ID subjects indicate that the quality of the immune response is linked to the iron status of mononuclear cells.
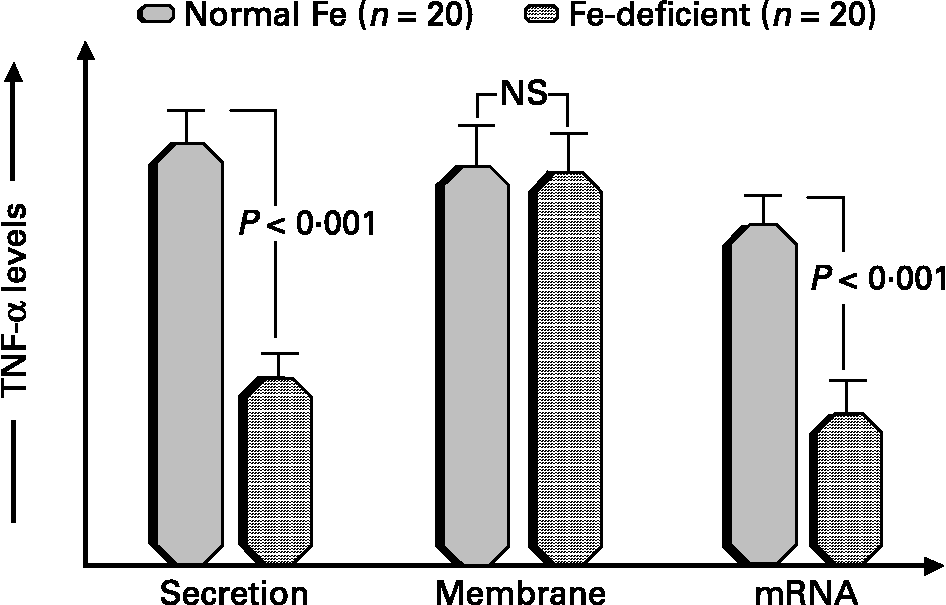
Fig. 1 TNF-α expression in human iron deficiency Blood mononuclear cells from iron deficient and iron replete women were stimulated and TNF-α protein concentration in the medium, TNF-α protein bound to the plasma membrane and TNF-α mRNA levels were measured. Data are mean ± SEM from 20 women. Data are taken from reference 33.
To summarise the present evidence, iron deficiency depresses certain aspects of cell-mediated immunity as well as cytokine secretion/production (Table 1); humoral immunity is unaffected and the significance of hypoferraemia (as opposed to normal transferrin saturation) on the growth of microorganisms is uncertain.
Table 1 Impaired immune functions in iron deficiency: present evidence
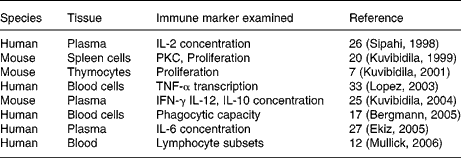
IL = interleukin, PKC = protein kinase C, TNF-α = tumour necrosis factor, IFN-γ = interferon-gamma.
Trace element supplementation, infections and morbidity
When analysing our current knowledge about the interaction between micronutrient supplementation, immunity and morbidity, several questions arise. For instance, does dietary trace element supplementation reduce infection? Or does the improvement of immunity by dietary mineral imply the recovery of health? Certainly, an evaluation of the risks and benefits of an oral mineral supplementation is of particular importance before implementing an intervention. The widespread occurrence of deficiencies of micronutrients in humans has served as the impetus to determine whether supplementation with these trace metals alone or as an adjuvant has the potential to prevent, attenuate and treat infectious diseases. However, it is also recognised that the host's needs must be balanced against the possibility that excessive amounts of redox-active metals such as iron and copper may induce free-radical-mediated damage, and that viruses and infectious microorganisms also require the same trace elements for their survival and replication as the host. Some representative clinical trials on trace element supplementation and infectious diseases are discussed below.
Isolated human copper deficiency is difficult to find due to other concomitant macro and micronutrient deficiencies. Several years ago, our group analysed the effect of copper therapy on leukocyte phagocytosis in infantile hypocupraemiaReference Heresi, Castillo-Duran, Munoz, Arevalo and Schlesinger15. Nineteen well-characterised hypocupraemic infants, 5–9 months of age, with normal weight/length ratio and free of infections, were fed daily with a cow's milk formula containing copper (40 μg/kg) for one month. Plasma copper and ceruloplasmin concentrations, and phagocytic activity of polymorphonuclear leukocytes were measured before and after therapy. After supplementation, copper and ceruloplasmin concentrations as well as the phagocytic index recovered to normal values. However, further clinical trials analysing the effect of copper supplementation in subjects with different infections are needed.
Since iron deficiency anaemia is a major worldwide health problem especially in malaria infected children from developing countries, several investigators have analysed the effect of supplementation of this micronutrient on infectious morbidity in randomised, double blind placebo-controlled trialsReference Harvey, Heywood and Nesheim34–Reference Shankar38. Most of these studies recorded important laboratory outcomes of the intervention, such as haemoglobin change and parasite prevalence. For instance, Berger and co-workersReference Berger, Dyck, Aplogan, Schneider, Traissac and Hereberg37 assessed the impact of a 3-month daily oral Fe supplementation on haematological status, cell-mediated immunity and susceptibility to infections in 6–36 month-old children from rural Africa living in an environment where Fe deficiency and malaria are frequent. Iron supplementation had significant and positive effects on iron status and some immune factors since haemoglobin and total T and Th cells were improved post-therapy. However, no impact on the incidence of malaria was found. These data suggest that control of ID by oral Fe supplementation in young children has to be conducted in association with prophylaxis and treatment of malaria and repeated deworming. Special series of papers dealing with micronutrient supplements on anaemia, growth and morbidity during infancy have been publishedReference Untoro, Karyadi, Wibowo, Erhardt and Gross39–Reference Hop and Berger42. These studies were carried out by the International Research on Infant Supplementation in 4 countries (Vietnam, Indonesia, Peru and South Africa) using a common protocol and data collection instruments. The daily administration of a multiple micronutrient supplement is more efficacious in improving micronutrient status, anaemia, and child weight growth than daily iron supplement or weekly multiple micronutrient supplements. However, the collected morbidity data show no difference between any supplement group and the placebo group. More recently, iron plus zinc supplementation, but not iron alone, was found to be protective against P. vivax in children younger than five years old in the Peruvian AmazonReference Richard, Zavaleta, Caulfield, Black, Witzing and Shankar43. Further research should also investigate whether there are combined benefits from the use of multiple micronutrient therapy in parallel with maternal and infant supplementations together with infection control measures. Additional controlled clinical studies are clearly warranted before definitive recommendations are made. Studies are also urgently needed to assess the effect of ID and iron supplementation on morbidity and mortality due to HIV, tuberculosis and typhoid fever.
Another interesting application of micronutrient intervention is related to vaccine response in older people, since mortality associated with influenza is more likely to occur in this population. The report of UK National Diet and Nutritional Survey found that up to 40 % of older, institutionalised people had low biochemical indices for certain micronutrients. The effect of an experimental nutritional formula on symptoms of upper respiratory tract infection (URTI) and immune function in healthy seniors has been investigatedReference Langkamp-Henken, Bender, Gardner, Herrlinger-Garcia, Kelley and Murasko44. It was found that subjects consuming the formula had significantly fewer days of symptoms of URTI, better antibody response to influenza, and greater lymphocyte proliferative response to influenza vaccine components postimmunization than control subjects. This study concludes that in an elderly population, in which only 30–70 % of those vaccinated are likely to be protected from hospitalization due to influenza, it may be possible to modulate immune function and ultimately reduce symptoms through micronutrient supplementation. Since nutritional intervention may be important in improving seroconversion, Allsup and associatesReference Allsup, Shenkin, Gosney, Taylor, Taylor, Hammond and Zambon45 recently investigated whether a short period of micronutrient therapy could improve antibody response to the influenza vaccine when administered to older people living in long-term facilities. One hundred and sixty-four residents aged 60 and older were randomised to receive either micronutrient supplement or placebo (one tablet twice a day for 8 weeks); the influenza vaccine was administered 4 weeks after their commencement. Results of seroconversion to each of the 3 antigens in the 2000/2001 influenza vaccine (serotypes: H1N1, H3N2, B) showed no differences between the supplemented and placebo groups. Thus, short micronutrient supplementation had no beneficial effect on the antibody response to the influenza vaccine. Larger trials are needed to investigate the effects of long-term therapy and clinical outcome in poorly nourished institutionalised older people.
Possible future directions
Micronutrient deficiencies are of clinical and public health importance in developing countries. Studies to date on micronutrients indicate they have as much potential to alter innate immunity as macronutrients do. In supplementation studies, micronutrients may have varied effects on inflammatory cells that may be either direct or indirect, making it difficult to link in vitro observations with those studies. While micronutrient deficiency, in general, may have a widespread effect on nearly all components of the innate immune response, that effect can be reversed by supplementation. Although preliminary work is promising, an extensive number of well-controlled studies need to be done to clarify which micronutrients (alone or in combination), and in what concentrations, are necessary to influence immunity. It would also be important to select appropriate methods and parameters for accurate assessment. Thus, an important area of focus should be on innate immunity, micronutrient status, and disease.
On the other hand, deficiencies of trace elements affect almost one-third of all elderly subjects. It is expensive and impractical to estimate dietary intake or blood levels of various nutrients in such individuals. Since there is no evidence to suggest that physiological amounts of trace elements given for prolonged periods have any toxic or adverse consequences, and given the high prevalence of deficiencies of several micronutrients in old age, it would be prudent to opt for a suitable micronutrient supplement in modest amounts for all elderly individuals in order to achieve the maximum physiological and health benefits with the least risk of toxicity.
Thus, a better understanding of the molecular and cellular changes caused by inadequate trace elements should lead to the development of effective immunotherapeutic interventions and to an improvement in quality of life for many human beings.
Conflict of interest statement
This study was supported by Fondecyt grant 1020693 and ENL 06/9. The authors have no conflicts of interest to report. All authors participated in the writing of the manuscript.




