Introduction
Habitat loss and fragmentation are considered the major causes of species extinction and biodiversity loss (Fahrig, Reference Fahrig2003). Despite the growing number of studies on the effects of these processes on various organisms, amphibians and reptiles are amongst the least studied groups, together representing only 4% of articles published in 1995–2000 in some of the major ecology journals (McGarigal & Cushman, Reference McGarigal and Cushman2002). However, habitat loss and fragmentation can contribute to population declines of reptiles (Gibbons et al., Reference Gibbons, Scott, Ryan, Buhlmann, Tuberville and Metts2000; Araújo et al., Reference Araújo, Thuiller and Pearson2006) and, as a consequence, may affect other species through trophic interactions (Brown & Nelson, Reference Brown and Nelson1993; Rodrigues, Reference Rodrigues2005; Whitfield & Donnelly, Reference Whitfield and Donnelly2006).
Reptiles have characteristics such as small home ranges and low energetic requirements (Pough et al., Reference Pough, Andrews, Cadle, Crump, Savitzky and Wells1998) that may allow them to maintain viable populations even in small habitat fragments (McGarigal & Cushman, Reference McGarigal and Cushman2002) and are therefore presumed to be less vulnerable to habitat loss and fragmentation than small mammals and birds (Dickman, Reference Dickman1987; McGarigal & Cushman, Reference McGarigal and Cushman2002), despite some evidence of their sensitivity (Sarre et al., Reference Sarre, Smith and Meyers1995; Smith et al., Reference Smith, Arnold, Sarre, Abenperg-Traun and Steven1996; Cosson et al., Reference Cosson, Ringuet, Claessens, De Massary, Dalecky and Villiers1999; Driscol, Reference Driscol2004; Bell & Donnelly, Reference Bell and Donnelly2006). Some studies have shown that habitat structure may be more important for reptiles than the size of a fragment (Kichener et al., Reference Kichener, Chapman, Dell and Muir1980; Jellinek et al., Reference Jellinek, Driscol and Kirkpatrik2004). There is currently no consensus about the relative influence of isolation, connectivity or presence of corridors on lizard communities (Smith et al., Reference Smith, Arnold, Sarre, Abenperg-Traun and Steven1996; Burbrink et al., Reference Burbrink, Phillips and Heske1998; Maisonneuve & Rioux, Reference Maisonneuve and Rioux2001; Driscol, Reference Driscol2004; Bell & Donnelly, Reference Bell and Donnelly2006).
Most studies of the effects of habitat loss and fragmentation on lizards have been in Australia (Kichener et al., Reference Kichener, Chapman, Dell and Muir1980; Sarre et al., Reference Sarre, Smith and Meyers1995; Smith et al., Reference Smith, Arnold, Sarre, Abenperg-Traun and Steven1996; Sumner et al., Reference Sumner, Moritz and Shine1999; Driscol, Reference Driscol2004; Jellinek et al., Reference Jellinek, Driscol and Kirkpatrik2004), with only a few in the neotropics (Dixo, Reference Dixo2001, Reference Dixo2005; Freire, Reference Freire2001; Silvano et al., Reference Silvano, Colli, Dixo, Pimenta, Wiederhecker, Rambaldi and Oliveira2003; Bell & Donnelly, Reference Bell and Donnelly2006). In the Atlantic Forest of South America, one of the global biodiversity hotspots (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000), now reduced to < 12% of its original extent (SOS Mata Atlântica/INPE, 2008; Ribeiro et al., Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009), little is known about the effect of fragmentation on lizards (Dixo, Reference Dixo2001; Freire, Reference Freire2001; Silvano et al., Reference Silvano, Colli, Dixo, Pimenta, Wiederhecker, Rambaldi and Oliveira2003; Dixo & Martins, Reference Dixo and Martins2008). In a study carried out in Bahia, north-east Brazil, no differences were found in reptile species richness, abundance and diversity between small (25–100 ha) and large (> 1,000 ha) fragments (Dixo, Reference Dixo2001; Pardini et al., Reference Pardini, Accacio, Laps, Mariano, Paciencia and Dixo2009). However, this study was in a landscape with high connectivity, where fragments are located in a landscape with 49% forest cover surrounded by an inter-habitat matrix dominated by forest formations (secondary forests, shaded cacao and rubber plantations, covering 23% of the landscape). This forested matrix could represent secondary habitats for the majority of the species (Faria et al., Reference Faria, Paciencia, Dixo, Laps and Baumgarten2007), thus contributing to the absence of a response to forest fragmentation (Dixo, Reference Dixo2001).
In the study reported here we investigated the effect of habitat fragmentation per se (Fahrig, Reference Fahrig2003), fragment size, corridor linkages and forest structure on the leaf-litter lizard community in an Atlantic Forest landscape with an old history of disturbance (> 150 years). The total forest cover is 31%, predominantly surrounded by an agricultural matrix. We compared the number of species, total abundance, and abundance of two species of leaf-litter lizards in fragments and continuous forest to test the hypotheses that: (1) fragmentation negatively affects this lizard community, (2), large fragments and the presence of corridors reduce the effects of fragmentation, and (3) forest structure influences the lizard community. Our results are important for the design of strategies for the conservation of lizards in the Brazilian Atlantic forest.
Study area
The Atlantic Forest biome formerly occupied the whole Brazilian coast but is currently represented by a mosaic of sparse and isolated forest fragments, often severely damaged and < 50 ha (Ribeiro el al., in press). Our study was on the Ibiúna Plateau, 40 km south-east of São Paulo, chosen because of the existence of a continuous forest and a fragmented landscape in similar conditions of relief, altitude, climate and forest succession. Altitudes are 870–1,030 m, climate is temperate hot and humid, type Cfa in the Köppen (Reference Köppen1948) system, and mean maximum and minimum temperatures are 27 and 11oC, respectively. Annual mean precipitation is c. 1,340 mm and varies seasonally, with colder and drier months in April–August. The original forest is classified as lower montane rainforest (Oliveira-Filho & Fontes, Reference Oliveira-Filho and Fontes2000), with elements of Araucaria mixed forest and semi-deciduous forest (Catharino et al., Reference Catharino, Bernacci, Franco, Durigan and Metzger2006).
The continuous forest, the Morro Grande Forest Reserve, of c. 9,400 ha, is connected to one of the largest Atlantic Forest remnants, on the slopes of the Paranapiacaba mountain range (> 760,000 ha). The reserve is composed of a mosaic of secondary forests (sensu Brown & Lugo, Reference Brown and Lugo1990) in different stages of succession, most with 60–80 years of regeneration, and others that are older and well structured (Metzger et al., Reference Metzger, Alves, Goulart, Teixeira, Simões and Catharino2006). The fragmented landscape is adjacent to the Reserve (Fig. 1) and is relatively heterogeneous, with a predominance of forests at an intermediate to advanced stage of succession (31%). Vegetation in early stages of succession and open areas (agriculture and pasture, including fallow lands and areas in early plant succession) covers an additional 43% of the landscape, reforested areas 7%, aquatic areas 3%, and built up areas (rural and urban) a further 16% (Seabra, Reference Seabra1971).
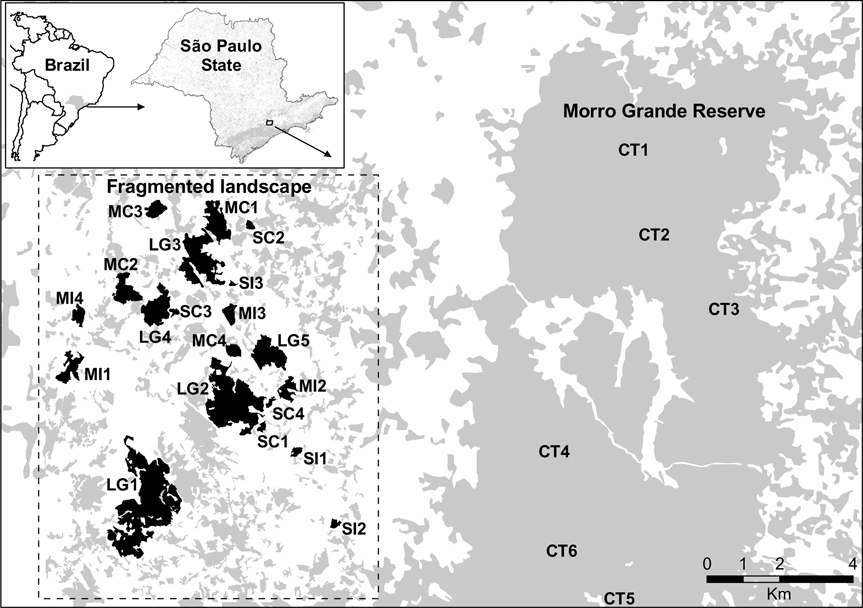
Fig. 1 The location of the studied fragmented landscape in the Ibiúna Plateau and the Morro Grande Reserve (abbreviations of the sites as in Table 1). The inset indicates the location of the study area in the state of São Paulo, Brazil.
Methods
We sampled leaf-litter lizards in 20 forest fragments and in six areas within the Morro Grande Forest Reserve. Fragments were selected to represent the range of fragment size and connectivity in the studied landscape (Table 1). Assessment of connectivity (connected or isolated) was based on the presence or absence of corridors to large fragments. Corridors are of native vegetation, mainly of secondary forest, and 25–100 m wide. Within each size and connectivity class selection of fragments was random after fragments with high levels of human disturbance (e.g. presence of cattle, understorey clearing, selective logging) or structural alteration were excluded. Fragments were at an intermediate to advanced stage of succession (60–80 years of regeneration; Teixeira et al., Reference Teixeira, Soares-Filho, Freitas and Metzger2009). Mean distance between selected forest fragments was c. 1.4 km. Within the Morro Grande Forest Reserve six sites in similar conditions of forest succession were selected, each at least 2.4 km apart. Fragment sizes were obtained from a detailed map produced from visual interpretation of 1:10,000 aerial photographs taken in April 2000 (Silva et al., Reference Silva, Metzger, Simões and Simonetti2007). Little alteration has occurred in the spatial distribution of the forests since 2000. The area of fragments was calculated with FRAGSTAT v. 3.3 (McGarigal & Marks, Reference McGarigal and Marks1995) using a raster image with a resolution of 10 m.
Table 1 The size, connectivity and area of the 26 study sites on the Ibiúna Plateau (Fig. 1).
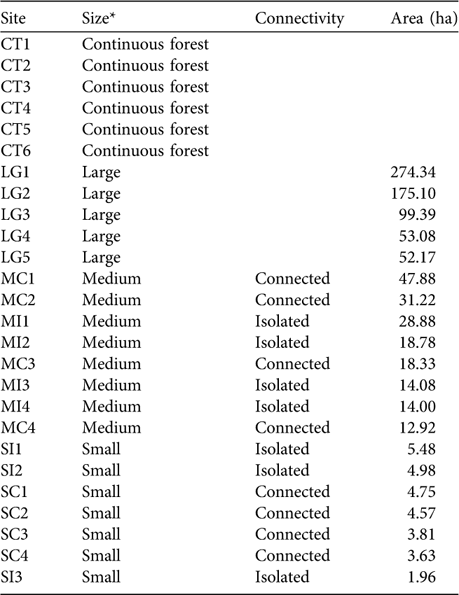
* Continuous forest, areas of the Morro Grande Forest Reserve (c. 9,400 ha); Large, fragments of 50.1–276 ha; Medium, 10–50 ha; Small, <5.5 ha
Sampling of leaf-litter lizards was with pitfall traps (Corn, Reference Corn, Heyer, Donnelly, McDiarmid, Hayek and Foster1994; Cechin & Martins, Reference Cechin and Martins2000). Captured lizards were marked and released individually. In each of the 26 sites 11 60-litre buckets were installed in a line, with a 10 m fence-guide (50 cm high) between traps, resulting in a 100-m line. Trap lines were placed a minimum distance of 50 m from the fragment or forest edge, except in the small fragments where buckets were installed as far away from the edge as possible. Because our objective was to investigate spatial and not seasonal patterns, we sampled only during summer (the wet season), the time of year when lizard capture success is higher for pitfall traps (Dixo, Reference Dixo2005). Surveys were in January–February 2002 and December 2002–January 2003. In each period the buckets were open for 16 days, resulting in a total of 352 pitfall trap days per site and a total of 9,152 pitfall trap days.
Forest structure
We measured foliage density and stratification using an adaptation of the method described in Malcolm (Reference Malcolm, Lowman and Nadkarni1995; for more details see Pardini et al., Reference Pardini, Souza, Braga-Neto and Metzger2005). Foliage density and stratification are good indicators of forest regeneration stages (De Walt et al., 2003) and level of forest disturbance (Malcolm & Ray, 2000). At each site 12 stations spaced 15 m apart were set in each of two parallel lines of 165 m, 20 m apart, overlaying the pitfall trap line. The stature of the inferior and superior limits of all foliage along the imaginary column was measured and used to calculate the length occupied by foliage in five strata (0–1, 1–5, 5–10, 10–15, > 15 m). For each site we calculated the mean foliage length in each stratum.
Data analysis
To estimate lizard species richness and evaluate the completeness of our inventory we used a first-order jacknife estimator calculated with EstimateS (Colwell, Reference Colwell1997) to determine the percentage of estimated richness that was actually observed. To describe forest structure a principal component analysis (PCA) was performed using the foliage density in the five strata in the 26 sites in a correlation matrix (centred and standardized per species) using CANOCO v. 4.0 (ter Braak & Smilauer, 1998). To examine the influence of forest structure, species richness, total abundance and abundance of individual species were regressed against the 26 site scores of the first axis of the PCA.
To test the effects of forest fragmentation on the lizard community we compared the richness, total abundance and abundance of species of leaf-litter lizards between continuous forest and isolated fragments (small- and medium-sized) using the independent t-test. To test the effect of the presence of corridors we carried out a two-way ANOVA considering two classes of fragment size (small- and medium-sized) and the presence or absence of corridors. Barlett's test of homogeneity of variance was calculated and, where necessary, data were log transformed. All statistical analyses were performed with Statistica v. 6.1 (StatSoft, 2001). Simple linear regressions were used to test the relationship of species richness, total abundance and abundance of the two most common species of lizards with fragment area. To minimize the influence of forest structure differences among sites, ANOVAs, t-tests and simple regressions were carried out using the residuals from the regressions of lizard variables against the first axis of the PCA.
We used the non-parametric Multi-Response Permutations Procedure (MRPP; Zimmerman et al., Reference Zimmerman, Goetz and Mielke1985) to test differences in lizard composition caused by fragmentation (isolated fragments vs continuous forest), fragment size reduction (small, medium and large fragments), and among the connected and isolated fragments (small- and medium-sized). These analyses were performed with PC-ORD v. 4.1 (McCune & Mefford, 1997).
Results
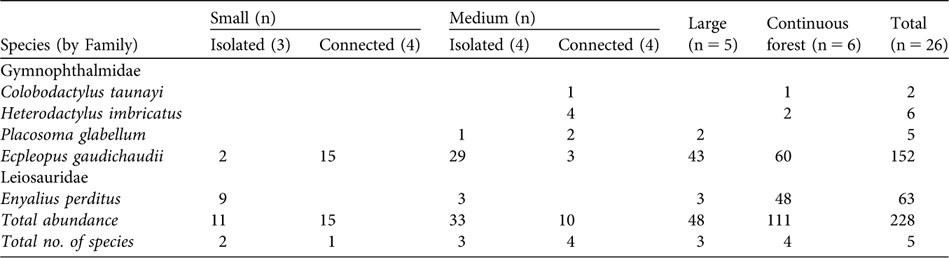
A total of 228 individuals of five species of leaf-litter lizards were captured, belonging to two families, Gymnophthalmidae and Leiosauridae (Table 2). The observed richness was equal to that estimated by the Jacknife procedure, indicating that we achieved a complete inventory of the leaf-litter lizard assemblage. The number of species per fragment was 0–4 (Table 2). Ecpleopus gaudichaudii and Enyalius perditus were the commonest species (95% of the total abundance).
Table 2 Occurrence and abundance of the five species of leaf-litter lizards in the different classes of fragment size (small, medium or large) and connectivity (isolated or connected), and in the continuous forest (Table 1, Fig. 1).
The first axis of the PCA explained 43.5% of the total variation in forest structure among the 26 sites. It represents a gradient of increasing foliage density in the lower strata and decreasing density in the higher strata. Number of lizard species, total abundance and abundance of E. gaudichaudii were not significantly related to the gradient of forest structure (R 2 = 0.008, P = 0.283; R 2 = 0.000, P = 0.511; R 2 < 0.001, P = 0.721, respectively, Fig. 2). The abundance of E. perditus was significantly negatively correlated with the first axis of the PCA, decreasing towards forest in earlier stages of regeneration or subjected to higher levels of disturbance (R 2 = 0.243, P = 0.006; Fig. 2).
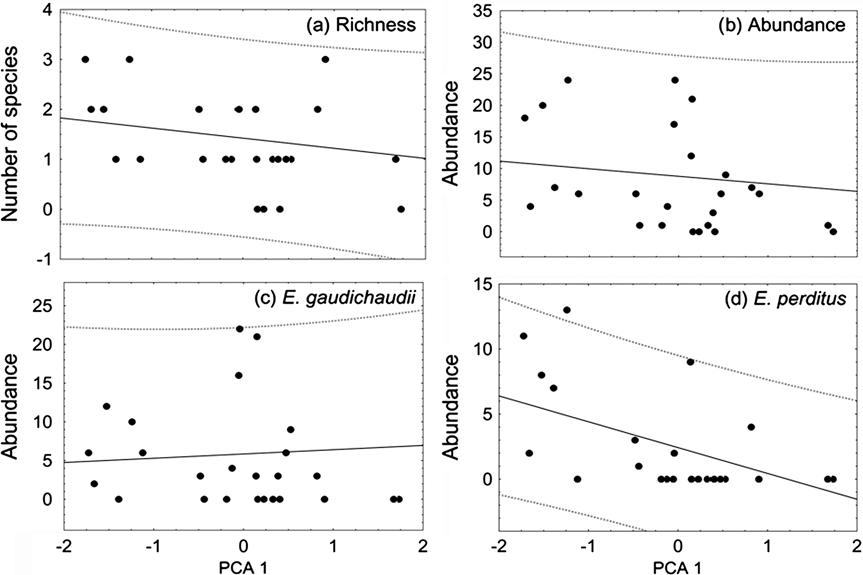
Fig. 2 Relationship of (a) lizard richness, (b) total abundance, (c) abundance of Ecpleopus gaudichaudii and (d) abundance of Enyalius perditus with forest structure in 26 sites (Fig. 1, Table 1), the latter summarized by scores on the first axis of a PCA of foliage density in five strata (see text for details). Forest in earlier stages of regeneration or subjected to higher levels of disturbance, with lower canopy and denser understorey, has higher scores on PCA axis 1.
The continuous forest had more species and more individuals of leaf-litter lizards than fragments (df = 11, t = -3.384, P = 0.006; df = 11, t = -3.184, P = 0.009, respectively). E. perditus was more abundant in continuous forest than in fragments (df = 11, t = -6.113, P < 0.001) but abundance of E. gaudichaudii did not vary between sites (df = 11, t = -1.432, P = 1.798). The composition of the lizard community also varied significantly between continuous forest and fragments (MRPP: n = 13, t = -2.718, P = 0.019).
Richness (n = 20, R 2 = 0.052, P = 0.170), total abundance (n = 20, R 2 = 0.025, P = 0.239), abundance of E. gaudichaudii (n = 20, R 2 = 0.003, P = 0.315), and abundance of E. perditus (n = 20, R 2 = 0.000, P = 0.787) were not correlated with log(area). The composition of the lizard community did not vary significantly between small, medium and large fragments (MRPP: n = 20, t = -0.206, P = 0.406).
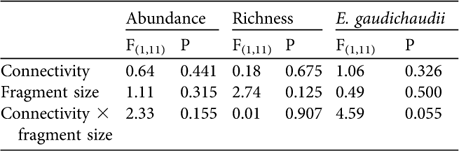
Species composition was not significantly different (MRPP: n = 15, t = -1.025, P = 0.148) nor were number of species, total abundance and the abundance of E. gaudichaudii significantly higher in connected than in isolated fragments, and did not differ between small and medium-sized fragments (Table 3). The abundance of E. perditus was not analysed because this species was not found in the connected fragments (Table 2).
Table 3 Two-way ANOVA comparing total lizard abundance, species richness and abundance of E. gaudichaudii between areas of different connectivity (presence/absence of corridors) and fragment size (small and medium-sized).
Discussion
The main conclusion from this study is that, in a comparison of continuous and fragmented forest, the leaf-litter lizards of the Ibiúna Plateau are sensitive to fragmentation per se (Hypothesis 1 is thus not refuted) but not to fragment size or connection by corridors (refuting Hypothesis 2), and only one species (E. perditis) is sensitive to forest structure (Hypothesis 3 therefore holds true only for some species). Despite the low species richness of this leaf-litter lizard community the number of species is that expected for the region, and is similar to that of other Atlantic Rainforest sites, such as the Japi Mountain Range and Fazenda Intervales, each with five species (Sazima & Haddad, Reference Sazima, Haddad and Morellato1992; Sazima, Reference Sazima and Leonel2001). The number of species is slightly less than recorded at the Ecological Station Juréia-Itatins but this latter area is more heterogeneous in habitat types and structure and has a broader altitudinal gradient (Marques & Sazima, Reference Marques, Sazima, Marques and Duleba2004).
Variation in forest structure did not influence lizard species richness, total abundance or abundance of E. gaudichaudii. These results were not expected given that previous studies have found that habitat structure, heterogeneity and vegetation complexity are essential for the maintenance of herpetofaunal diversity (Burbrink et al., Reference Burbrink, Phillips and Heske1998; Maisonneuve & Rioux, Reference Maisonneuve and Rioux2001) and were, in some cases, more important than fragment size (Kichener et al., Reference Kichener, Chapman, Dell and Muir1980; Jellinek et al., Reference Jellinek, Driscol and Kirkpatrik2004). Our results suggest that the Gymnophthalmidae in Ibiúna, and particularly E. gaudichaudii, can live in secondary habitats as long as the leaf-litter stratum remains relatively undisturbed.
The only species in Ibiúna that appears to be sensitive to forest structure is E. perditus. This species was also sensitive to fragmentation and was more abundant in preserved and less disturbed areas. The genus is known to be restricted to closed-canopy forest (Jackson, Reference Jackson1978). The different microhabitats used by E. perditus and E. gaudichaudii may influence their responses to fragmentation and changes in forest structure. E. gaudichaudii appears to be dependent on leaf-litter, regardless of forest age, cover, area or canopy density and height (but may not tolerate silting, burning or removal of leaf-litter, even if the arboreal stratum is left undisturbed) whereas E. perditus seems to be more sensitive to logging and forest degradation.
However, fragment area had no influence on the abundance of E. perditus. As it is sensitive to forest structure but can persist in small fragments, it probably has a small home range and low energetic requirements. Preliminary results from an ongoing study of E. perditus indicate that it is not a very active species and that its movements are greater in the vertical than the horizontal forest stratum (Liou, Reference Liou2008).
Regardless of variation in forest structure, lizards were sensitive to fragmentation per se but not to fragment size or connectivity. This fragmentation effect is consistent with other studies (Sarre et al., Reference Sarre, Smith and Meyers1995; Smith et al., Reference Smith, Arnold, Sarre, Abenperg-Traun and Steven1996; Driscol, Reference Driscol2004; Bell & Donnelly, Reference Bell and Donnelly2006) but differs from findings in the north-east Atlantic Forest (Dixo, Reference Dixo2001; Silvano et al., Reference Silvano, Colli, Dixo, Pimenta, Wiederhecker, Rambaldi and Oliveira2003), where the landscape has a larger percentage of forest (49%) and a permeable matrix that decreases isolation between fragments. In a situation such as that at Ibiúna, where the agricultural matrix is not permeable to movement of leaf-litter lizards, continuous areas will be necessary for the preservation of the complete lizard community.
The absence of a relationship between fragment size and lizard species richness may be related to the capacity of these leaf-litter species to live in secondary or disturbed forest sites. Alternatively, these species, which have small body sizes (Dixo & Verdade, Reference Dixo and Verdade2006), may have low mobility and small area requirements, enabling them to survive in fragments of different sizes, including the small fragments of < 5.5 ha surveyed at Ibiúna.
The matrix of Ibiúna is a hostile environment to leaf-litter lizards (Dixo, Reference Dixo2005) and one would expect corridors linking forest fragments to favour an increase in the richness and abundance of lizards through movement between fragments (Meffe & Carrol, Reference Meffe and Carrol1997; Rosemberg et al., Reference Rosemberg, Noon and Meslow1997). Several studies show that herpetofauna are able to use riparian forests (Burbrink et al., Reference Burbrink, Phillips and Heske1998; Maisonneuve & Rioux, Reference Maisonneuve and Rioux2001). However, the presence of corridors did not influence species richness, total abundance or abundance of E. gaudichaudii in the Ibiúna landscape, as has been observed in Australia (Driscol, Reference Driscol2004). The lack of influence of corridors is probably related to the absence of an effect of fragment size. The leaf-litter lizards of the fragments that we studied at Ibiúna are therefore functionally isolated (Trakhtenbrot et al., Reference Trakhtenbrot, Nathan, Perry and Richardson2005).
In conclusion, the effects of forest fragmentation in Ibiúna on the two dominant sympatric lizard species E. perditus and E. gaudichaudii are influenced by differences in their microhabitat use, and E. perditus could be a good indicator of disturbance. In addition, the conservation of this assemblage of leaf-litter lizards will depend, at a regional scale, on the maintenance of large tracts of continuous forest because neither the size of fragments nor the presence of connections that could favour regional (metapopulation) dynamics seemingly influence lizard occurrence. Strategies for conservation of leaf-litter lizards in such highly fragmented Atlantic Forest landscapes will need to consider greater connectivity between fragments and continuous forest, allowing the latter areas to act as sources of individuals for fragments.
Acknowledgements
We are grateful to Henning Steinicke, Guarino R. Colli, Marcio Martins, Ricardo J. Sawaya, Ana Carolina O.Q. Carnaval and two anonymous reviewers for helpful comments, to Miguel T. Rodrigues for identifying lizards, Jose Mario B. Ghellere, Marcelo Awade, Maria Cristina Peruzin, Ricardo Braga-Neto and Sergio Marques de Sousa for help in the field, José Roberto Nali and SABESP for facilitating our research at the Morro Grande Reserve, and the many landowners who authorized access to their properties. This work was carried out with permits for herpetofauna capture and collection from the Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis. Funding was provided by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazilian Council for Research and Technology (CNPq), German Federal Ministry of Education and Research (BMBF) and Fundação O Boticário de Proteção à Natureza.
Biographical sketches
Marianna Dixo has been involved in conservation biology and biodiversity research on amphibians and reptiles since 1999. Her current research focus is the diversity of leaf herpetofauna in three fragmented landscapes of the Brazilian Atlantic forest with different amounts of remaining habitat, investigating the importance of fragment size and connectivity for the conservation of leaf-litter herpetofauna. Jean Paul Metzger carries out research on the biological effects of forest fragmentation and connectivity, particularly in the Brazilian Atlantic rainforest.







