Introduction
Dementia and parkinsonism are the two most common neurodegenerative syndromes globally Reference de Lau and Breteler1,2 . Individuals with dementia and parkinsonism often experience impaired functional and work capacity, reduced quality of life, and shortened lifespans Reference Koerts, Konig and Tucha3–Reference Rao, Suliman and Vuik8 . These conditions are also costly to patients, their families, caregivers, and society Reference Koerts, Konig and Tucha3,Reference Martinez-Martin, Rodriguez-Blazquez and Paz9–Reference Wimo, Guerchet and Ali15 . In many high-income countries, there is a growing population of ethnic minority and immigrant groups Reference Rechel, Mladovsky and Ingleby16,Reference Singh and Hiatt17 , which underscores the importance of understanding the distribution of neurological disease burden in populations with varying immigrant and ethnic backgrounds over time. Understanding these time trends will help identify potential priority groups affected by these conditions, which would better inform health care planning, resource allocation, policy decisions, and research priorities.
Little is known as to whether the incidence and prevalence of dementia and parkinsonism vary among different ethnic populations in high-income countries such as Canada and the USA. Ethnicity refers to the social group a person belongs to and identifies with, or with which others identify the person Reference Bhopal18 . This identification stems from a multitude of cultural and other factors, including ancestry, religion, language, and physical features associated with race Reference Bhopal18 . The prevalence of dementia is forecasted to increase by more than 300% between 2001 and 2040 in China and India, compared to 100% in developed countries based on projected population estimates, incidence, remission, and mortality Reference Ferri, Prince and Brayne19 . Chinese and South Asians constitute the fastest growing ethnic groups in many parts of Canada and the USA 20–Reference Passel and Cohn22 . Identifying differences in disease burden by ethnicity is critical to guide prevention and health care strategies, given the greatest burden of deaths and disability from neurological disorders is in low- and middle-income countries Reference Feigin, Vos and Nichols23 , and increasing migration of these populations to high-income countries. When assessing immigrant health, it is important to consider the healthy immigrant effect, whereby immigrants recently landed in Canada tend to be healthier than Canadian-born residents, but this health advantage appears to reduce over time Reference Lu and Ng24 . It is unclear whether this health advantage also disappears over time among recent immigrants in terms of dementia and parkinsonism.
Emerging evidence suggests that the epidemiology of dementia and parkinsonism varies across ethnic and immigrant groups Reference Mehta and Yeo25–Reference Parlevliet, Uysal-Bozkir and Goudsmit30 . A systematic review reported higher dementia incidence rates in the USA for Blacks and Caribbean Hispanic populations than other ethnic groups Reference Mehta and Yeo25 . For Parkinson’s disease, Van Den Eeden et al. reported lower incidence rates for Asians and Blacks than Hispanics and non-Hispanic Whites in the USA Reference Van Den Eeden, Tanner and Bernstein26 , although this could be related to care disparities as Blacks tend to present with more severe disease Reference Hemming, Gruber-Baldini and Anderson31 . There is also considerable burden of neurodegenerative conditions among immigrants in Italy, Norway, the Netherlands, and the UK Reference Canevelli, Lacorte and Cova27–Reference Parlevliet, Uysal-Bozkir and Goudsmit30 . Parlevliet et al. reported that dementia was three to four times more prevalent in most non-Western immigrant groups compared to the native Dutch population Reference Parlevliet, Uysal-Bozkir and Goudsmit30 . Notably, studies are needed to assess the incidence and prevalence of dementia and parkinsonism among ethnic and immigrant groups in Canada. Approximately 250,000 immigrants (0.8% of the population) arrive annually to Canada from other countries globally, and the greatest proportion settles in the province of Ontario 32 . As the proportion of immigrants continues to grow, exploring these trends and potential drivers impacting health among immigrant and ethnic groups would inform public health and health care strategies to improve outcomes.
We thus conducted a population-based study to assess the time trends of the incidence and prevalence of dementia and parkinsonism in Ontario across major ethnic groups (i.e., Chinese origin, South Asian origin, and the General Population) and immigrant populations (i.e., recent immigrants, longer-term immigrants, and long-term residents). We also explored the influence of risk factors on dementia and parkinsonism incidence trends among persons of Chinese and South Asian origin. These ethnic origins were selected because they represent the two largest ethnic minority groups in Canada 20 .
Methods
Study Design and Population
We established two population-based open cohorts in Ontario, one for dementia from 2001 to 2014 and one for parkinsonism from 2001 to 2015, by linking population-based health administrative and vital statistics databases held at ICES (formerly the Institute for Clinical Evaluative Sciences) using unique encoded identifiers. These open cohorts comprised a series of annual cohorts from 2001 to 2015 fiscal years (i.e., April 1, 2001–March 31, 2016) for calculating annual incidence and prevalence of dementia and parkinsonism. To define the numerator for each cohort, we included all Ontario residents who were: (1) aged 20 to 100 years; (2) registered with the Ontario Health Insurance Plan (OHIP); and (3) diagnosed with incident dementia or parkinsonism for calculating annual incidence, or prevalent cases of dementia or parkinsonism for calculating annual prevalence. To define the denominator for calculating annual prevalence, we included all Ontario residents aged 20 to 100 years who were registered with OHIP as the overall population each year (i.e., those with and without dementia or parkinsonism). For calculating annual incidence, we further restricted to the population at risk of dementia or parkinsonism at the beginning of each year as the denominator by excluding prevalent cases. OHIP is a universal publicly funded health insurance plan for the entire Ontario population. We reported the incidence and prevalence of dementia up to March 31, 2014 (2001 to 2014), and of parkinsonism up to March 31, 2015 (2001 to 2015) due to differences in case ascertainment Reference Jaakkimainen, Bronskill and Tierney33,Reference Butt, Tu and Young34 as described below.
Data Sources
We assembled this cohort using Ontario’s Registered Persons Database (RPDB), a registry of all Ontario residents who have ever had provincial health insurance Reference Chan35 . We used the following databases to ascertain cases of dementia or parkinsonism: (1) OHIP for physician visits; (2) Ontario Drug Benefit for drug benefit claims for those aged 65 years and older; and (3) Canadian Institute for Health Information (CIHI) Discharge Abstract Database and Same Day Surgery database for hospitalizations and same day surgeries, respectively (for dementia only). We linked the following databases to obtain demographics, ethnicity, and immigration status: (1) RPDB (age and sex); (2) Census (neighborhood income quintile, residence [urban versus rural]); and (3) Immigration, Refugees, and Citizenship Canada Permanent Resident (IRCC-PR) Database for immigration data (i.e., time since immigration versus long-term residence). This database includes immigration application records for people who initially applied to land in Ontario with records dating from 1985. Landed immigrants who resided in Ontario for at least three months or longer are eligible for OHIP coverage. Specifically, the IRCC-PR database captures immigrants and refugees who landed in Ontario since 1985 and became permanent residents. Immigrants prior to 1985 were combined with Canadian-born in the long-term residence category since IRCC-PR records date from 1985 and onward; and (4) ICES-derived ETHNIC cohort, described below, for ethnic origin. These databases cover virtually the entire Ontario population Reference Chen, Kwong and Copes36 .
Case Ascertainment for Dementia
We used an administrative database algorithm to ascertain cases of dementia that was previously validated against primary care electronic medical records Reference Jaakkimainen, Bronskill and Tierney33 . Dementia included Alzheimer’s disease, vascular dementia, dementia in other diseases classified elsewhere (frontotemporal dementia and idiopathic normal pressure hydrocephalus), and unspecified dementia (senile dementia and presenile dementia). Dementia was ascertained based on either one hospital admission with a diagnosis of dementia, or at least three OHIP claims for dementia within 2 years (with date of first OHIP claim as incidence date), or at least one prescription for drugs specific to dementia Reference Jaakkimainen, Bronskill and Tierney33 . Drugs used to identify dementia included donepezil, galantamine, rivastigmine, tacrine, and memantine. This algorithm has a sensitivity of 79.3%, specificity of 99.1%, positive predictive value of 80.4%, and negative predictive value of 99.0% Reference Jaakkimainen, Bronskill and Tierney33 .
Case Ascertainment for Parkinsonism
A validated administrative database algorithm was used to ascertain cases of parkinsonism, which included Parkinson’s disease, idiopathic or primary parkinsonism, parkinsonian syndrome, atypical Parkinson’s, and secondary Parkinson’s Reference Butt, Tu and Young34 . Parkinsonism was determined based on one physician claim with a diagnostic code for parkinsonism and one drug claim for a medication used to treat parkinsonism within a 6-month period, or two physician claims within a 1-year period Reference Butt, Tu and Young34 . Drugs used to identify parkinsonism included levodopa drugs, monoamine oxidase B inhibitors, dopamine agonists, and catechol-O-methyltransferase inhibitors. This algorithm has a sensitivity of 77.8%, specificity of 99.9%, positive predictive value of 76.9%, and negative predictive value of 99.9% Reference Butt, Tu and Young34 .
Incident cases of dementia and parkinsonism were defined as the first recorded dementia or parkinsonism billing or hospitalization code, respectively. This served as a proxy for incident diagnosis of dementia and parkinsonism similar to previous studies assessing incidence trends of these conditions ascertained using administrative databases Reference Kosteniuk, Morgan and O’Connell37–Reference Vanderkruk, Eberg and Mahootchi39 . We created two cohorts to identify cases of dementia and parkinsonism because dementia and parkinsonism require, respectively, a 2-year and 1-year look-forward window for case ascertainment Reference Jaakkimainen, Bronskill and Tierney33,Reference Butt, Tu and Young34 .
Ethnicity
For ethnicity, we used a validated approach to identify persons of Chinese and South Asian origins Reference Shah, Chiu and Amin40 . As health administrative data in Canada do not include ethnic identifiers, the approach uses lists of surnames that are highly specific for each ethnic group and validated against self-reported Chinese and South Asian ethnicity from the Canadian Community Health Survey (CCHS). These surname lists use the earliest recorded surname, which minimizes misclassification of ethnicity related to changes in surname after marriage. The surname list for Chinese has a sensitivity of 80.2%, specificity of 99.7%, positive predictive value of 91.9%, and negative predictive value of 99.2% Reference Shah, Chiu and Amin40 . The South Asian surname list has a sensitivity of 50.4%, specificity of 99.7%, positive predictive value of 89.3%, and negative predictive value of 97.2% Reference Shah, Chiu and Amin40 .
Ethnicity refers to cultural and racial backgrounds that individuals identify with or to which ancestors belonged. We selected these ethnic origins because the two largest ethnic minority groups in Canada are Chinese (ancestry from China, Hong Kong, or Taiwan) and South Asian (ancestry from the Indian subcontinent) 20 . Persons with surnames not on the two lists for Chinese or South Asian origins were considered to be from the General Population 20 . Only 11% of the Ontario population is comprised of other ethnic minority groups (i.e., not Chinese or South Asian) 20 .
Immigration Status
Immigration status refers to whether individuals were Canadian-born or born outside of Canada and subsequently moved to Canada. Immigrant status was defined as follows: (1) recent immigrant, which was less than 10 years after immigration; (2) longer-term immigrant, which was at least 10 years after immigration; or (3) long-term resident.
Selected Risk Factors of Dementia and Parkinsonism
We a priori selected health conditions and behaviors that are known or suspected risk factors for dementia and parkinsonism (Supplementary File). We ascertained these over a 5-year period before the diagnosis. To ascertain traumatic brain injury, diabetes, hypertension, chronic obstructive pulmonary disease (COPD), and congestive heart failure, we used data on hospital discharges, physician office visits, and emergency room visits with administrative database algorithms as used in previous studies (Supplementary File) Reference Tu, Chen and Lipscombe41–Reference Schultz, Rothwell and Chen44 . In addition, we used hospitalization data to ascertain the presence of stroke and coronary artery disease.
We used six cycles of the CCHS (i.e., 2000–2001, 2003–2004, 2005–2006, 2007/2008, 2009/2010, and 2011/2012) to ascertain the prevalence of body mass index, education level, smoking status, and physical activity level in the Ontario population, stratified by self-reported ethnicity and immigration status.
Analysis
We described the cohort with respect to sex, age, income quintile, residence (rural versus urban), ethnic origin (Chinese origin, South Asian origin, or the General Population), and immigration status. We calculated crude and age- and sex-standardized incidence and prevalence of dementia and parkinsonism each year between April 1, 2001 and March 31, 2014 for dementia or March 31, 2015 for parkinsonism. To conduct direct standardization, we used 2001 Census data for the Ontario population on July 1 of each year. We stratified estimates by ethnic group and immigration status. To calculate annual incidence, we used incident cases of dementia or parkinsonism as the numerator and person-years at risk (excluding prevalent cases) as the denominator. We calculated annual prevalence using prevalent cases of dementia or parkinsonism as the numerator, excluding previously incident cases that died, and the Ontario population aged 20 to 100 years on July 1 as the denominator.
Time Trend Analysis
We assessed time trends in incidence of dementia or parkinsonism using Poisson regression models, stratified by ethnic group and immigration status. The dependent variable was the number of incident cases of dementia or parkinsonism with person-years as the offset. We used the two-sided Cochran–Armitage trend test to evaluate time trends of the age- and sex-standardized prevalence of dementia and parkinsonism, stratified by ethnic group and immigration status, over four epochs: 2002–2004 (epoch 1); 2005–2007 (epoch 2); 2008–2010 (epoch 3); and 2011–2013 (epoch 4). We computed an average prevalence in each epoch to assess these time trends. In addition, we adjusted for selected preexisting conditions in the Poisson regression model to explore drivers of dementia and parkinsonism incidence over time. Prevalence estimates based on CCHS data were weighted using CCHS sampling weights to provide population estimates for Ontario. Statistical significance was set at an alpha level of 0.05. All statistical analyses were conducted using the SAS statistical software package version 9.445.
Results
Characteristics of Incident Dementia
From 2001 to 2013/14 (population ˜13.5 million in 2013, with 50.4% female and median age of 40.3 years), we identified 387,937 incident cases of dementia in Ontario, with 39% male and a mean age at diagnosis of 80.0 years (Table 1). The proportion of incident cases who were of Chinese or South Asian origin was 2% and 1%, respectively, and who were recent or longer-term immigrants was 1% and 4%, respectively, during the study period. The mean age at diagnosis of dementia among recent immigrants was 74.9 years (SD 13.3) and 39.5% were male. The mean age at diagnosis of dementia among longer-term immigrants was 77.8 years (SD 11.5) and 38.3% were male. Among incident cases of dementia in 2013/14, similar proportions of patients of Chinese or South Asian origin were recent immigrants (approximately 10%), longer-term immigrants (approximately 40%), and long-term residents (approximately 50%) (Supplemental File).
Table 1: Characteristics of persons with incident dementia from 2001/02 to 2013/14, aged 20 years and older in Ontario, Canadaa
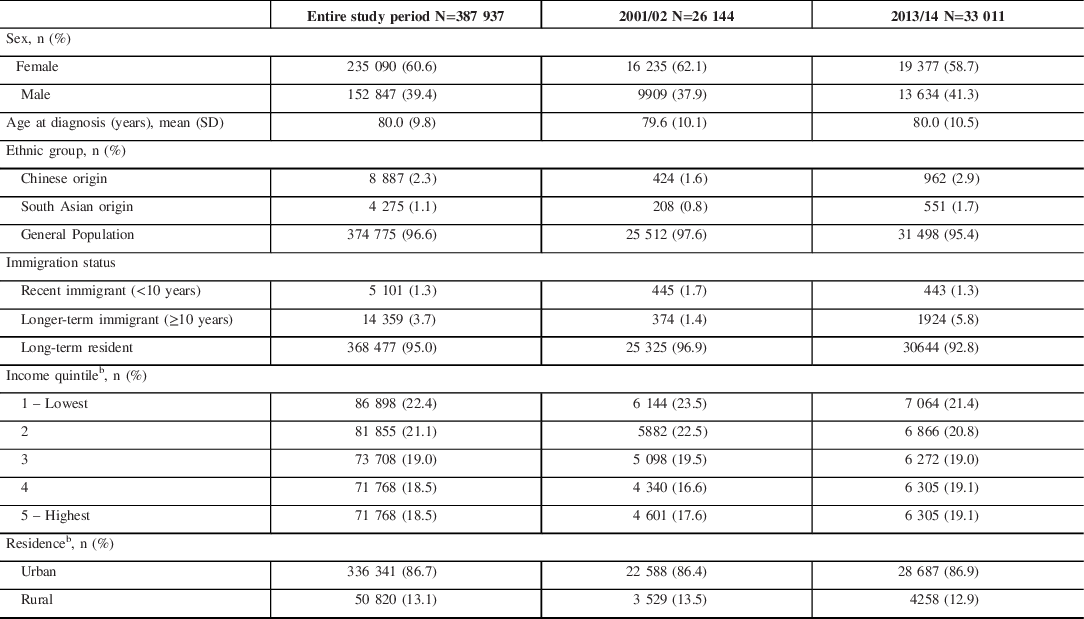
SD = standard deviation.
a Fiscal year starts on April 1.
b Percentages do not add up to 100% due to missing values.
Characteristics of Incident Parkinsonism
We identified 59,617 incident cases of parkinsonism in Ontario from 2001 to 2015, with 56% male and a mean age at diagnosis of 73 years (Table 2). During the study period, the proportion of incident cases who were of Chinese or South Asian origin was 4% and 2%, respectively, and who were recent or longer-term immigrants was 3% and 5%, respectively. The mean age at diagnosis of parkinsonism among recent immigrants was 67.6 years (SD 12.6) and 54.2% were female. The mean age at diagnosis of parkinsonism among longer-term immigrants was 69.9 years (SD 12.8) and 55.9% were female. Among incident cases of parkinsonism in 2014/15, comparable proportions of patients of Chinese or South Asian origin were recent immigrants (11%), longer-term residents (34% and 42%, respectively), and long-term residents (55% and 47%, respectively) (Supplemental File).
Table 2: Characteristics of persons with incident parkinsonism (including Parkinson’s disease) from 2001/02 to 2014/15, aged 20 years and older in Ontario, Canadaa
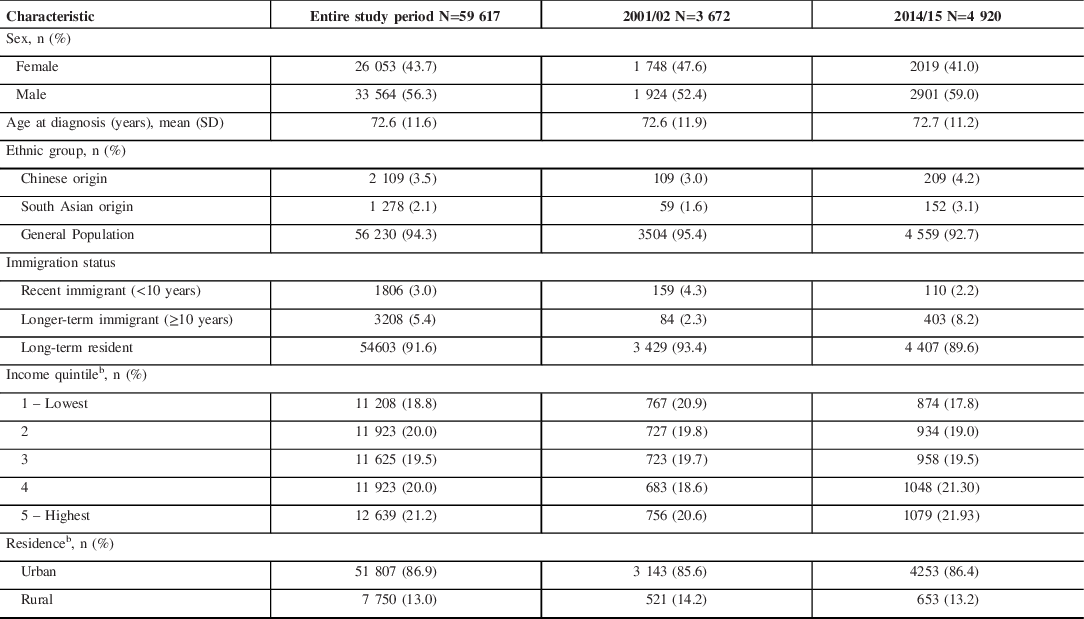
SD = standard deviation.
a Fiscal year starts on April 1.
b Percentages do not add up to 100% due to missing values.
Age- and Sex-standardized Incidence and Prevalence of Dementia
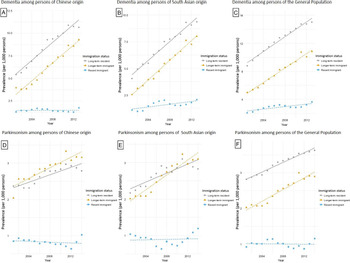
The incidence and prevalence of dementia among Chinese and South Asian were lower compared to that of the General Population across all immigrant groups (Table 3; Figures 1 and 2). When assessing by immigration status, the incidence and prevalence of dementia was higher among long-term residents than recent or longer-term immigrants across selected ethnic groups. For Chinese origin, dementia incidence increased 11.3% for recent immigrants from 2001 to 2014, 41.4% for longer-term immigrants, and 28.1% for long-term residents (Figure 1; Supplementary File). For South Asian origin, dementia incidence increased 42.2% in recent immigrants, 58.2% for longer-term immigrants, and 7.0% for long-term residents. For the General Population, dementia incidence increased 6.4% for recent immigrants, 41.7% for longer-term immigrants, and 4.4% for long-term residents.
Table 3: Age- and sex-standardizeda incidence and prevalence of (A) dementia in 2001/02 and 2013/14 and (B) parkinsonism in 2001/02 and 2014/15 in Ontario, Canada, by ethnicity and immigration statusb
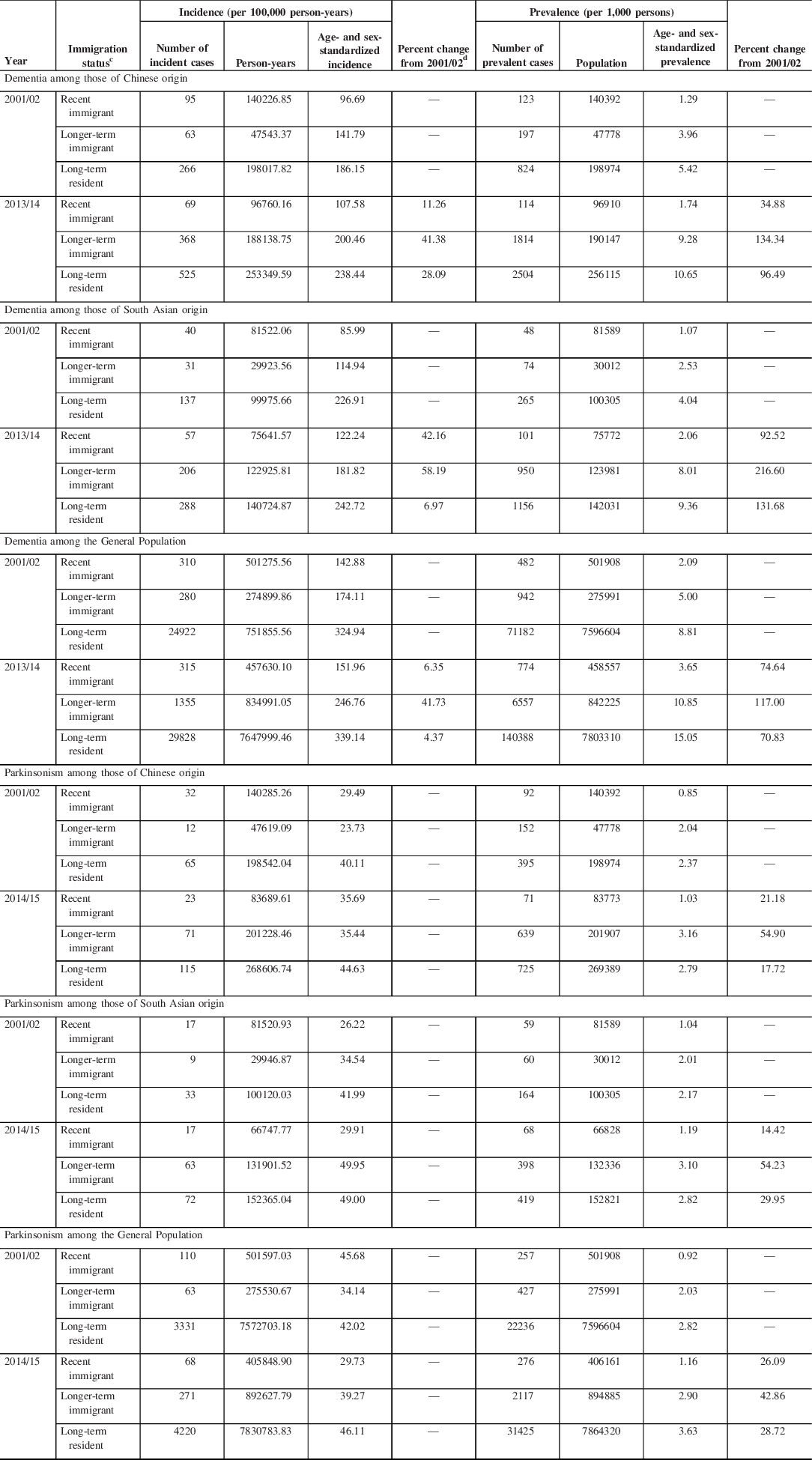
a Standardized using the 2001 Census data for the Ontario population.
b Fiscal year starts on April 1.
c Recent immigrant is <10 years since immigration; longer-term immigrant is ≥10 years since immigration.
d Incidence trends for parkinsonism not assessed due to the relatively small number of cases.

Figure 1: Age- and sex-standardized incidence of dementia among persons of (A) Chinese origin; (B) South Asian origin; and (C) the General Population in Ontario, Canada, stratified by immigration status (recent immigrant: <10 years after immigration; longer-term immigrant: ≥10 years after immigration).
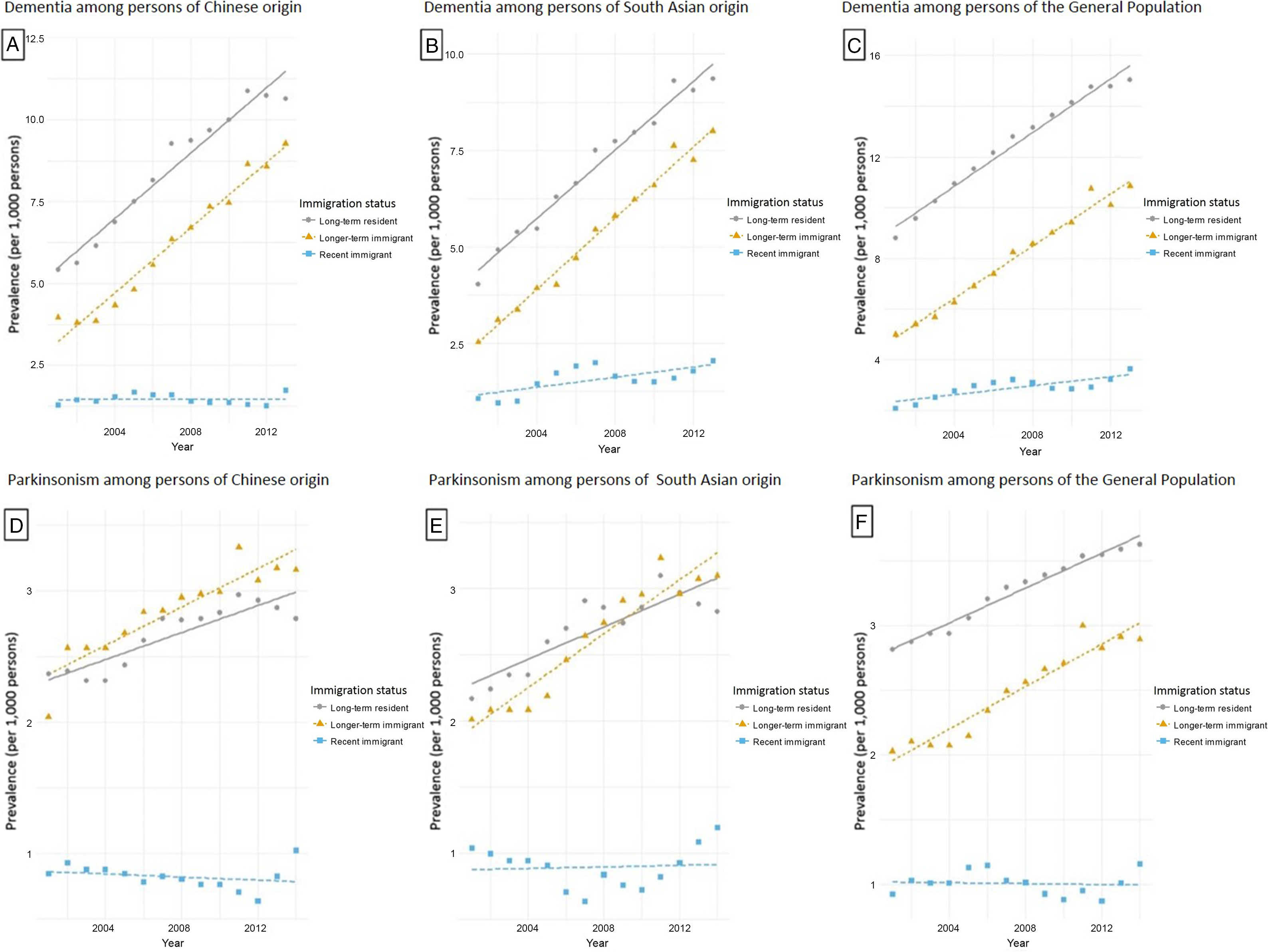
Figure 2: Age- and sex-standardized prevalence of dementia among persons of (A) Chinese origin; (B) South Asian origin; and (C) the General Population, and prevalence of parkinsonism among persons of (D) Chinese origin; (E) South Asian origin; and (F) the General Population in Ontario, Canada, stratified by immigration status (recent immigrant: <10 years after immigration; longer-term immigrant: ≥10 years after immigration).
For persons of Chinese origin, prevalence of dementia increased 34.9% for recent immigrants during the study period, increased 134.3% for longer-term immigrants, and increased 96.5% for long-term residents (Table 3; Figure 2). For South Asian origin, prevalence increased 92.5% for recent immigrants, 216.6% for longer-term immigrants, and 131.7% for long-term residents. For the General Population, prevalence increased 74.6% for recent immigrants, 117.0% for longer-term immigrants, and 70.8% for long-term residents over the study period.
Age- and Sex-standardized Incidence and Prevalence of Parkinsonism
The incidence and prevalence of parkinsonism among Chinese and South Asian were lower than that of the General Population among long-term residents (Table 3; Figures 1 and 2). The relatively small number of cases precluded us from assessing incidence trends stratified by ethnicity and immigration status. For Chinese origin, parkinsonism prevalence increased 21.2% for recent immigrants from 2001 to 2015, 54.9% for longer-term immigrants, and 17.7% for long-term residents (Table 3; Figure 2). For South Asian origin, prevalence increased 14.4% for recent immigrants, 54.2% for longer-term immigrants, and 30.0% for long-term residents. For the General Population, prevalence increased 26.1% over time for recent immigrants, 42.9% for longer-term immigrants, and 28.7% for long-term residents.
Adjustment for stroke and coronary artery disease decreased the dementia incidence trends among persons of Chinese origin, suggesting that these conditions may be drivers of these incidence trends (Supplemental File). Adjustment for other pre-existing medical conditions known to affect neurological conditions did not appear to explain the incidence of dementia or parkinsonism trends over time. In addition, further adjustment for other health-related or behavioral factors (body mass index, educational attainment, smoking, and physical activity level) had little influence on the time trends of the incidence of dementia and parkinsonism (Supplementary File).
Discussion
Across selected ethnic groups in Ontario, dementia incidence and prevalence were considerably higher in long-term residents than recent or longer-term immigrants. Dementia incidence had modest increases among recent immigrants from 2001 to 2014 regardless of their ethnic group, but had larger increases among longer-term immigrants. Similarly, the prevalence of dementia and parkinsonism had modest increases over time for recent immigrants but significantly increased for longer-term immigrants and long-term residents across selected ethnic groups. Adjustment for a number of risk factors for brain health conditions, except for stroke and coronary artery disease, did not appear to explain incidence trends of dementia or parkinsonism over time.
Comparison of Results with Previous Literature
This study provides more recent information on ethnic variations in dementia and parkinsonism compared to previous studies Reference Mehta and Yeo25,Reference Van Den Eeden, Tanner and Bernstein26,Reference Mayeux, Marder and Cote46 . Our study results in the Canadian context suggest that dementia and parkinsonism estimates vary by ethnicity, similar to previous studies reporting ethnic variations in the USA Reference Mehta and Yeo25,Reference Van Den Eeden, Tanner and Bernstein26,Reference Mayeux, Marder and Cote46 . Mehta et al. reported higher dementia incidence among Blacks and Caribbean Hispanic populations than Mexican American, Japanese Americans, and non-Latino White populations, and varied prevalence across ethnic groups in the USA Reference Mehta and Yeo25 . We found lower incidence and prevalence of dementia among Chinese and South Asian than that of the General Population; thus, our study provides new knowledge on these estimates among Chinese and South Asian groups. For parkinsonism, our results generally agree with previous findings but extend our understanding by adding information on immigration status. Van Den Eeden et al. reported lower incidence rates of Parkinson’s disease for Asians and Blacks compared to Hispanics and non-Hispanic Whites in Northern California Reference Van Den Eeden, Tanner and Bernstein26 . We found lower incidence and prevalence of parkinsonism for Chinese and South Asian groups than that of the General Population among long-term residents.
There are potential reasons why differences between ethnic groups could arise. Ethnic groups may have different gene-environment exposures related to the development of neurodegenerative disorders Reference Correia Guedes, Ferreira and Rosa47 . A previous systematic review reported consistent evidence that minority ethnic groups with dementia accessed diagnostic services later in their illness and were less likely to access certain care such as antidementia medication Reference Mukadam, Cooper and Livingston48 . Another systematic review reported considerable barriers to help seeking in minority ethnic groups, which may lead to receiving diagnostic services at a late stage in the neurodegenerative disease Reference Hemming, Gruber-Baldini and Anderson31,Reference Cooper, Tandy and Balamurali49 . Our results suggest that stroke and coronary artery disease are potential drivers of incidence of dementia among persons of Chinese origin. Previous systematic reviews found stroke and coronary artery disease to be independent, potentially modifiable risk factors for dementia Reference Deckers, Schievink and Rodriquez50,Reference Kuźma, Lourida and Moore51 . Further studies are needed to elucidate the role that ethnicity and these risk factors plays on incidence and prevalence trends of neurodegenerative disorders.
Our findings suggest that recent immigrants across major ethnic groups had generally lower incidence and prevalence of dementia and parkinsonism than long-term residents after accounting for age and sex, but this difference diminished with longer-term immigrants. Our results on incidence are consistent with a previous study that reported lower risk of dementia and Parkinson’s disease among immigrants compared to the Swedish-born population Reference Wändell, Fredrikson and Carlsson52,Reference Wändell, Carlsson and Li53 . Previous literature reported inconsistent results on whether prevalence of dementia was higher or lower among immigrants compared to non-immigrant populations Reference Diaz, Kumar and Engedal29,Reference Moon, Badana and Hwang54,Reference Parlevliet, Uysal-Bozkir and Goudsmit55 . Our study results are novel in exploring trends of incidence and prevalence of dementia and parkinsonism among recent and longer-term immigrants, which may help elucidate disease patterns in immigrant populations.
Our findings are consistent with the healthy immigrant effect, whereby recent immigrants to high-income countries such as Canada appear relatively healthier than long-term residents Reference Singh and Hiatt17,Reference Kennedy, McDonald and Biddle56 . There is a growing body of literature reporting immigrants to be healthier than Canadian-born residents at the time of arrival, but this health advantage diminishes over time, potentially from challenges adjusting to new environments, stress, or adopting unhealthy behaviors Reference Lu and Ng24 . Since key pre-existing medical conditions and behavioral factors (e.g., smoking, physical activity) were accounted for in our study, this suggests that changes in other factors over time may have contributed to the increased incidence trends in longer-term immigrants. Other potential drivers to consider in future research includes environmental exposures (e.g., air pollution and toxins), health care access, stress, adjustments to new environments, and gene-environment exposures Reference Lu and Ng24,Reference Feldman, Johansson and Lambert57–Reference Breckenridge, Berry and Chang59 . Protective effects may also have been present in the countries of origin. Future research to identify other drivers of dementia and parkinsonism trends across ethnic and immigrant groups is warranted. It is important for decision-makers and health care providers to consider prevention and health care strategies to help prevent the incidence of dementia and parkinsonism in immigrant populations across selected ethnic groups. These findings may guide early detection, management, equitable access to health services, and monitoring of health over time among immigrants related to these neurological disorders.
In addition, previous studies identified barriers to care among recent immigrants with neurodegenerative conditions, which may delay or hinder an appropriate diagnosis Reference Sagbakken, Spilker and Nielsen60–Reference Czapka and Sagbakken64 . Health care providers reported challenges in the assessment and early detection of disease, including language barriers and difficulties involving family members or interpreters Reference Sagbakken, Spilker and Nielsen60–Reference Tillmann, Just and Schnakenberg63 . Immigrants and their families may experience barriers to help-seeking or accessing care, lack of knowledge on neurodegenerative conditions or services, or difficulties communicating due to language Reference Czapka and Sagbakken64 . In our study, the mean age of physician diagnosis for dementia and parkinsonism were similar between recent and longer-term immigrants. While barriers to health services among recent immigrants may potentially delay diagnosis, it does not appear to explain entirely our observed trends of dementia and parkinsonism in these subpopulations. Nonetheless, decision-making and future research should consider culturally tailored and patient-centered approaches to provide information, treatment, and services for these patient populations.
Strengths and Limitations
Our study has strengths. First, we computed population-based incidence and prevalence using a large sample over a long time frame (387,937 incident cases of dementia and 59,617 incident cases of parkinsonism from 2001 to 2014/15). Canada has the highest per-capita rates of immigration and Ontario is the most populous province in Canada Reference Fearon65,66 . Approximately 250,000 immigrants (0.8% of the population) arrive annually to Canada from other countries globally, and the greatest proportion settles in the province of Ontario 32 . Second, we used population-based health administrative databases with validated algorithms to ascertain cases of dementia and parkinsonism Reference Jaakkimainen, Bronskill and Tierney33,Reference Butt, Tu and Young34 . These algorithms have relatively high accuracy Reference Jaakkimainen, Bronskill and Tierney33,Reference Butt, Tu and Young34 . Finally, we used a validated approach to identify persons of Chinese and South Asian origins Reference Shah, Chiu and Amin40 .
Our study has some limitations. First, incident cases of dementia and parkinsonism in this study as ascertained using algorithms may have had an earlier diagnosis before the study period. Using administrative databases may not capture individuals without clinically recognized symptoms and those who have not sought health care. Previous studies reported barriers to care among recent immigrants with neurodegenerative conditions, which may delay or hinder an appropriate diagnosis Reference Sagbakken, Spilker and Nielsen60–Reference Czapka and Sagbakken64 . The imperfect sensitivity of the algorithms for dementia or parkinsonism may have led to underestimates. On the contrary, there may be overestimating since outcomes are based on physician billing codes that may not be accurate. It is important to note that drug claims data do not capture those aged less than 65 years, so rates may be underestimated in younger age groups. Second, despite using the entire Ontario population, this study may still have been underpowered to detect significant trends in individuals with dementia or parkinsonism of Chinese or South Asian origin, particularly among recent immigrants (<5% of incident cases). Nevertheless, we provided stratified estimates of incidence and prevalence trends by ethnicity and immigration status to address important knowledge gaps for dementia and parkinsonism. Third, immigrant data were limited to immigrants and refugees who landed in Ontario since 1985 and became permanent residents. Immigrants who landed in Ontario before 1985 were combined with long-term residents since IRCC-PR data records are only available from 1985. However, many health and behavioral factors of immigrants living in Canada over 15 to 20 years become similar to those of non-immigrants Reference Chiu, Austin and Manuel67,Reference Perez68 . Finally, we were unable to ascertain other risk factors (e.g., environmental exposures and drug exposure) for dementia and parkinsonism. Future research is needed to examine the effects of other risk factors on time trends in dementia and parkinsonism incidence among different ethnic and immigrant groups.
Conclusion
Our study found that recent immigrants across major ethnic groups (i.e., Chinese, South Asian, and the General Population) in Ontario had considerably lower rates of dementia and parkinsonism than long-term residents. However, this difference diminished with longer-term immigrants in selected ethnic groups. This information will help public health professionals and health policy decision-makers tailor public health strategies to identified priority groups, including longer-term immigrants across selected ethnic groups. As the proportion of immigrants continues to grow, further research exploring these trends and potential drivers impacting health over time among ethnic and immigrant populations would facilitate culturally-tailored public health and intervention strategies to improve health outcomes.
Acknowledgments
This study was supported by Public Health Ontario (PHO) and ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this paper are those of the authors and are independent from the funding sources. Parts of this material are based on data and/or information compiled and provided by Canadian Institute for Health Information (CIHI) and by Immigration, Refugees and Citizenship Canada (IRCC). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the author(s), and not necessarily those of CIHI or IRCC. No endorsement by ICES, Ontario MOHLTC, CIHI, or IRCC is intended or should be inferred.
Funding
Funding for this study is provided by Health Canada (MOA-4500314182). The funding agency was not involved in the study design, analysis or interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Dr. Jessica Wong is supported by tuition assistance from the Canadian Memorial Chiropractic College. Drs. Jeff Kwong, Karen Tu, and Debra Butt are supported by Investigator Awards from the Department of Family and Community Medicine, University of Toronto.
Disclosures
All authors report no disclosures.
Statement of Authorship
JJW, HC, JCK, KT, DAB, and BRS contributed to the study design. JJW, HC, ASW, and AK prepared and cleaned the data. JJW, HC, JCK, KT, DAB, ASW, BRS, and AK contributed to the data analyses. JJW took the lead in drafting the manuscript. All authors contributed to interpretation of data, provided critical revisions to the manuscript, and approved the final draft.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.7.